Abstract
Purpose
To determine the maximum tolerated dose (MTD) of radiation (RT) with concurrent temozolomide (TMZ) in patients with newly diagnosed glioblastoma (GBM), to estimate their progression free (PFS) and overall survival (OS), and to assess the role of 11C methionine PET (MET-PET) imaging in predicting recurrence.
Methods and Materials
Intensity modulated RT (IMRT) doses of 66–81 Gy, assigned to patients by the time-to-event continual reassessment method, were delivered over 6 wks with concurrent daily TMZ (75 mg/m2) followed by adjuvant cyclic TMZ (200 mg/m2 d1-5 q28d x6 cycles). Treatment was based on gadolinium-enhanced MRI. Pretreatment MET-PET scans were obtained for correlation with eventual sites of failure.
Results
38 patients were analyzed with a median follow-up of 54 months for patients who remain alive. Late CNS grade≥3 toxicity was observed at 78 (2 pts of 7) and 81 Gy (1 pt of 9). None of 22 patients receiving ≤75 Gy developed radiation necrosis. Median OS and PFS were 20.1(14.0, 32.5) and 9.0 (6.0, 11.7) months, respectively. Twenty-two of 32 patients with pretreatment MET PET uptake showed uptake beyond the contrast-enhanced MRI. Patients whose treatment did not include the region of increased MET-PET uptake demonstrated an increased risk of non-central failure (p<0.001).
Conclusions
GBM patients can safely receive standard TMZ with 75 Gy in 30 fractions, delivered using IMRT. The median OS of 20.1 months is promising. Furthermore, MET-PET appears to predict regions of high risk of recurrence not defined by MRI, suggesting that further improvements may be possible by targeting metabolically active regions.
INTRODUCTION
Patients with glioblastoma (GBM) treated with a standard radiation therapy (RT) dose of 60 Gy, typically progress in the high dose region.1–3 Although the addition of concurrent and adjuvant temozolomide (TMZ) has improved over all survival, a majority of tumors continue to progress locally.4–5 One possible explanation for this lack of local control is that the current standard dose of 60 Gy is insufficient. Recent advances in RT delivery, such as intensity modulated radiation (IMRT),6 might permit us to safely escalate RT doses by limiting the RT dose to normal tissues7 which, when combined with effective chemotherapy, may improve outcome.
An important obstacle to the effectiveness of dose escalated RT is the inability to precisely target the tumor. Evidence now suggests that amino acid positron emission tomography (PET) using 11C methionine (MET-PET) may identify glioma beyond the region identified by conventional magnetic resonance imaging (MRI).8–11 However, no large prospective studies have yet correlated MET-PET uptake with patterns of failure after treatment, although such information could be very useful in optimizing the regions requiring high-dose RT for primary GBM.12
We hypothesized that the use of IMRT would permit us to escalate the dose of RT with concurrent TMZ substantially above the 60 Gy currently used. Thus, the primary objective of this study was to determine the maximum tolerated dose (MTD) of IMRT delivered over 6 weeks with concurrent TMZ in primary GBM, as well as to make a preliminary estimate of the overall survival (OS) and the progression free survival (PFS). A second important goal of this study was to assess the patterns of failure in patients who had prospectively undergone MET-PET imaging. We hypothesized that a subset of patients might progress in MET-PET avid regions outside the standard target defined by T1 gadolinum-enhanced MRI. In such cases, MET-PET might improve standard targeting by identifying areas at highest risk of recurrence in future studies.
METHODS AND MATERIALS
Patient eligibility
This study was approved by the University of Michigan’s Institutional Review Board. Eligible patients were 18 years or older, with a Karnofsky performance status (KPS) of 70 or greater, newly diagnosed with histologically-confirmed supratentorial (World Health Organization) Grade IV gliomas including glioblastoma and gliosarcoma, and had adequate bone marrow reserve, liver and renal function. Treatment was required to begin within 5 weeks of surgical resection. Exclusion criteria precluded multifocal, recurrent gliomas, infratentorial tumors, evidence of cerebrospinal fluid (CSF) dissemination, severe concurrent disease, prior malignancy requiring cytotoxic chemotherapy within one year, prior RT therapy leading to overlap of RT fields, planned final boost exceeding one-third of the brain, or inability to undergo magnetic resonance imaging (MRI). All patients underwent baseline MRI (T1, post-gadolinium T1, and fluid attenuated inversion recovery (FLAIR) etc), and MET-PET.
PET Imaging Parameters
MET-PET was obtained on a Siemens ECAT EXACT HR+ whole body tomography (axial resolution 4.1 mm full width at half maximum in the center of the field of view.13 Following intravenous injection of approximately 740 MBq of 11C MET in a dynamic acquisition, emission scans were obtained in a three-dimensional mode. Summed image data obtained between 10 and 30 minutes post injection were utilized for further analysis. PET uptake was defined by automatic segmentation using a threshold of 1.5 times mean activity and normalized to the mean activity of the normal brain, defined as the cerebellum as previously described.14
Image registration
A treatment planning computed tomography (CT) scan was obtained with the patient immobilized in an individualized thermoplastic mask. . Image registration of research scans was performed using functional imaging analysis tools (FIAT), a software package developed at the University of Michigan.15 Registration of the accumulated RT dose plan was accomplished by applying the same transformation. These methods are able to detect discrepancies in registration with a magnitude of 1 voxel or approximately 2–3 mm.
TITE-CRM RT dose allocation
Individual RT dose levels were allocated according to the TITE-CRM algorithm.16Per protocol a dose limiting toxicity was defined as any grade 3 or 4 irreversible CNS toxicity, non-hematologic, non-CNS grade 4 toxicity or any grade 5 toxicity. For each patient, the probability of dose-limiting toxicity (DLT) was estimated based on the expected risk, as well as the incidence of DLT in patients already treated, weighted by the amount of time patients had been followed. At the time of study enrollment, each patient was assigned RT dose with estimated probability of DLT closest but less than the target rate of 25%. RT dose escalation was restricted to one level between sequential patients. Prior to assigning patients to the next higher RT dose level, at least one patient treated at the previous level completed three months of observation without any DLT.
RT Volumes
Gross tumor volumes (GTVs) were defined as the residual gross tumor or resection cavity, based on the contrast enhancing T1-weighted MRI. GTVs were expanded within the skull uniformly by 1.5 cm to form the clinical target volume (CTV). CTV and GTV were expanded uniformly by 0.5 cm to generate planning target volumes, (PTV1 and PTV2, respectively). IMRT plans were generated to deliver 60 Gy in 30 fractions to PTV1 and a simultaneous higher dose (range, 66–81 Gy) to the smaller target, PTV2. T2/FLAIR signal abnormality was not targeted. The maximum dose limits to normal tissue organs at risk were defined as 60 bioGy to the optic nerves and chiasm, and brainstem was limited to 65 bioGy using alpha/beta ratio of 2.5.
Chemotherapy
Patients received concomitant TMZ 75 mg/m2 daily for six weeks. Four weeks following completion of RT, patients without evidence of disease progression continued to receive adjuvant TMZ 200 mg/m2 days 1–5 every 28 days, for six to twelve cycles or until evidence of disease progression. Additional cycles were prescribed at the discretion of the treating neuro-oncologist. Pneumocytis jirovecii prophylaxis using aerosolized pentamidine was maintained monthly during daily TMZ chemotherapy. During the later phase of the study, CD4 counts were closely monitored in patients who developed grade 3 lymphopenia. Pneumocystis prophylaxis was continued during adjuvant TMZ in patients with low CD4 counts during the final 3 weeks of concomitant TMZ. Anti-seizure medications and steroids were given as clinically indicated. Doses were recorded at each treatment evaluation.
Toxicity
Chemotherapy and radiation toxicities were graded using the Common Toxicity Criteria (CTC) version 3.0.17Acute toxicity was assessed during the first 90 days following RT. Late toxicity was assessed every 3 months during the first year and every 6 months thereafter. All patients were monitored for late CNS toxicity until death. Additional imaging studies including MR Spectroscopy, MET-PET and if possible stereotactic biopsies were obtained at time of suspected tumor progression to differentiate it from radiation necrosis.
Response Criteria
Conventional MRI was obtained at 1 month, and every 2–3 months thereafter. Response was defined using standard Macdonald criteria.18 As published data regarding pseudo-progression became available, a finding of worsening enhancement noted within 3 months of treatment completion was followed closely for progression versus pseudo-progression. Second line therapy was given at the physician’s discretion following progression.
Pattern of failure
A pattern of failure was determined by registering the MRI at progression with the delivered RT dose distribution as previously described.19 The location of failure was classified according to the proportion of the volume of the rVOI contained within the 95% high dose prescription isodose surface: central (>95%), in field (>80–95%), marginal (20–80%), or distant (<20%). MET PET images were co-registered with the baseline MR post-gadolinium T1 as well as recurrence MRI.
Statistical analysis
The primary endpoint was to evaluate the rate of acute and late treatment-related toxicities. A standard two-parameter logistic regression model was used at the end of the trial to obtain estimated probabilities of toxicity by dose. Secondary endpoints included PFS and OS. Kaplan-Meier estimates of median PFS and OS functions were determined with 95% confidence intervals. PFS was measured from the date of resection to progression, death, or last follow-up. Univariate proportional hazards regression models were used to assess the relation of dose and other clinical covariates to PFS and/or OS. Fisher’s exact test was used to test for an association between coverage of PET GTV and subsequent non-central failure.
RESULTS
Patient characteristics
Between November 2003 and August 2007, 42 consecutive patients were enrolled. Three patients did not participate due to a subsequent determination of ineligibility (delay in RT > 5 wks in 2 patients) or withdrawn consent (1 patient). One patient was non-evaluable for DLT per protocol due to clinical deterioration following the initial five RT fractions. MR scan showed tumor progression; all therapies were halted and the patient was transferred to hospice. This patient was included for survival analysis. The remaining thirty-eight patients were analyzed for long-term toxicity, with a median follow-up of 54 months (range: 42–62) for patients who remain alive. Patient characteristics are listed in Table 1. The median patient age was 56 years (range: 23–75).
Table 1.
Patient Characteristics (N=38)
| Age (years), median (range) | 56 (23–75) | |
| Gender | ||
| Male | 19 | |
| Female | 19 | |
| KPS | ||
| 90–100 | 33 | |
| 80 | 3 | |
| 70 | 2 | |
| RPA classification | ||
| III | 13 | |
| IV | 17 | |
| V | 8 | |
| Extent of Surgery | ||
| Gross total resection | 15 | |
| Subtotal resection | 17 | |
| Biopsy only | 6 | |
| Radiation Prescription Dose | ||
| 66 Gy | 1 | |
| 72 Gy | 12 | |
| 75 Gy | 9 | |
| 78 Gy | 7 | |
| 81 Gy* | 9 |
Abbreviations: KPS=Karnofsky performance score, RPA=recursive partitioning analysis
One pt assigned to 81 Gy received less than prescribed dose due to early termination of treatment
Toxicities
Acute toxicities were primarily hematologic toxicities and infections (Table 2). Three grade 5 acute toxicities due to TMZ were observed: (1 patient) sepsis, 75 Gy; (1 patient) prolonged and severe thrombocytopenia with pancytopenia, 75Gy; (1 patient) aplastic anemia assigned to 81 Gy. Aplastic anemia is a rare but reported adverse event with TMZ.21Neither of the patients was receiving trimethoprim-sulfamethoxazole or other drugs known to suppress blood counts.
Table 2.
Chemotherapy and Radiation Toxicities (N=38 patients)
| Acute Toxicities | Late Toxicities | |||||
|---|---|---|---|---|---|---|
| Grade | Grade | |||||
| Category | 3 | 4 | 5 | 3 | 4 | 5 |
| Blood/Bone Marrow | 5 | 4 | 2 | 0 | 0 | 0 |
| Gastrointestinal | 1 | 0 | 0 | 0 | 0 | 0 |
| Infection | 3 | 0 | 1 | 0 | 0 | 0 |
| Neurologic | 6 | 0 | 0 | 3 | 0 | 0 |
| Pulmonary | 1 | 0 | 0 | 0 | 0 | 0 |
| Auditory (Hearing) | 0 | 0 | 0 | 1 | 0 | 0 |
Of the 38 patients evaluated for DLT, one patient did not complete the prescribed RT course. This patient was assigned to 81 Gy but received only 75 Gy because of development of aplastic anemia. Late CNS Grade 3 toxicity was reported at 78 Gy (2 patients of 7) and 81 Gy (1 patient of 9). No case of radiation necrosis was observed at or below 75 Gy. Median time to radiation necrosis was 7 months (range: 5.4–8.9). Additional late RT toxicities included one patient with a Grade 3 otitis with conductive hearing loss.
The number of dose-limiting toxicities noted was: 0 of 1 patient at 66 Gy, 0 of 12 at 72 Gy, 2 of 9 at 75 Gy, 3 of 7 at 78 Gy and 2 of 9 at 81 Gy. The estimated probabilities of DLT with 90% confidence intervals are: 66 Gy, 0.02 (0.00, 0.23), 72 Gy, 0.08 (0.02, 0.25); 75 Gy, 0.14 (0.06, 0.28); 78 Gy, 0.23 (0.13, 0.38) and 81 Gy, 0.36 (0.17, 0.61).
Adjuvant and Salvage Therapy
33 patients received adjuvant TMZ while five patients did not receive any adjuvant treatment due to clinical deterioration or death. 17 patients completed at least six cycles.16 patients were discontinued earlier due to tumor progression, toxicity or patient choice. 24 patients (60%) of the 33 patients received salvage chemotherapy at time of progression; nine of whom received salvage bevacizumab.
Fifteen patients underwent a second resection due to either new imaging findings to confirm tumor progression (9) or progressive clinical symptoms (6). Pathology review showed recurrent GBM in 5 patients; RT dose delivered was 66, 72(2 patients), 75, and 78 Gy. Multifocal radiation necrosis with no evidence of residual glioma was noted in 2 patients treated to 78 Gy. Both recurrent GBM and radiation changes with areas of vascular hyalinization, gliosis, and coagulative necrosis were noted in 8 patients RT dose was delivered to72 Gy (3 patients), 78 Gy (2 patients), and 81 Gy (3 patients).
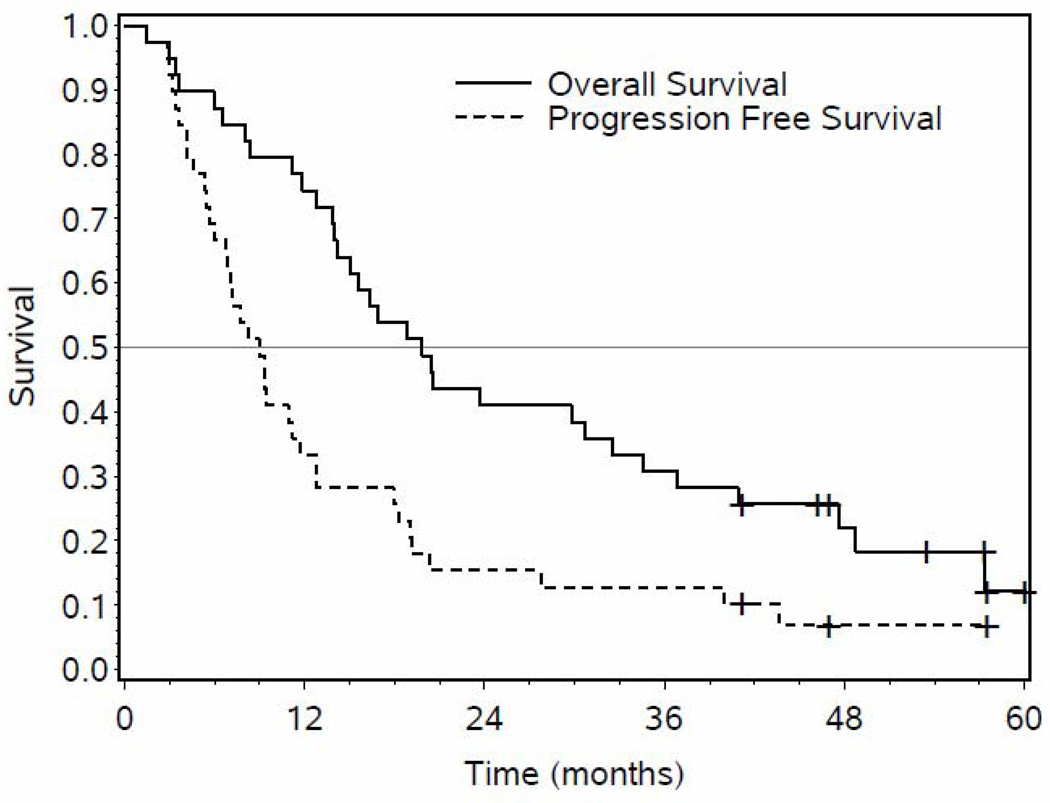
Survival
Median PFS was 9.0 months (95% CI: 6.0, 11.7) and median OS was 20.1 months (95% CI: 14.0, 32.5) (Figure 1). With a median follow-up of 54 months, seven patients remain alive, three patients without evidence of disease progression. Two patients developed other cancers: (1 patient) primary hepatocellular carcinoma two years after completing RT, succumbing shortly thereafter; and (1 patient) Stage IB non-small cell lung cancer 14 months post-treatment eventually succumbing to diffuse metastases. An additional patient died from cardiopulmonary arrest shortly after re-resection.
Figure 1.
Kaplan Meier overall (solid) and progression free survival curves (dotted line) of all patients (n=39) receiving study treatment is shown above.
We performed exploratory analyses to determine if the escalated RT dose affected either PFS .OS, or the risk of central failure. There was no statistically significant relationship between RT dose and PFS or OS (p> 0.5). We did note a change in the pattern of failure with decreased probability of central failures with increased RT dose (p=0.05). Younger age (p<0.03), resection (p<0.03), and RTOG RPA class 3 (p<0.0003) were associated with improved survival. Both smaller PET (p<0.005) and MRI volumes (p<0.02) were associated with improved PFS but not OS. (Table 3)
Methionine PET GTV and Patterns of Failure
We then explored the potential utility of MET-PET in predicting eventual recurrence to determine if there were regions of PET uptake beyond conventional MRI that may benefit from additional boosting with radiation.
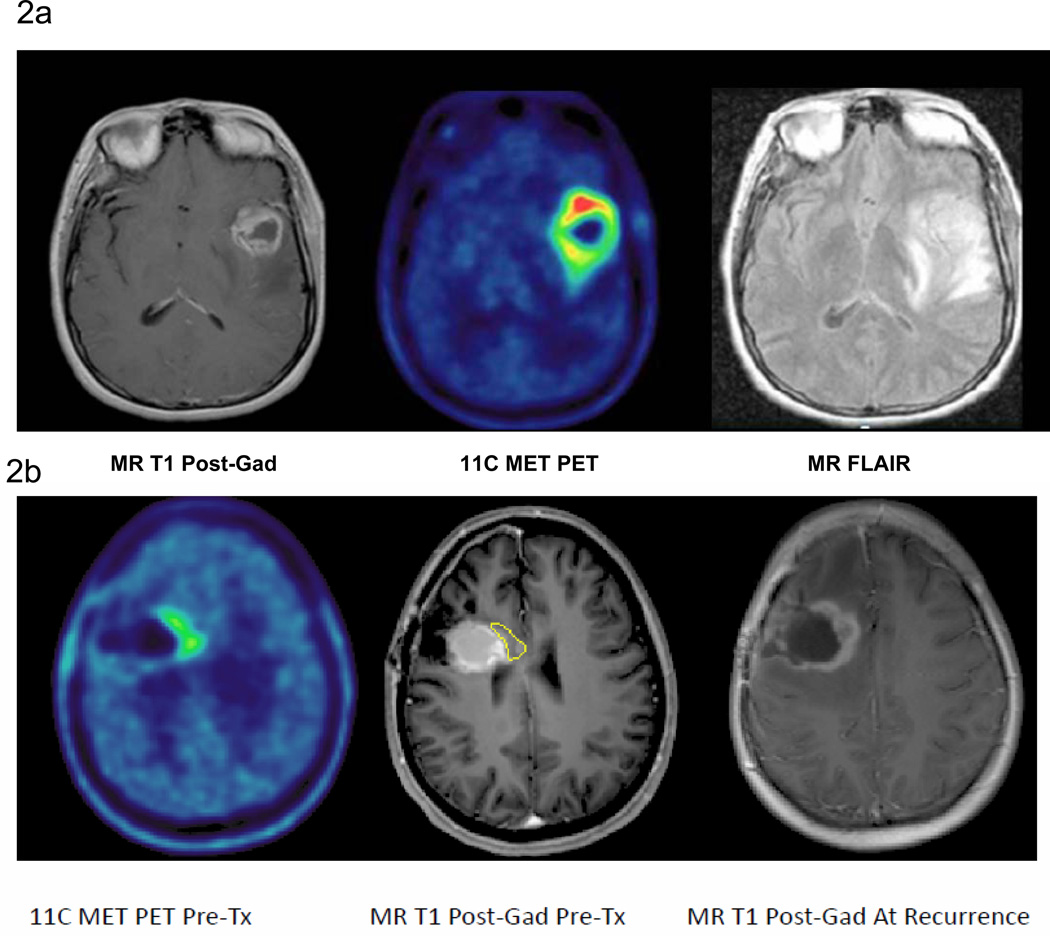
MET PET was particularly helpful in distinguishing residual tumor after resection from post-surgical changes. The tumor volume estimated by MET PET was generally smaller than the contrast-enhancing volume on MRI in patients that underwent resection. The median MRI GTV (defined as areas of peripheral enhancement excluding regions of central necrosis) was 16.4 cm3 (range: 0.8–57.9) while the median MET PET tumor volume was 5.7 cm3 (range: 0.5–43.8 cm3). Of the 32 patients who had appreciable pre-treatment MET PET uptake (region of uptake >1cm3), 22 showed MET PET uptake that extended beyond the gadolinium-enhanced MRI target volume. Among these 22 cases, the mean distance the MET PET uptake extended beyond the gadolinium-enhancingtumor volume was 1cm (range: 0.8–3.5 cm). In the vast majority of cases, the pretreatment MET PET uptake volume fell within the MR FLAIR volume because the MR FLAIR volumes are considerably larger due to peritumoral edema (Figure 2a). In four cases, the PET GTV extended beyond the MR FLAIR volume by a maximum distance of 1.5 cm.
Figure 2.
a 11C MET PET (middle panel) clearly demonstrates areas of increased metabolic uptake extending beyond the contrast-enhancing lesion on MRI (left panel) but not beyond MR FLAIR.(right panel) MR FLAIR volume also includes surrounding peritumoral edema.
b Pre-treatment post gadolinium T1-weighted MRI (middle panel) has been co-registered to the pre-treatment 11CMET-PET scan (left panel) as well as the post gadolinium T1-weighted MRI at recurrence (right panel). This example shows the overlap between the area of initial increased PET uptake (yellow) and eventual area of recurrence.
Of the 28 CNS recurrences noted, 16 were central, 2 were in-field, 8 were marginal and 2 were distant. Suboptimal coverage of the tumor defined by MET PET (defined as less than 95% isodose coverage of MET PET tumor) resulted in a higher risk of subsequent non-central failure (p <0.001). Seven of eight patients with suboptimal PET GTV coverage recurred with non-central failures while 5 of 20 patients with adequate PET GTV coverage developed non-central failures. Furthermore, all of the non-central failures showed overlap with the initial PET GTV. Overlap between the areas of initial increased PET uptake and the eventual area of recurrence on MRI is demonstrated in Figure 2b4.
DISCUSSION
In this study, we have shown that the use of highly conformal radiation techniques permits the safe administration of substantially higher RT doses than the standard 60 Gy. The observed median survival of 20.1 months and the change in pattern of failure with higher RT doses suggest improved efficacy. Late CNS toxicity was not observed with RT doses at or below 75 Gy with concurrent TMZ. Furthermore, we found that patients tended to progress in regions of inadequate coverage of the tumor defined by MET-PET uptake. This suggests that MET-PET is useful in determining tumor extent, and encourages further exploration of using functional imaging to define target volumes as a method of decreasing recurrence in primary GBM.
Prior RT dose escalation trials using RT alone or less effective chemotherapy, including UM 90 Gy dose escalation study 22, RTOG phase III stereotactic radio-surgery boost trial,23RTOG phase I 3D dose escalation study,24 and BTCG randomized phase III interstitial brachytherapy boost trial,25all failed to demonstrate improved survival. Most likely, these studies failed because the RT doses required for tumor cell kill exceeded normal brain tolerance limits. The EORTC/NCIC phase III trial of concurrent TMZ with standard 60 Gy RT followed by adjuvant TMZ confirmed modest gains in long-term outcome.5 The combination of highly conformal, dose escalated RT and effective chemotherapy that radiosensitizes may improve the effectiveness of radiation at dose levels tolerable to the normal brain.
A key finding of our study is that MET-PET appears to define regions of active tumor not apparent using T1 gadolinium-enhanced MRI.11C Methionine (MET-PET) imaging identifies metabolic activity through increased transport mediated by type L amino acid carriers at the level of the blood-brain barrier, which are highly expressed in malignant tumors compared to normal brain.26 Biopsy studies have confirmed that increased MET PET uptake correlates with Ki-67 staining, proliferating cell nuclear antigen expression, and microvessel density.27–29 These studies suggest that MET-PET may improve delineation of tumor extent in gliomas compared to conventional MRI imaging.11These findings are consistent with our observation that inadequate coverage of the region of MET-PET uptake, which can occur when using only standard targeting of the T1-gadolinium enhanced region was associated with area of eventual recurrence.
Our median OS compares favorably with other recently reported GBM trials. However, our study was conducted in an era prior to the routine use of salvage bevacizumab. A number of patients underwent stereotactic biopsies, and a number of patients older than seventy years of age were included. MGMT analysis was performed in a limited number of patients.(26%) MGMT analysis is currently underway in the remaining patients. A recent phase II study examining the use of a novel anti-angiogenic agent, bevacizumab in combination with standard TMZ and RT, reported a median OS of 19.6 months, a significant improvement over the previous EORTC/NCIC data.30In comparison, recently RT/TMZ treated groups have also shown a median OS of 18–20 months in patients treated with either an additional novel chemotherapeutic or received salvage bevacizumab at recurrence.30–31
Although this is a small prospective single institutional study, this is the first study to confirm the safety and tolerability of delivering higher radiation doses with concurrent TMZ delivered in six weeks. We found that 75 Gy in 30 fractions was the maximum radiation dose delivered with concurrent TMZ without a substantial risk of radiation necrosis. It is possible that still higher doses could be safely administered by limiting radiation boost volumes to areas of initial 11C MET PET imaging as well in combination with bevacizumab. A recent study showed that the addition of bevacizumab combined with a highly hypofractionated course of re-irradiation over 5 treatments in recurrent gliomas surprisingly reported no cases of radiation necrosis.32The addition of bevacizumab to chemoradiation may increase the therapeutic ratio through possible anti-tumor effects as well as by allowing the safe escalation of RT by reducing the toxicity associated with radiation necrosis. Thus, it would be reasonable to investigate adding bevacizumab to the combination of TMZ and dose intensified radiation, with the target volume defined using MET-PET. This is a study we are now initiating. This strategy may be of particularly interest in patients predicted to have poor outcome, i.e non-methylated MGMT tumors.
Statement of Translational Relevance.
Recent advancements in RT treatment planning and imaging have improved our ability to deliver highly conformal radiotherapy. Here, we sought to translate these new developments in order to facilitate a new approach to treating GBM. We demonstrate the safety and tolerability of delivering higher radiation (RT) doses with concurrent temozolomide. We evaluate the ability of molecular imaging such as 11-C methionine PET (MET PET) to identify tumor beyond regions identified on standard MRI. We show that suboptimal coverage of the MET-PET target volume can occur when using only standard MR imaging and is associated with increased non-central failures. Therefore, MET PET may identify tumor sub-volumes that are at highest risk of recurrence. From this, we hypothesize that a new strategy that identifies these sub-regions and then targets these regions in conjunction with effective chemotherapy and other novel targeted agents will achieve higher, non-complicated tumor control probabilities than currently obtainable.
Acknowledgments
Supported by: NIH PO1 CA59827, P01 CA87634, and PO1 CA85878
Footnotes
Presented in part at the ASTRO meeting, Chicago, Illinois, 2009
References
- 1.Wallner KE, Galicich JH, et al. Patterns of failure following treatment for glioblastoma multiforme and anaplastic astrocytoma. International journal of radiation oncology, biology, physics. 1989;16(6):1405–1409. doi: 10.1016/0360-3016(89)90941-3. [DOI] [PubMed] [Google Scholar]
- 2.Garden AS, Maor MH, Yung WK, et al. Outcome and patterns of failure following limited-volume irradiation for malignant astrocytoma. Radiother Oncol. 1991;20(2):99–110. doi: 10.1016/0167-8140(91)90143-5. [DOI] [PubMed] [Google Scholar]
- 3.Lee SW, Fraass BA, et al. Patterns of failure following high-dose 3-D conformal RT for high-grade astrocytomas: a quantitative dosimetric study. International journal of radiation oncology, biology, physics. 1999;43(1):79–88. doi: 10.1016/s0360-3016(98)00266-1. [DOI] [PubMed] [Google Scholar]
- 4.Stupp R, Mason WP, van den Bent MJ, et al. Radiotherapy plus concomitant and adjuvant TMZ for glioblastoma. N Engl J Med. 2005;352:987–996. doi: 10.1056/NEJMoa043330. [DOI] [PubMed] [Google Scholar]
- 5.Stupp R, Hegi M, et al. Effects of RT with concomitant and adjuvant temozolomide versus RT alone on survival in glioblastoma in a randomised phase III study: 5-year analysis of the EORTC-NCIC trial. Lancet oncology. 2009;10(5):459–466. doi: 10.1016/S1470-2045(09)70025-7. [DOI] [PubMed] [Google Scholar]
- 6.MacDonald SM, Ahmad S, Kachris S, et al. Intensity modulated radiation therapy versus three dimensional conformal radiation therapy for the treatment of high grade glioma: a dosimetric comparison. J Appl. Clin Med Phys. 2007;8(2):47–60. doi: 10.1120/jacmp.v8i2.2423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ruben JD, Dally M, Bailey M, et al. Cerebral radiation necrosis: incidence, outcomes and risk factors with emphasis on radiation parameters and chemotherapy. Int J Radiat Oncol Biol Phys. 2006;65:499–508. doi: 10.1016/j.ijrobp.2005.12.002. [DOI] [PubMed] [Google Scholar]
- 8.Grosu AL, Weber WA, Riedel E, et al. L-(methyl-11C) methionine positron emission tomography for target delineation in resected high-grade gliomas before radiotherapy. Int J Radiat Oncol Biol Phys. 2005;63(1):64–74. doi: 10.1016/j.ijrobp.2005.01.045. [DOI] [PubMed] [Google Scholar]
- 9.Grosu AL, Weber WA, Franz M, et al. Reirradiation of recurrent high-grade gliomas using amino acid PET (SPECT)/CT/MRI image fusion to determine gross tumor volume for stereotactic fractionated radiotherapy. Int J Radiat Oncol Biol Phys. 2005;63(2):511–519. doi: 10.1016/j.ijrobp.2005.01.056. [DOI] [PubMed] [Google Scholar]
- 10.Nuutinen J, Sonninen P, Lehikoinen P, et al. Radiotherapy treatment planning and long-term follow-up with [(11)C]methionine PET in patients with low-grade astrocytoma. Int J Radiat Oncol Biol Phys. 2000 Aug 1;48(1):43–52. doi: 10.1016/s0360-3016(00)00604-0. [DOI] [PubMed] [Google Scholar]
- 11.Kracht LW, Miletic H, Busch S, et al. Delineation of brain tumor extent with 11C-L-methionine positron emission tomography: local comparison with stereotactic histopathology. Clin Cancer Res. 2004;10(21):7163–7170. doi: 10.1158/1078-0432.CCR-04-0262. [DOI] [PubMed] [Google Scholar]
- 12.Weber D, Casanova N, Zilli T, et al. Recurrence pattern after 18F-positron Emission Tomography-guided radiotherapy for high-grade glioma: A prospective study. Radiotherapy and Oncology. 2009;93:586–592. doi: 10.1016/j.radonc.2009.08.043. [DOI] [PubMed] [Google Scholar]
- 13.Wienhard K, Dahlbom M, Eriksson L, Michel C, Bruckbauer T, Pietrzyk U, et al. The ECAT EXACT HR: performance of a new high resolution positron scanner. J Comput Assist Tomogr. 1994;18(1):110–118. [PubMed] [Google Scholar]
- 14.Torii K, Tsuyuguchi N, Kawabe J, et al. Correlation of amino-acid uptake using methionine PET and histological classifications in various gliomas. Ann Nucl Med. 2005;19:677–683. doi: 10.1007/BF02985116. [DOI] [PubMed] [Google Scholar]
- 15.Cao Y. Development of Image Software Tools for Radiation Therapy Assessment. Medical Physics. 2005;32(6):2136–2136. [Google Scholar]
- 16.Normolle D, Lawrence T. Designing dose-escalation trials with late-onset toxicities using the time-to-event continual reassessment method. Journal of clinical oncology. 2006;24(27):4426–4433. doi: 10.1200/JCO.2005.04.3844. [DOI] [PubMed] [Google Scholar]
- 17.CTCAEv3.0. The revised common toxicity criteria: Version 3.0. 2006 < http://ctep.info.nih.gov>.
- 18.Macdonald DR, Cascino TL, Schold SC, Jr, et al. Response criteria for phase II studies of supratentorial malignant glioma. J Clin Oncol. 1990;8(7):1277–1280. doi: 10.1200/JCO.1990.8.7.1277. [DOI] [PubMed] [Google Scholar]
- 19.Lee IH, Piert M, et al. Association of 11C-Methionine PET Uptake With Site of Failure After Concurrent Temozolomide and Radiation for Primary Glioblastoma Multiforme. IJROBP. 2009;73(2):479–485. doi: 10.1016/j.ijrobp.2008.04.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rietschel Petra, Wolchok Jedd D, Krown Susan, et al. Phase II Study of Extended-Dose Temozolomide in Patients With Melanoma. J Clin Oncol. 2008;26(14):2299–2304. doi: 10.1200/JCO.2007.14.5292. [DOI] [PubMed] [Google Scholar]
- 21.Villano JL, Collins CA, Manasanch EE, et al. Aplastic anaemia in a patient with glioblastoma multiforme treated with temozolomide. Lancet Oncol. 2006;7(5):436–438. doi: 10.1016/S1470-2045(06)70696-9. [DOI] [PubMed] [Google Scholar]
- 22.Chan JL, Lee SW, Fraass BA, et al. Survival and failure patterns of high-grade gliomas after three-dimensional conformal radiotherapy. J Clin Oncol. 2002;20:1635–1642. doi: 10.1200/JCO.2002.20.6.1635. [DOI] [PubMed] [Google Scholar]
- 23.Tsien C, Moughan J, et al. Phase I three-dimensional conformal radiation dose escalation study in newly diagnosed glioblastoma: Radiation Therapy Oncology Group Trial 98-03. International journal of radiation oncology, biology, physics. 2009;73(3):699–708. doi: 10.1016/j.ijrobp.2008.05.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Souhami L, Seiferhold W, Brachman D. Randomized comparison of stereotactic radiosurgery followed by conventional radiotherapy with carmustine to conventional radiotherapy with carmustine for patients with glioblastoma multiforme:report of Radiation Therapy Oncology Group 93-05 protocol. Int J radiat Oncol Biol Phys. 2004;60(3):853–860. doi: 10.1016/j.ijrobp.2004.04.011. [DOI] [PubMed] [Google Scholar]
- 25.Selker RG, Shapiro WR, Burger P. The Brain Tumor Cooperative Group NIH Trial 87-01: a randomized comparison of surgery, external radiotherapy, and carmustine versus surgery, interstitial radiotherapy boost, external radiation therapy, and carmustine. Neurosurgery. 2002;51(2):342–355. [PubMed] [Google Scholar]
- 26.Jacobs AH, Thomas A, Kracht LW, et al. 18F-fluoro-L-thymidine and 11C-methylmethionine as markers of increased transport and proliferation in brain tumors. J Nucl Med. 2005;46:1948–1958. [PubMed] [Google Scholar]
- 27.Torii K, Tsuyuguchi N, et al. Correlation of amino-acid uptake using methionine PET and histological classifications in various gliomas. Annals of nuclear medicine. 2005;19(8):677–683. doi: 10.1007/BF02985116. [DOI] [PubMed] [Google Scholar]
- 28.Kracht LW, Friese M, Herholz K, et al. Methyl-[11C]-l-methionine uptake as measured by positron emission tomography correlates to microvessel density in patients with glioma. Eur J Nucl Med Mol Imaging. 2003;30:868–873. doi: 10.1007/s00259-003-1148-7. [DOI] [PubMed] [Google Scholar]
- 29.Sato N, Suzuki M, Kuwata N, et al. Evaluation of the malignancy of glioma using 11C-methionine positron emission tomography and proliferating cell nuclear antigen staining. Neurosurg Rev. 1999;22:210–214. doi: 10.1007/s101430050018. [DOI] [PubMed] [Google Scholar]
- 30.Lai A, Tran A, Nghiemphu PL, et al. Phase II Study of Bevacizumab Plus Temozolomide During and After Radiation Therapy for Patients with Newly Diagnosed Glioblastoma Multiforme. J Clin Oncol. 2011;29(2):142–148. doi: 10.1200/JCO.2010.30.2729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Grossman SA, Ye X, Piantadosi S, et al. Survival of Patients with newly Diagnosed Glioblastoma treated with radiation and temozolomide in researc studies in the United States. Clin Cancer Res. 2010;16(8):2443–2449. doi: 10.1158/1078-0432.CCR-09-3106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gutin PH, Iwamoto FM, Beal K, et al. Safety and efficacy of bevacizumab with hypofractionated stereotactic irradiation for recurrent malignant gliomas. Int J Radiat Oncol Biol Phys. 2009;75:156–163. doi: 10.1016/j.ijrobp.2008.10.043. [DOI] [PMC free article] [PubMed] [Google Scholar]