Abstract
Right ventricular failure (RVF) is the main cause of death in patients with pulmonary artery hypertension (PAH). Sildenafil, a phosphodiesterase type 5 (PDE5) inhibitor, was recently approved for treatment of PAH patients. However, the mechanisms underlying RV contractile malfunction and the benefits of sildenafil on RV function are not well understood. We aimed to investigate 1) the ultrastructural and excitation-contraction coupling alterations underlying PAH-induced RVF; 2) whether the ultrastructural changes are reversible; 3) the mechanisms underlying the therapeutic benefits of sildenafil in PAH-RVF. We used a single injection of monocrotaline (MCT) in Wistar rats to induce pulmonary vascular proliferation, which led to PAH and RVF. RV myocytes displayed severe T-tubule loss and disorganization as well as blunted and dys-synchronous SR Ca2+ release. Sildenafil prevented and reversed the MCT-induced PAH and LV filling impairment. Early intervention with sildenafil prevented RV hypertrophy and the development of RVF, T-tubule remodeling and Ca2+ handling dysfunction. While late treatment with sildenafil did not reverse RV hypertrophy in animals with established RVF, RV systolic function was improved. Furthermore, late intervention partially reversed both the impairment of myocyte T-tubule integrity and Ca2+ handling protein and SR Ca2+ release function in MCT-treated rats. In conclusion, PAH-induced increase in RV afterload causes severe T-tubule remodeling and Ca2+ handling dysfunction in RV myocytes, leading to RV contractile failure. Sildenafil prevents and partially reverses ultrastructural, molecular and functional remodeling of failing RV myocytes. Reversal of pathological T-tubule remodeling, although incomplete, is achievable without the regression of RV hypertrophy.
Keywords: right ventricle failure, pulmonary artery hypertension, PDE5 inhibitor, calcium, t-tubule, excitation-contraction coupling
Introduction
Numerous previous studies on heart failure have focused on the left ventricle (LV). Our recent work and the work of others have demonstrated characteristic alterations in the myocyte Transverse (T)-tubule membrane system and excitation-contraction (EC) coupling function in animal models of LV heart failure. Failing LV myocardium is characterized by T-tubule loss and disorganization; reduction in amplitude and synchrony of Ca2+ release; reduction in coupling gain function; reduction in sarcoplasmic reticulum (SR) Ca2+ content; and alterations in Ca2+ handling proteins, including downregulation of SR Ca-ATPase (SERCA) and upregulation of Na+-Ca2+ exchanger (NCX).1–6 In contrast to LV failure, there is a paucity of research in right ventricular (RV) failure (RVF). Little is known about the mechanisms underlying RVF, although more attention has been paid to recently.7–9 The RV is thought to be different from the LV in embryologic origin.10 The crescent shaped RV is much thinner than the LV in free wall and has greater compliance. Because of these differences, the RV is more sensitive than the LV to stresses, including afterload increase. For example, patients with systemic hypertension can compensate for increased afterload for many years, whereas adult patients with pulmonary arterial hypertension (PAH) often rapidly progress to RVF, and patients with untreated, severe PAH have a high rate of morbidity and a life expectancy of only 2 to 3 years.7
PAH is the most severe form of pulmonary hypertension. RVF is the main cause of death in patients with PAH.11 However, the cellular and molecular mechanisms underlying RV contractile malfunction in PAH are not well understood. In the present study, we used a well-established monocrotaline (MCT) -induced PAH – RVF model in rats 12 to investigate the structure – function relationship of RV myocytes to PAH and RVF. Sildenafil, a phosphodiesterase type 5 (PDE5) inhibitor, recently approved for the treatment of PAH, is associated with reduced pulmonary vascular resistance, improved exercise capacity and increased survival in patients.13 Accordingly, the most important determinant of survival in PAH is RV function.14
In the present study, we investigated the effects of sildenafil on PAH-induced RV dysfunction, using MCT-induced PAH-RVF rat model and laser scanning confocal microscopy. One of the key questions we are asking is whether alterations in ultrastructure and EC coupling function are reversible in PAH-induced RVF upon therapeutic intervention.
Methods
An expanded Materials and Methods section is available in the online Data Supplement (Please see http://hyper.ahajournals.org). Animal experiments were performed according to the protocol approved by the University of Iowa Institutional Animal Care andUse Committee. Male Wistar rats (Charles River Laboratory, Inc.) weighing 175 g – 200 g were given a single subcutaneous injection of monocrotaline (MCT, 60 mg/kg, Sigma-Aldrich) or the same volume of 0.9% saline.12 Rats receiving MCT were subdivided into 3 groups: MCT alone, SilMCT/D1 (treated with sildenafil beginning one day after MCT injection, daily, s.c, 100 mg/kg/day); SilMCT/D23 (treated with sildenafil beginning day 23 after MCT injection, for 2 weeks, daily, s.c., 100 mg/kg/day). MCT alone and SilMCT/D1 groups were sacrificed at day 24–25 for histology, confocal imaging (Ca2+ and T-tubules) and western blotting. SilMCT/D23 group was sacrificed two weeks after sildenafil treatment for histology, confocal imaging (Ca2+ and T-tubules) and western blotting. Echocardiography, lung histology, confocal Ca2+ imaging of field stimulated Ca2+ transients in single isolated myocytes, in situ confocal imaging of myocyte T-tubule structure on intact heart, western blotting assay of EC coupling proteins and statistical methods were described in the online Data Supplement.
Results
MCT-induced PAH-RVF and effects of sildenafil in Wista rats
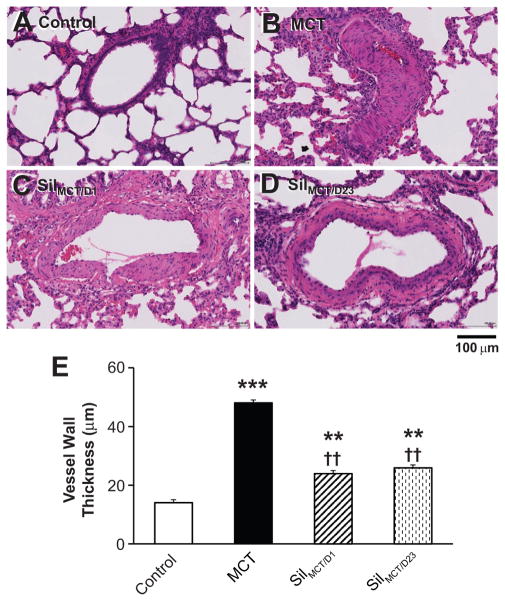
A single injection of MCT (60 mg/kg) in rats caused massive remodeling of the pulmonary arteries. The pulmonary artery wall thickness, as examined at day 24–25, was increased by more than three-fold over saline treated controls, leading to the obstruction of the pulmonary arteries (Figure 1A, B, E). In control rats, there was no tricuspid regurgitation. Pulmonary artery pressure (PAP) could not be estimated with echocardiography, whereas at day 24–25, all MCT-treated rats had high systolic PAP (sPAP, 82±6 mmHg, n=18). These results confirm development of PAH in MCT-treated rats.
Figure 1. Effects of MCT and sildenafil on vascular wall thickness in lung pulmonary arteries.
A–D, Representative images of pulmonary vessels in the lungs of control (A), MCT (B), MCT rats treated with sildenafil beginning at day 1 (C) and MCT rats treated with sildenafil beginning at day 23 for two weeks (D). E, Vascular wall thickness in pulmonary vessels under 500 μm in length. **, p < 0.01; ***, p<0.001 vs. saline control; ††, p < 0.01 vs. MCT group. N=3, 3, 4, 4 lungs for each group, respectively.
We next examined the ability of sildenafil to either prevent or reverse PAH. Sildenafil, 100 mg/kg/day, when given one day after MCT injection (SilMCT/D1), prevented PAH (Figure 1C). Only 2 out of 14 rats in SilMCT/D1 group had detectable sPAP (37 and 40 mmHg). Pulmonary artery wall thickness was significantly reduced with early intervention (Figure 1C, E), suggesting that sildenafil prevents PAH development in MCT rats. Next, we administered sildenafil to MCT rats beginning at day 23 (SilMCT/D23), at which point RV dysfunction was evident by echocardiography, and treatment continued for two weeks. This delayed sildenafil intervention reversed MCT-induced pulmonary artery remodeling, similar to that of the SilMCT/D1 group (Figure 1D, E), and echocardiography detected PAP in only one out of 8 rats in this group (30 mmHg).
Echocardiography and morphometric data demonstrated that MCT-treated rats developed RV hypertrophy (Figure 2A–C), marked RV dilation (Online Supplemental Figure S1 & Figure 2D, E) and a severe decline in RV fractional shortening (Figure 2F). SilMCT/D1 prevented both RV hypertrophy and dilation and maintained RV systolic function (Figure 2A–F). RV weight (i.e, dry RV weight, dry RV/(LV+septum)) and RV wall thickness were not significantly different between control and SilMCT/D1 group (Figure 2A, B, C). While pulmonary artery remodeling in SilMCT/D23 group was reversed to levels similar to that of SilMCT/D1 group (Figure 1), SilMCT/D23 failed to reverse RV hypertrophy (Figure 2A–C). Surprisingly, SilMCT/D23 rats showed significantly better RV contractile function compared to MCT-treated rats (Figure 2F). 62.5% (5 out of 8) of rats in the SilMCT/D23 group had improved RV systolic function following two-week sildenafil treatment. Their averaged RV fractional shortening was significantly improved from 15.6±3.4% to 26.9±3.5% (p<0.05). However, echocardiographic evidence showed that acute exposure of sildenafil (before and 1 hour after a single dose injection, 100 mg/kg) did not affect either LV or RV function in MCT-induced PAH-RVF rats (Online Data Supplement, Figures S2–3). These data suggest that the improvement in RV function was not attributed to acute effects of sildenafil.
Figure 2. Effects of MCT and sildenafil treatments on RV morphometric and RV systolic function.
A–B, RV weight was measured after 24 hours of drying at 37°C (A) and normalized to LV+septum weight (B). N=5, 6, 7, 5 hearts per group, respectively. C–D, RV dimensions during systole (C) and diastole (D), and RV wall thickness during diastole (E). F, RV fractional shortening (FS, %), calculated as (RV dimension during diastole – RV dimension during systole) divided by RV dimension during diastole. N=11, 10, 9, 8 per group, respectively. MCT treated rats developed RVF with significant reduction in RV FS. Sildenafil early treatment prevented MCT induced RVF with maintained FS. Sildenafil late treatment improved RV systolic function, comparing to those of MCT alone. **, p < 0.001 vs. saline control; †, p<0.05; ††, p < 0.001 vs. MCT group; ‡, p<0.05 vs. SilMCT/D1 group.
Interestingly, LV filling was impaired in the MCT group as evidenced by reduction in LV end diastolic volume (Figure 3A), stroke volume (Figure 3B) and cardiac output (Figure 3C). Sildenafil (both SilMCT/D1 and SilMCT/D23) restored LV filling and cardiac output to normal levels (Figure 3A–C), due to normalization of PAH and RV afterload (Figure 2). Nonetheless, the LV ejection fraction remained unaffected by MCT and sildenafil treatments (Figure 3D).
Figure 3. MCT-induced PAH impairs LV filling.
A, End diastolic volume; B, LV stroke volume; C, cardiac output. MCT induced PAH - RV afterload increase caused impairment of LV filling. Both early and late treatments with sildenafil normalized LV filling and output. D, LV ejection fraction (EF) was normal among all groups. ***, p<0.001 vs. saline control; †, p < 0.05; ††, p < 0.01 vs. MCT group. N=11, 10, 9, 8 per group, respectively.
Sildenafil normalized Ca2+ handling defects in RV myocytes from MCT-treated rats
To investigate the cellular mechanism underlying PAH-RVF, we next measured intracellular Ca2+ with laser scanning confocal microscope in isolated RV myocytes. RV myocytes from MCT-treated rats had smaller and slower Ca2+ transients compared to control RV myocytes (Figure 4). The slowed Ca2+ transients in RV myocytes from the MCT-treated group was reflected by a prolonged Ca2+ activation (time to peak, Tpeak, Figure 4F) and decay phase (time to 50% decay from peak transient, T50, Figure 4G). These data indicate that PAH-RVH is associated with compromised SR Ca2+ release function. In contrast, RV myocytes from the SilMCT/D1 group displayed normal Ca2+ transients, suggesting that early treatment prevents the development of RV hypertrophy/failure and preserves cellular Ca2+ handling. Furthermore, we found that sildenafil treatment at a late stage (SilMCT/D23) partially improved Ca2+ handling (Figure 4E–G). However, the improvement of myocyte Ca2+ handling function is not likely due to the acute effect of sildenafil. Ca2+ transients in RV myocytes from MCT-treated rats were not altered by local perfusion of sildenafil (10 μM for 10 minutes) (Online Data Supplement, Figure S4), supporting the echocardiographic finding from acute sildenafil-treated MCT rats (Figures S2–3).
Figure 4. Confocal imaging of Ca2+ transients in single isolated RV myocytes.
A–D, representative Ca2+ transients from control, MCT, SilMCT/D1, and SilMCT/D23 groups, respectively. Shown underneath each panel of Ca2+ image is the spatially averaged Ca2+ profile. RV myocyte from MCT-treated rat (B) exhibited defects in the activation of Ca2+ transients (red arrows), prolonged plateau (green doublehead arrows) and slower decay phase (pink arrows). E–G, Summary data on parameters of Ca2+ transients, including amplitude (F/F0), time to peak (Tpeak) and decay rate (time to 50% decay, T50). *, p<0.05 vs control; **, p<0.01 vs control; †, p<0.05 vs MCT; ††, p<0.01 vs MCT. n=30, 54, 61, 52 cells (from 3–6 hearts), respectively.
Sildenafil prevented and partially reversed T-tubule remodeling in RV myocytes from MCT-treated rats
The myocyte T-tubule system is an important determinant of cardiac EC coupling function.15 We next examined whether T-tubule remodeling occurs in the MCT-induced PAH-RVF model and whether this ultrastructural remodeling is reversible upon therapeutic treatment. In MCT-treated group, we consistently observed drastic T-tubule remodeling in RV myocytes in contrast to the highly organized T-tubule network in RV myocytes from control rats (Figure 5A, B). As shown in a representative T-tubule image in Figure 5B, RV myocytes from MCT-treated rats lost a majority of T-tubules, resulting in a severe decrease in T-tubule power (TTpower, Figure 5E), an index of T-tubule integrity.4 Surprisingly, RV myocytes from the SilMCT/D1 group displayed normal T-tubule organization with similar TTpower to that of the control group (Figure 5C,E). RV myocytes from the SilMCT/D23 group had improved T-tubule integrity and organization compared to the MCT alone group (Figure 5D, E), suggesting this ultrastructure alteration is reversible even with delayed sildenafil therapy. Histogram analysis of TTpower distribution further confirmed our findings on MCT-induced RV myocyte T-tubule remodeling and beneficial effects of sildenafil in this model (See Online Data Supplement, Figure S5). Collectively, our data demonstrate that improvements in Ca2+ transients in sildenafil-treated RV myocytes are associated with the prevention and partial reversal of T-tubule ultrastructure remodeling.
Figure 5. In situ confocal imaging of RV myocyte T-tubule system from Langendorff perfused intact hearts.
A–D, representative T-tubule images from control, MCT, SilMCT/D1, and SilMCT/D23 group, respectively. B, MCT-treated rats exhibited severe T-tubule loss and disorganization in RV myocytes. C, Early treatment of sildenafil (SilMCT/D1) prevented MCT-induced T-tubule remodeling. D, Late treatment of sildenafil (SilMCT/D23) partially reversed MCT induced T-tubule remodeling. E. Average data of TTpower (T-tubule power, an index of the strength of T-tubule regularity, for analysis, See reference Wei S, et al, 2010). TTpower of SilMCT/D1 group is not different from control. TTpower of SilMCT/D23 group is significantly higher than that of MCT rats, but lower than those of control and SilMCT/D1 groups. **, p<0.01 vs control; ††, p<0.01 vs MCT; ‡, p<0.05 vs. SilMCT/D1 group. N=7, 9, 7, 5 hearts per group, respectively.
In addition, analysis of cardiomyocyte surface area from T-tubule images revealed that early sildenafil treatment (SilMCT/D1) fully protected RV myocytes from developing cellular hypertrophy, whereas late treatment failed to suppress hypertrophy in myocytes from the SilMCT/D23 group (See Online Data Supplement, Figure S6). These cellular data are consistent with our observations at the organ and tissue levels (i.e., echocardiography and morphometric measurements, Figure 2).
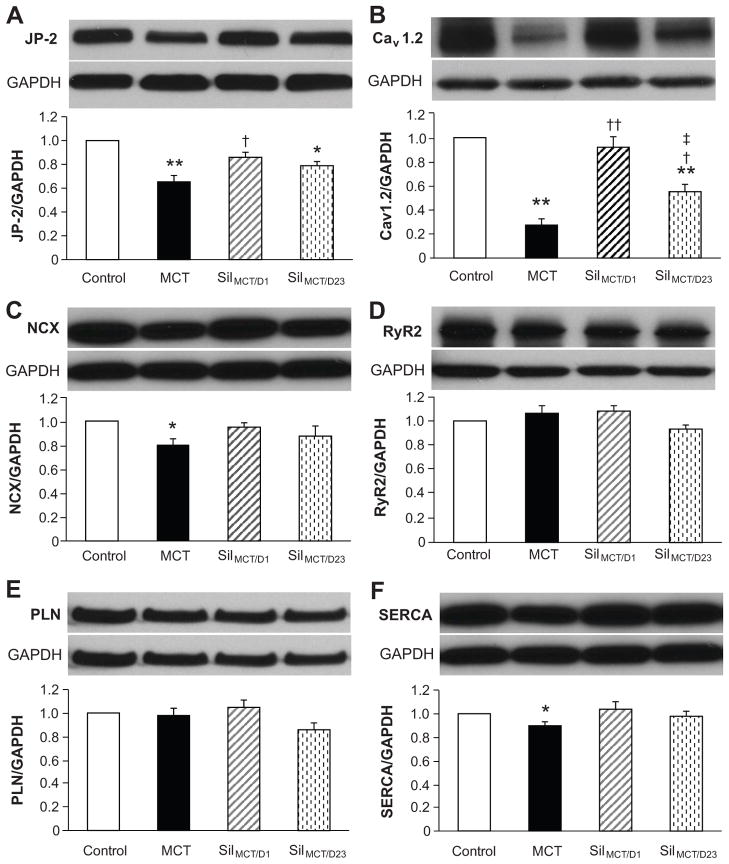
Effects of sildenafil on Ca2+ handling proteins in RV myocytes
Our recent data 4 and that of the Wehrens group 16 indicate that junctophilin-2 (JP-2) is involved in T-tubule remodeling during cardiomyopathy. In comparison to control RV myocytes, JP-2 was reduced by 35% in MCT-treated RV myocytes, unchanged in the SilMCT/D1 group, and partially restored by SilMCT/D23 treatment (Figure 6A). The trend in JP-2 protein expression levels correlates with the RV T-tubule changes described above.
Figure 6. Alterations in expression of Ca2+ handling proteins in RV myocytes in response to sildenafil treatment.
RV tissue lysates were extracted for Western blotting assay. Representative blots and quantitation of JP-2 (A), Cav1.2 (B), NCX1 (C), RyR2 (D), PLN (E) and SERCA (F) levels. *, p<0.05 vs control; **, p<0.01 vs control; †, p<0.05 vs MCT; ††, p<0.01 vs MCT; ‡, p<0.05 vs. SilMCT/D1 group. N=4–5 hearts for each group.
Myocyte Ca2+ handling function is also associated with several important ion channels and transporters. We identified a significant reduction in Cav1.2 Ca2+ channels (Cav1.2, Figure 6B) and sodium-calcium exchanger (NCX, Figure 6C) expression in the MCT-treated group, compared to control. The marked reduction in Cav1.2 is likely to contribute to decreasing the Ca2+ transient amplitude and reducing synchrony of SR Ca2+ release (i.e., prolonged Tpeak) in RV myocytes from MCT-induced PAH-RVF rats. Consistent with the prevention/recovery in T-tubule integrity, Cav1.2 levels were normal or partially recovered by sildenafil treatments in SilMCT/D1 and SilMCT/D23 groups, respectively. SR-associated proteins RyR2 and phospholamban (PLN) remained largely unchanged, and SR Ca2+-ATPase (SERCA) was slightly but significantly downregulated in MCT-treated group (Figure 6D–F). These data suggest that the molecular mechanism by which sildenafil improves myocardial function is, at least partly, through normalization of Ca2+ handling protein expression.
Discussion
The pulmonary circulation is intimately coupled with RV function in health and disease. RV function is the most important determinant of survival in patients with PAH.14, 17 Despite the significant advances in our understanding and clinical practice in PAH over the past decades, RVF remains the common fatal pathway and consequence of PAH. However, our understanding of RVF is limited due to a paucity of research in this area.
In this study, we used an established, MCT-induced PAH model to study the structure-function relationship of T-tubules and intracellular Ca2+ in RVF. The major findings are as follows: 1) MCT-induced RVF manifested with marked RV hypertrophy, RV dilation and systolic dysfunction, blunted and dyssynchronous Ca2+ transients, severe T-tubule loss and disorganization, and alterations in levels of Ca2+ handling proteins; 2) sildenafil administered at an early stage prevented the development of PAH and RV hypertrophy and failure, preserved normal T-tubule ultrastructure, normal Ca2+ transients and expression of key Ca2+ handling proteins; 3) when given at a delayed stage (with established RV hypertrophy and failure), sildenafil reversed MCT-induced PAH but not RV hypertrophy, and partially restored RV contractile function, RV myocyte Ca2+ transients, T-tubule ultrastructure and Ca2+ handling proteins; 4) sildenafil treatment corrected MCT-induced impairment of LV filling; and 5) sildenafil did not acutely affect RV contractile function in live animals and intracellular Ca2+ handling in isolated RV myocytes of MCT-induced PAH-RVF rats. Our data provide evidence that T-tubule ultrastructural remodeling is reversible upon therapeutic treatment, independent of cardiac hypertrophy remodeling.
Our recent data suggested that (LV) T-tubule remodeling may represent a key mechanism underlying the transition from hypertrophy to (LV) heart failure.4 Severe T-tubule loss leads to a significant reduction or redistribution in Cav1.2 Ca2+ channel, increase in orphaned RyR2s, reduction in coupling efficacy between Ca2+ channels on T-tubule membrane and RyR2s on SR membrane, and therefore reduction in Ca2+ transient amplitude and synchrony.2, 3, 18 In this study, we proposed that RV myocyte T-tubule disruption during PAH-induced RV remodeling is an important factor in the development of RVF. On the other hand, improvement of the RV myocyte T-tubule ultrastructure was anticipated to increase RV contractile function. Consistently, our Ca2+ handling and echocardiographic results did support this notion. Future studies to further establish the temporal relationship between T-tubule disruption and changes in Ca2+ handling in MCT-injected rats, e.g., in the early stage, may provide a better mechanistic understanding of T-tubule remodeling and Ca2+ handling dysfunction in heart disease.
Sildenafil was recently approved for use in patients with PAH. The beneficial effects of sildenadil or other PDE5 inhibitors in PAH are thought to result from relatively selective vasodilatory and antiproliferative effects on the pulmonary vasculature.19 Our data reveal a potent effect of sildenafil on pulmonary vascular remodeling, consisted with the literature.20 A recent work from Michelakis group provided elegant evidence that PDE5 is increased in hypertrophied human RV myocardium, and acute inhibition of PDE5 improves RV myocyte contractility, suggesting a direct, acute effect of PDE5 inhibition on RV myocyte function in disease.19 However, our data showed that acute exposure of sildenafil did not increase contractile function of both ventricles (Figure S2 & S3) nor did it enhance SR Ca2+ release function in RV myocytes (Figure S4) from MCT-treated rats. Moreover, we showed that early treatment with sildenafil maintained normal pulmonary artery resistance and prevented afterload increase-induced RV structural (hypertrophy, wall thickness, chamber dimension) and ultrastructural (T-tubule) remodeling as well as functional alterations. In addition, our data from late treatment group (SilMCT/D23) revealed a potential benefit of a PDE5 inhibitor on RV function: improving or reversing RV myocyte T-tubule remodeling and therefore Ca2+ handling in “advanced” disease. This represents a heretofore unappreciated mechanism of sildenafil as a therapeutic for PAH-RVF disease. Finally, we believe that benefit of PDE5 inhibitor therapy for PAH and RV dysfunction is mainly through its chronic effects on decreasing RV afterload and ensuing RV structural and functional improvement.
Sildenafil is effective in improving PAH-related RV dysfunction; however, it is still debating whether sildenafil exerts its therapeutic effects via its action on the pulmonary vasculature (e.g., afterload unloading) or by a direct anti-hypertrophic effect on RV remodeling. Kass and colleagues have published a number of studies suggesting that sildenafil prevents, arrests, and even reverses LV hypertrophy, fibrosis, and dilatation in mice subjected to LV pressure overload induced by transverse aortic constriction.21, 22 Recently, two independent groups 23, 24 examined whether sildenafil provides similar, direct protection against RV remodeling as shown in LV by the Kass group. Surprisingly, they both provided evidence that sildenafil does not prevent, but even exacerbates RV hypertrophy in a pre-established RV hypertrophy model induced by pulmonary trunk artery banding, indicating that sildenafil prevents myocardial remodeling in PAH mainly through an indirect action via RV unloading. The discrepant effects of sildenafil on pressure overload-induced LV vs. RV hypertrophy could possibly be due to different mechanisms involved in the development of hypertrophy in the RV and LV. It is being increasingly recognized that, in addition to important differences in gene expression, embryology, and physiology, the RV and LV may have divergent responses to stress, including activation of different signaling cascades.10, 25
The beneficial effects of sildenafil on T-tubule remodeling, particularly in the setting of un-improved RV hypertrophy, suggest that unloading of RV afterload (due to sildenafil-induced normalization of PAP) may play an important role. Increasing evidence demonstrates the occurrence of reversal remodeling in failing hearts after mechanical unloading (e.g., in patients after left ventricle assisted device (LVAD) surgery or lung transplantation). LVAD has been associated with improved cardiac function and a broad spectrum of reverse remodeling events in LV myocytes (e.g., changes in morphology and at the cellular, biochemical, molecular, and transcriptional levels).26–28 Our finding of reverse remodeling of the T-tubule ultrastructure reflects a new layer of cardiovascular benefits upon mechanical unloading in sildenafil-treated PAH-RVH rats. However, mechanical unloading is not always beneficial. Unwanted chronic mechanical unloading may lead to damage of the T-tubule system and defects in Ca2+ handling.29
Perspective
In patients with idiopathic PAH, the clinical course is determined by progressive loss of cross-sectional area in the pulmonary arterial tree, and by RVF resulting from increased afterload. Sildenafil was introduced into the treatment armamentarium for PAH by virtue of its known vasodilator properties. Here in this study, we elucidated the mechanisms of RVF in a PAH model and uncovered a new mechanism by which sildenafil provides therapeutic benefit in PAH. Specifically, RV myocytes rapidly develop maladaptive, ultrastructural remodeling and EC coupling dysfunction in response to an increase in pulmonary arterial resistance, leading to RV contractile failure during PAH. Early intervention with sildenafil prevents PAH and preserves RV structure and function at the (sub)cellular and whole-organ levels. Late intervention promotes partially reverse structural and functional remodeling in RV failing myocytes. The prevention and repair of maladaptive changes to RV T-tubules appears to be an important benefit of sildenafil in PAH. Although therapeutic effects were observed when sildenafil was introduced late in the disease course, it appears that early intervention is required to prevent pathological cellular and ultrastructural remodeling of the RV – a finding with important translational implications.
Supplementary Material
Acknowledgments
Sildenafil was kindly provided by Pfizer Inc.
Funding Sources
This work was supported by NIH R01 HL090905 (L.S.S.), American Heart Association Scientific Development Grant 0635056N (L.S.S.), and NIH RR026293 (R.M.W.).
Abbreviations
- EC
excitation-contraction
- LV
left ventricle/ventricular
- MCT
monocrotaline
- PAH
pulmonary artery hypertension
- PAP
pulmonary artery pressure
- PDE5
phosphodiesterase type 5
- RV
right ventricle/ventricular
- RVF
right ventricle failure
- SR
sarcoplasmic reticulum
- T-tubule
transverse-tubule
- TTpower
the power of transverse tubule regularity
- Tpeak
time to peak Ca2+ transients
- T50
time to 50% decay from peak transients
Footnotes
Disclosures
No conflict of interest to be disclosed.
References
- 1.Louch WE, Mork HK, Sexton J, Stromme TA, Laake P, Sjaastad I, Sejersted OM. T-tubule disorganization and reduced synchrony of Ca2+ release in murine cardiomyocytes following myocardial infarction. J Physiol. 2006;574:519–533. doi: 10.1113/jphysiol.2006.107227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Song LS, Sobie EA, McCulle S, Lederer WJ, Balke CW, Cheng H. Orphaned ryanodine receptors in the failing heart. Proc Natl Acad Sci USA. 2006;103:4305–4310. doi: 10.1073/pnas.0509324103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Heinzel FR, Bito V, Biesmans L, Wu M, Detre E, von Wegner F, Claus P, Dymarkowski S, Maes F, Bogaert J, Rademakers F, D’Hooge J, Sipido K. Remodeling of t-tubules and reduced synchrony of Ca2+ release in myocytes from chronically ischemic myocardium. Circ Res. 2008;102:338–346. doi: 10.1161/CIRCRESAHA.107.160085. [DOI] [PubMed] [Google Scholar]
- 4.Wei S, Guo A, Chen B, Kutschke W, Xie YP, Zimmerman K, Weiss RM, Anderson ME, Cheng H, Song LS. T-tubule remodeling during transition from hypertrophy to heart failure. Circ Res. 2010;107:520–531. doi: 10.1161/CIRCRESAHA.109.212324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Balijepalli RC, Lokuta AJ, Maertz NA, Buck JM, Haworth RA, Valdivia HH, Kamp TJ. Depletion of t-tubules and specific subcellular changes in sarcolemmal proteins in tachycardia-induced heart failure. Cardiovasc Res. 2003;59:67–77. doi: 10.1016/s0008-6363(03)00325-0. [DOI] [PubMed] [Google Scholar]
- 6.He J, Conklin MW, Foell JD, Wolff MR, Haworth RA, Coronado R, Kamp TJ. Reduction in density of transverse tubules and l-type Ca2+ channels in canine tachycardia-induced heart failure. Cardiovasc Res. 2001;49:298–307. doi: 10.1016/s0008-6363(00)00256-x. [DOI] [PubMed] [Google Scholar]
- 7.Banerjee D, Haddad F, Zamanian RT, Nagendran J. Right ventricular failure: A novel era of targeted therapy. Curr Heart Fail Rep. 2010;7:202–211. doi: 10.1007/s11897-010-0031-7. [DOI] [PubMed] [Google Scholar]
- 8.Bogaard HJ, Natarajan R, Henderson SC, Long CS, Kraskauskas D, Smithson L, Ockaili R, McCord JM, Voelkel NF. Chronic pulmonary artery pressure elevation is insufficient to explain right heart failure. Circulation. 2009;120:1951–1960. doi: 10.1161/CIRCULATIONAHA.109.883843. [DOI] [PubMed] [Google Scholar]
- 9.Toldo S, Bogaard HJ, Van Tassell BW, Mezzaroma E, Seropian IM, Robati R, Salloum FN, Voelkel NF, Abbate A. Right ventricular dysfunction following acute myocardial infarction in the absence of pulmonary hypertension in the mouse. PLoS One. 2011;6:e18102. doi: 10.1371/journal.pone.0018102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zaffran S, Kelly RG, Meilhac SM, Buckingham ME, Brown NA. Right ventricular myocardium derives from the anterior heart field. Circ Res. 2004;95:261–268. doi: 10.1161/01.RES.0000136815.73623.BE. [DOI] [PubMed] [Google Scholar]
- 11.Voelkel NF, Quaife RA, Leinwand LA, Barst RJ, McGoon MD, Meldrum DR, Dupuis J, Long CS, Rubin LJ, Smart FW, Suzuki YJ, Gladwin M, Denholm EM, Gail DB. Right ventricular function and failure: Report of a national heart, lung, and blood institute working group on cellular and molecular mechanisms of right heart failure. Circulation. 2006;114:1883–1891. doi: 10.1161/CIRCULATIONAHA.106.632208. [DOI] [PubMed] [Google Scholar]
- 12.Redout EM, Wagner MJ, Zuidwijk MJ, Boer C, Musters RJ, van Hardeveld C, Paulus WJ, Simonides WS. Right-ventricular failure is associated with increased mitochondrial complex ii activity and production of reactive oxygen species. Cardiovasc Res. 2007;75:770–781. doi: 10.1016/j.cardiores.2007.05.012. [DOI] [PubMed] [Google Scholar]
- 13.Galie N, Manes A, Negro L, Palazzini M, Bacchi-Reggiani ML, Branzi A. A meta-analysis of randomized controlled trials in pulmonary arterial hypertension. Eur Heart J. 2009;30:394–403. doi: 10.1093/eurheartj/ehp022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bristow MR, Zisman LS, Lowes BD, Abraham WT, Badesch DB, Groves BM, Voelkel NF, Lynch DM, Quaife RA. The pressure-overloaded right ventricle in pulmonary hypertension. Chest. 1998;114:101S–106S. doi: 10.1378/chest.114.1_supplement.101s. [DOI] [PubMed] [Google Scholar]
- 15.Brette F, Orchard C. T-tubule function in mammalian cardiac myocytes. Circ Res. 2003;92:1182–1192. doi: 10.1161/01.RES.0000074908.17214.FD. [DOI] [PubMed] [Google Scholar]
- 16.van Oort RJ, Garbino A, Wang W, Dixit SS, Landstrom AP, Gaur N, De Almeida AC, Skapura DG, Rudy Y, Burns AR, Ackerman MJ, Wehrens XH. Disrupted junctional membrane complexes and hyperactive ryanodine receptors after acute junctophilin knockdown in mice. Circulation. 2011;123:979–988. doi: 10.1161/CIRCULATIONAHA.110.006437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.D’Alonzo GE, Barst RJ, Ayres SM, Bergofsky EH, Brundage BH, Detre KM, Fishman AP, Goldring RM, Groves BM, Kernis JT, Levy PS, Pietra GG, Reid LM, Reeves JT, Rich S, Vreim CE, Williams GW, Wu M. Survival in patients with primary pulmonary hypertension. Results from a national prospective registry. Ann Intern Med. 1991;115:343–349. doi: 10.7326/0003-4819-115-5-343. [DOI] [PubMed] [Google Scholar]
- 18.Wu CY, Jia Z, Wang W, Ballou LM, Jiang YP, Chen B, Mathias RT, Cohen IS, Song LS, Entcheva E, Lin RZ. Pi3ks maintain the structural integrity of t-tubules in cardiac myocytes. PLoS One. 2011;6:e24404. doi: 10.1371/journal.pone.0024404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nagendran J, Archer SL, Soliman D, Gurtu V, Moudgil R, Haromy A, St Aubin C, Webster L, Rebeyka IM, Ross DB, Light PE, Dyck JR, Michelakis ED. Phosphodiesterase type 5 is highly expressed in the hypertrophied human right ventricle, and acute inhibition of phosphodiesterase type 5 improves contractility. Circulation. 2007;116:238–248. doi: 10.1161/CIRCULATIONAHA.106.655266. [DOI] [PubMed] [Google Scholar]
- 20.Schermuly RT, Kreisselmeier KP, Ghofrani HA, Yilmaz H, Butrous G, Ermert L, Ermert M, Weissmann N, Rose F, Guenther A, Walmrath D, Seeger W, Grimminger F. Chronic sildenafil treatment inhibits monocrotaline-induced pulmonary hypertension in rats. Am J Respir Crit Care Med. 2004;169:39–45. doi: 10.1164/rccm.200302-282OC. [DOI] [PubMed] [Google Scholar]
- 21.Takimoto E, Champion HC, Li M, Belardi D, Ren S, Rodriguez ER, Bedja D, Gabrielson KL, Wang Y, Kass DA. Chronic inhibition of cyclic gmp phosphodiesterase 5a prevents and reverses cardiac hypertrophy. Nat Med. 2005;11:214–222. doi: 10.1038/nm1175. [DOI] [PubMed] [Google Scholar]
- 22.Nagayama T, Hsu S, Zhang M, Koitabashi N, Bedja D, Gabrielson KL, Takimoto E, Kass DA. Sildenafil stops progressive chamber, cellular, and molecular remodeling and improves calcium handling and function in hearts with pre-existing advanced hypertrophy caused by pressure overload. J Am Coll Cardiol. 2009;53:207–215. doi: 10.1016/j.jacc.2008.08.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schafer S, Ellinghaus P, Janssen W, Kramer F, Lustig K, Milting H, Kast R, Klein M. Chronic inhibition of phosphodiesterase 5 does not prevent pressure-overload-induced right-ventricular remodelling. Cardiovasc Res. 2009;82:30–39. doi: 10.1093/cvr/cvp002. [DOI] [PubMed] [Google Scholar]
- 24.Andersen A, Nielsen JM, Peters CD, Schou UK, Sloth E, Nielsen-Kudsk JE. Effects of phosphodiesterase-5 inhibition by sildenafil in the pressure overloaded right heart. Eur J Heart Fail. 2008;10:1158–1165. doi: 10.1016/j.ejheart.2008.09.016. [DOI] [PubMed] [Google Scholar]
- 25.Kaufman BD, Desai M, Reddy S, Osorio JC, Chen JM, Mosca RS, Ferrante AW, Mital S. Genomic profiling of left and right ventricular hypertrophy in congenital heart disease. J Card Fail. 2008;14:760–767. doi: 10.1016/j.cardfail.2008.06.002. [DOI] [PubMed] [Google Scholar]
- 26.Ambardekar AV, Buttrick PM. Reverse remodeling with left ventricular assist devices: A review of clinical, cellular, and molecular effects. Circ Heart Fail. 2011;4:224–233. doi: 10.1161/CIRCHEARTFAILURE.110.959684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Terracciano CM, Hardy J, Birks EJ, Khaghani A, Banner NR, Yacoub MH. Clinical recovery from end-stage heart failure using left-ventricular assist device and pharmacological therapy correlates with increased sarcoplasmic reticulum calcium content but not with regression of cellular hypertrophy. Circulation. 2004;109:2263–2265. doi: 10.1161/01.CIR.0000129233.51320.92. [DOI] [PubMed] [Google Scholar]
- 28.Soppa GK, Barton PJ, Terracciano CM, Yacoub MH. Left ventricular assist device-induced molecular changes in the failing myocardium. Curr Opin Cardiol. 2008;23:206–218. doi: 10.1097/HCO.0b013e3282fc7010. [DOI] [PubMed] [Google Scholar]
- 29.Ibrahim M, Al Masri A, Navaratnarajah M, Siedlecka U, Soppa GK, Moshkov A, Al-Saud SA, Gorelik J, Yacoub MH, Terracciano CM. Prolonged mechanical unloading affects cardiomyocyte excitation-contraction coupling, transverse-tubule structure, and the cell surface. FASEB J. 2010;24:3321–3329. doi: 10.1096/fj.10-156638. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.