Abstract
The identification of micro-organisms carried by ticks is an important issue for human and animal health. In addition to their role as pathogen vectors, ticks are also the hosts for symbiotic bacteria whose impact on tick biology is poorly known. Among these, the bacterium Wolbachia pipientis has already been reported associated with Ixodes ricinus and other tick species. However, the origins of Wolbachia in ticks and their consequences on tick biology (known to be very diverse in invertebrates, ranging from nutritional symbionts in nematodes to reproductive manipulators in insects) are unknown. Here we report that the endoparasitoid wasp Ixodiphagus hookeri (Hymenoptera, Chalcidoidea, Encyrtidae) – strictly associated with ticks for their development - is infested at almost 100% prevalence by a W. pipientis strain belonging to a Wolbachia supergroup that has already been reported as associated with other hymenopteran parasitoids. In a natural population of I. ricinus that suffers high parasitism rates due to I. hookeri, we used specific PCR primers for both hymenopteran and W. pipientis gene fragments to show that all unfed tick nymphs parasitized by I. hookeri also harbored Wolbachia, while unparasitized ticks were Wolbachia-free. We demonstrated experimentally that unfed nymphs obtained from larvae exposed to I. hookeri while gorging on their vertebrate host also harbor Wolbachia. We hypothesize that previous studies that have reported W. pipientis in ticks are due to the cryptic presence of the endoparasitoid wasp I. hookeri. This association has remained hidden until now because parasitoids within ticks cannot be detected until engorgement of the nymphs brings the wasp eggs out of diapause. Finally, we discuss the consequences of this finding for our understanding of the tick microbiome, and their possible role in horizontal gene transfer among pathogenic and symbiotic bacteria.
Introduction
As ticks are considered to be second only to mosquitoes as vectors of human infectious diseases in the world, the identification of micro-organisms carried by ticks is an important issue for human or animal health [1]. Among arthropod vectors, ticks harbor the largest diversity of micro-organisms, ranging from viruses (i.e. arboviruses such as Tick Borne Encephalitis…), prokaryotes (including spirochaetes such as Borrelia burgdorferi) and eukaryotes (including Apicomplexa - such as Babesia spp. or Theileria spp. – or even metazoans - such as some filarial nematodes -) [2]. This large diversity of micro-organisms may be related to the fact that all three stages (larvae, nymphs and adults) take a blood meal over several days, ingesting a large volume of blood [3], and that a wide range of different vertebrate host species is used for the blood meal (at least for the most common species in Europe or in North-America – i.e. Ixodes ricinus and I. scapularis respectively). Moreover, because of the long life cycle of ticks (usually 2 to 3 years for species living in temperate areas), these arthropods are reservoirs for pathogens (especially for vertically transmitted pathogens, i.e. trans-ovarial – transmission)in addition to vertebrate primary hosts, facilitating micro-organism persistence through unfavorable seasons [4].
However, ticks do not only harbor pathogens; they also host symbiotic bacteria whose effects on tick fitness and on vertebrate health are poorly known. For example, among Rickettsia spp., several species that are commonly found associated with ticks have never been found associated with disease [5], [6]. While Coxiella burnetti is known as the etiological agent of the Q-Fever responsible for recent outbreaks in Europe, an unnamed Coxiella species has been found in 100% of the individuals of Amblyomma americanum [7]. Antibiotic treatment of these ticks resulted in reduced reproductive fitness, suggesting that the Coxiella sp. is a primary endosymbiont [8]. Midichloria mitochondrii is an endosymbiotic alpha-proteobacterium found in the ovarian tissues of almost all Ixodes ricinus females from natural populations [9], [10]. This bacterium has a unique intramitochondrial lifestyle [11], [12], [13] but its consequences on tick fitness or reproduction remain unknown [13]. More recently, an obligate intracellular gamma-proteobacterium (Diplorickettsia massiliensis) has been isolated from I. ricinus, again with unknown consequences for its host tick [14]. The recent development of metagenomic approaches on ticks will probably allow the identification of numerous new bacteria species [15], [16].
Even if some of those micro-organisms are not pathogenic for vertebrate hosts, their presence within ticks may have some consequences for the epidemiology of some tick-borne diseases either by acting on vector fitness or through synergic or antagonistic interactions with known pathogens. Recent studies have highlighted the fact that species interactions in a parasite community drive infection risk [17] or that competitive exclusion may be detrimental to one pathogen species in case of co-infections [18]. In the case of blood-feeding insects, symbiotic bacteria having a role in the nutrition of their host have been reported in an increasing number of cases [19], [20]. Moreover, some bacterial symbionts can have direct consequences on the transmission of some pathogens by vectors. For example, the refractoriness of some Anopheles gambiae populations from Zambia in transmission of Plasmodium falciparum is due to the presence of an Enterobacter species in the mosquito's gut [21]. In the mosquito species Aedes albopictus, it has been demonstrated that the replication of the Chikungunya virus is modulated by the presence of endosymbiotic Wolbachia [22]. Wolbachia is also known to induce resistance to dengue virus in Aedes aegypti [23]. Wolbachia are now also known to interfere with the innate immune system of their arthropod host, hence conferring virus protection in some cases [24].
Wolbachia are endosymbiotic bacteria manipulating host reproductive systems (through cytoplasmic incompatibility or parthenogenesis for example) or as mutualists when they are associated with nematodes [25]. The presence of Wolbachia may have direct consequences on the development of other pathogens in the same arthropod vector, and also indirect effects on pathogen epidemiology through impacts on the dynamics and genetic diversity of the vector [26]–[27]. Thanks to the development of molecular tools to look for microorganisms within ticks, Wolbachia have been reported from ticks in an increasing number of studies (reviewed in table 1). However, their frequency is usually low (but see [28] where W. pipientis reach 7.6% in nymphs) and some studies designed to look for Wolbachia in ticks have failed to find any [29]. The consequences of the presence of Wolbachia in those ticks are unknown.
Table 1. List of studies reporting the presence of Wolbachia pipientis associated with ticks.
| Tick species | Origin of the tick population | Frequency of ticks harboring Wolbachia | Reference |
| Ixodes ricinus | France | 7,6% (n = 131) | [28] |
| Ixodes ricinus | Germany | 2 clones out of 77, obtained from 27 nymphs | [34] |
| Ixodes ricinus | Germany | 0,9% (n = 5424) | [33] |
| Ixodes ricinus | Italy | >1% (two samples of 100 nymphs) | [16] |
| Ixodes ricinus | Morocco | 0,5% (n = 221) | [35] |
| Ixodes scapularis | Massachusetts | 1 clone out of 106 from 7 nymphs | [46] |
| Rhipicephalus microplus | Texas | ? | [15] |
| Rhipicephalus sanguineus | Japan | 12,5% (n = 32) | [62] |
The purpose of this study was to look for Wolbachia in natural populations of I. ricinus and to identify their origin within ticks. We discovered that natural populations of the wasp Ixodiphagus hookeri (Hymenoptera, Chalcidoidea, Encyrtidae), an endoparasitoid of Ixodes ricinus, ,are infected at almost 100% prevalence by Wolbachia pipientis. Using PCR primers specific to I. hookeri and W. pipientis respectively, we showed that in natural tick populations, all individual ticks harboring Wolbachia were also parasitized by I. hookeri, while ticks that were not parasitized were Wolbachia-free. We showed experimentally that some unfed nymphs, exposed to the parasitoids while they were larvae gorging on their vertebrate host, also harbored Wolbachia. We hypothesize that the presence of Wolbachia, already reported in several studies using 16S PCR primers to identify bacteria within ticks, is due to the presence of Ixodiphagus in those ticks, which is externally cryptic (as the parasitized tick nymphs looks healthy until their engorgement that bring the parasitoid eggs out of their diapause). Finally, we discuss the consequences of these findings for our understanding of tick-microbiota and possible consequences for tick-borne diseases epidemiology.
Results
The parasitoid Ixodiphagus hookeri harbors endosymbiotic Wolbachia pipientis
Based on both (i) engorged nymphs collected on roe-deer and (ii) host-seeking nymphs collected on vegetation that were subsequently engorged in the lab, a total of 54 ticks collected in 4 natural populations were found to be parasitized by I. hookeri (Table 2; Figure 1). Variation of parasitism rates among years and sites range from 3,2% to 12,5% in Chizé (tick nymphs collected on roe deer), and from 19,6% to 20% in Gardouch (tick nymphs collected on roe-deer and on vegetation respectively). Among the I. hookeri individuals obtained from those 54 ticks, a total of 121 I. hookeri individuals (74 females and 47 males) were screened for Wolbachia pipientis by PCR using Wsp primers. Wolbachia DNA was found in 120 (99,2%) of those individuals (a single wasp male from the Gardouch population was Wolbachia-free, even after a second PCR trial).
Table 2. Parasitism rates of Ixodes rcinus due to Ixodiphagus hookeri and presence of Wolbachia in the wasps.
| Tick origin | Sampling site | Sampling date | No. nymphs collected | No. nymphs parasitized by I. hookeri | Parasitism rates % | No. nymphs parasitized by I. hookeri studied | No. I. hookeri individuals studied | No. I. hookeri individuals with Wsp PCR + | ||
| Female | Male | Total | ||||||||
| Roe deer | Chizé | Feb. 2007 | 128 | 16 | 12,5 | 0 | - | - | - | - |
| Roe deer | Chizé | Feb. 2008 | 14 | 1 | 7,1 | 0 | - | - | - | - |
| Roe deer | Chizé | March 2009 | 221 | 7 | 3,2 | 7 | 9 | 6 | 15 | 15 |
| Roe deer | Chizé | Feb. 2010 | 86 | 6 | 7 | 5 | 5 | 4 | 9 | 9 |
| Roe deer | Chizé | Feb. 2011 | 205 | 16 | 7,8 | 0 | - | - | - | - |
| Roe deer | Trois-Fontaines | Dec. 2009 | 20 | 1 | 5 | 1 | 1 | 1 | 2 | 2 |
| Roe deer | Gardouch | March 2009 | 40 | 8 | 20 | 8 | 27 | 8 | 35 | 35 |
| Vegetation | Gardouch | Oct. 2009 | 101 | 25 | 24,8 | 21 | 21 | 18 | 39 | 39 |
| Vegetation | Gardouch | April 2010 | 102 | 20 | 19,6 | 11 | 7 | 8 | 15 | 14 |
| Vegetation | Fougère | Sept. 2010 | 6 | 1 | 16,7 | 1 | 4 | 2 | 6 | 6 |
| Total | 923 | 101 | 10,9 | 54 | 74 | 47 | 121 | 120 | ||
Figure 1. Ixodiphagus hookeri (Hymenoptera : Chalcidoidea, Encyrtidae).
A Female habitus. B Female ovipositing in an engorged nymphs of Ixodes ricinus (ovipositor indicated by the arrow). C Adults of I. hookeri around the dead body of an engorged nymph of I. ricinus. D Emergence hole from which parasitoids exit the dead body of the engorged nymph.
Tick individuals from natural populations parasitized by I. hookeri are also harboring Wolbachia pipientis
Among 250 unfed nymphs collected in April 2010 in Gardouch, 102 were engorged and the parasitism rate due to I. hookeri observed in this natural population was 19,6%. DNA was extracted from 52 of these 250 nymphs, and the presence of both I. hookeri and W. pipientis DNA was investigated using of hymenopteran CO1 and Wolbachia Wsp specific PCR primers. Ten out of those 52 nymphs (19,2%) were found to harbor both I. hookeri and W. pipientis. All the 42 ticks that were found without any wasp DNA (i.e. no PCR amplification with the CO1 primers) were also found without W. pipientis (i.e. no PCR amplification with the Wsp primers), except for a single tick DNA extract found to be Wolbachia-positive but wasp-negative.
Experimental demonstration that parasitim of I. ricinus by I. hookeri is associated with the presence of Wolbachia in ticks
To demonstrate experimentally that I. hookeri is responsible for the presence of Wolbachia in some I. ricinus individuals, we set up a laboratory rearing of this parasitoid species and exposed some laboratory strain ticks to the parasitoids. Under experimental conditions, parasitism rate was extremely reduced when unfed nymphs were exposed to parasitoids (parasitism rate estimated by the engorgement of unfed nymphs exposed to parasitoids was 2,5% - i.e. 3 nymphs produced wasps of 171 exposed to adult parasitoids and subsequently engorged to reveal parasitism). Parasitoids obtained from those experimentally parasitized ticks again harbored Wolbachia as attested by the results of PCR amplification with Wsp primers, hence demonstrating experimentally the vertical transmission of Wolbachia in I. hookeri. Additionnaly, Wsp primers were tested successfully on DNA extracted from groups of about 50 I. hookeri eggs that were isolated after the dissection of female parasitoid gaster, demonstrating that the parasitoid eggs contain some Wolbachia.
Parasitoid rates were higher when nymphs were exposed to parasitoids while they were feeding on gerbils (40,5%; n = 84). However, these engorged nymphs are not the tick stage for which the primers are the most useful (preventing the engorgement of ticks in the lab to determine the parasitism rate), i.e. the unfed nymphs. To produce unfed nymphs in the lab that harbor I. hookeri, we exposed some tick larvae to parasitoids during engorgement on gerbils. Previous studies [30], [31] have shown that such tick larvae produce living nymphs that harbor parasitoid eggs and only the engorgement of those parasitized nymphs brings the wasp eggs out of diapause, allowing the parasitoids to emerge from those engorged nymphs. The set of nymphs exposed to parasitoids during engorgement was divided in two. Half were used to estimate the parasitism rate due to I. hookeri. Two of 33 nymphs produced some parasitoids after engorgement, corresponding to a parasitism rate of 6%. The second half was used to look for Wolbachia using the Wsp primers; 8 of the 106 unfed nymphs (5,7%) harbored Wolbachia. Two out of those 8 ticks harboring Wolbachia were also positive with the CO1 primers, confirming that nymphs containing Wolbachia also contained I. hookeri eggs. We interpreted the lack of amplification with the CO1 primers for 6 out of those 8 ticks as due to lower sensitivity of the CO1 primers and the low quantity of I. hookeri DNA expected in a parasitized nymph (containing 5 to 10 eggs). PCR screening with appropriate primers showed none of the 32 unfed nymphs used as a negative control (corresponding to larvae that were not exposed to parasitoids during their engorgement) to contain Wolbachia or I. hookeri DNA.
Wolbachia pipientis associated with I. hookeri belongs to a Wolbachia clade found in hymoneptera or other insects
Sequence data for Wsp and ftsZ genes of Wolbachia found in I. hookeri from ticks was used to determine whether these bacteria belong to a clade already associated with other insects (including parasitoids), or instead fall within Wolbachia groupings associated with ticks (Acari) or other Arachnida and Chelicerata. Six Wsp sequences (ranging from 515 to 379 pb) were obtained from PCR products based on I. hookeri individuals from the Gardouch and Chizé populations. The alignment revealed no polymorphism (in the 351 pb common to all 6 sequences or in the other part of the alignment). A consensus sequence of 533 pb was generated (515 pb from the longest sequence, to which 18 pb were added from another sequence) and compared to Wsp sequences already deposited in GenBank with BLAST. Over those 533 pb, this consensus sequences has 100% identity with a Wolbachia associated with I. ricinus (accession n° EF219192.1 from a dutch population; Huigens,M.E. et al. unpublished) and 99% identity with 16 Wsp sequences of Wolbachia associated with insects and particularly Hymenoptera (2 with another parasitoid chalcid – Spalangia cameroni [Hymenoptera: Pteromalidae], 10 with the parasitoid Asobara tabida [Hymenoptera : Braconidae], 1 with the parasitoid Trichopria drosophilae [Hymenoptera: Diapriidae], 2 with the moths Dichocrocis punctiferalis and Ostrinia furnacalis [Lepidoptera : Crambidae] respectively and 1 with the bug Elasmucha signoreti [Heteroptera : Acanthosomatidae]).
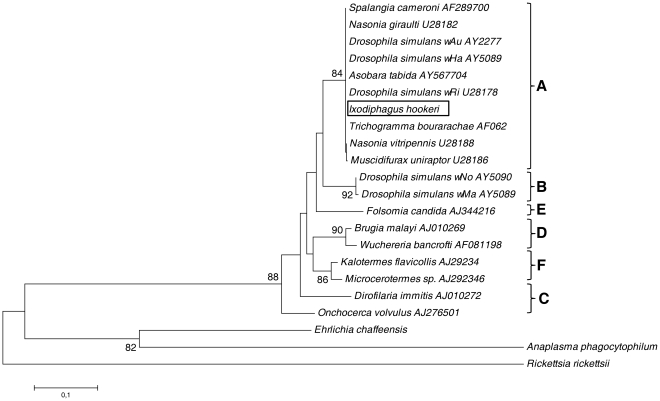
Because the Wsp gene is known to exhibit a high recombination rate [32], we also sequenced the Ftsz gene to identify the Wolbachia supergroup to which the Wolbachia associated with I. hookeri belong. The FtsZ sequence (947 bp long) was obtained from a wasp individual from the Gardouch population. In the phylogenetic tree (Figure 2), this sequence clearly belongs to supergroup A (with only one difference with the sequence found in the Wolbachia associated with other hymenopteran parasitoids such as Spalangia cameroni, Trichogramma bourarachae (Hymenoptera; Chalcidoidea; Trichogrammatidae), Asobara tabida, and the fruitfly Drosophila simulans. The Ftsz and the Wsp sequences have a 100% similarity with the allele ftsz-3 (435 bp long) and Wsp-40 (493 bp long) respectively in the Wolbachia MLST database (Wolbachia MLST website: http:pubmlst.org/wolbachia).
Figure 2. Phylogenetic tree of Wolbachia pipientis.
A Neighbor-Joining tree based on the the Ftsz sequence obtained with MEGA5 using the Maximum Composite Likelihood distance. Only bootstrap values greater than 80% are shown. Letters A to F (at the right of the name of the host from which the Wolbachia was sequenced and of its accession number) refer to the Wolbachia supergroups already described [58].
Discussion
Presence of I. hookeri in I. ricinus nymphs is correlated with presence of W. pipientis in ticks
By the use of specific PCR primers for the parasitoid I. hookeri and for the endosymbiotic bacteria W. pipientis, we demonstrated that almost all (99,2%) of the I. hookeri individuals investigated, emerging from 5 different natural french populations of the tick I. ricinus, harbored W. pipientis. The analysis of a natural tick population suffering from high parasitism due to I. hookeri (evaluated at 20%, based on the engorgement in the lab of nymphs collected on the vegetation) revealed that all the DNA extracts of nymph ticks showing evidence of the presence of I. hookeri (based on CO1 hymenopteran specific primers) also harbored W. pipientis (based on Wsp Wolbachia specific primers), while all but one of the tick DNA extracts without any evidence of I. hookeri were also Wolbachia-free. We also showed experimentally that unfed nymphs developing from larvae exposed to wasps while gorging on their host, also harbored Wolbachia. Moreover, the Wolbachia sequence of the Wsp and Ftsz genes associated with I. ricinus were identical or very similar to Wolbachia sequences already reported as associated with parasitoid hymenoptera, including other chalcids.
Based on these observations, we suggest that the presence of W. pipientis in I. ricinus, already reported by several authors (but, to date, without any hypothesis to explain the presence of this bacterium in ticks), is due to the parasitism of those ticks by the hymenopteran endoparasitoid I. hookeri. Because of the koinobiontic development of those parasitoids, the presence of the parasitoids in nymphs cannot be detected visually prior to their engorgement (bringing the parasitoid eggs out of diapause). In all of these studies (Table 1), Wolbachia has been identified from the sequencing of 16S rRNA gene amplicons. Ticks were sampled from natural populations (and not laboratory reared) and thus these ticks could have been exposed to I. hookeri parasitoids. Moreover, our hypothesis is corroborated by the fact that most of these studies are based on tick DNA extracts obtained from nymphs [16], [33], [34], the tick stage most attacked by the parasitoids [28]. Two other studies have reported the presence of W. pipientis from adults of I. ricinus, but at a much lower frequency (i.e 1,0% (n = 98) compared to 27,3% in nymphs (n = 33) in a French population [28]; 0,45% (n = 221) in a Moroccan population; [35]; see also [15] for the report of W. pipientis associated with adults of Rhipicephalus (Boophilus) microplus). Although emergence of I. hookeri has never been reported from engorged I. ricinus females [36] (but oviposition on adults occurs in the lab on adult Rhipicephalus sanguineus [37]), the presence of W. pipientis in a few adult ticks may still be explained by parasitism of nymphs by I.hookeri. Indeed, it is likely that not all ovipositions of I. hookeri result in successful development of parasitoids. In particular, the innate immune system of ticks may be able to kill some parasitoid eggs (by the encapsulation of parasitoid eggs resulting in its melanization and subsequent death) that could prevent the development of parasitoids but explain the presence of W. pipientis DNA in tick adults. The fact that W. pipientis has not been found in some studies that have looked for it in I. ricinus [29], [38] can also be explained by the absence of parasitoid in those ticks.
Use of Wolbachia specific primers to estimate parasitism rates due to I. hookeri
In subsequent studies, Wolbachia-specific Wsp primers could be used to determine parasitism rates due to I. hookeri in natural populations of I. ricinus using host-seeking nymphs (and thus avoiding the time-consuming engorgement of nymphs in the lab to determine parasitism rates). Because parasitoid diapause occurs at the egg stage [39], the amount of I. hookeri and Wolbachia DNA in unfed I. ricinus nymphs is probably reduced (5 to 10 eggs per ticks considering the mean number of parasitoid adults emerging from an engorged nymph; 6,5 according to [40]; n = 54). Fortunately, the use of mitochondrial primers (the DNA mitochondrial molecules being present in numerous copies in each single egg cell) is more sensitive to report the presence of I. hookeri than nuclear markers. However, the use of Wsp primers seems to be even more sensitive to report the presence of I. hookeri in ticks than the specific mitochondrial CO1 primers (c.f. the few cases where tick extracts were positive with the Wsp and not the CO1 primers). This may be due to a high number of Wolbachia bacteria in I. hookeri eggs (for example, between 116 and 292 bacteria per egg in Nasiona vitripennis [41].
Our results show that parasitism rates due to I. hookeri in natural population of I. ricinus can reach at least 20%, inflicting significant mortality on tick populations. This explains why I. hookeri was considered as a possible biological control agent to reduce tick populations, soon after its original description [42]. The higher parasitism rates observed in Gardouch may be associated with the higher roe deer density (as already observed in the USA for Odocoileus virginiae, Ixodes scapularis and I. hookeri [43]) maintained in a 14 hectare fenced area. In Chizé, the parasitism rates are relatively stable across years (again fenced, but covering a much larger area of 2580 hectares). However, the month of tick sampling (February), constrained by roe-deer capture, may not be representative of the parasitism rates of I. ricinus during other months.
Consequences of the presence of Wolbachia on tick and tick-borne diseases
The phenotypic consequences of Wolbachia infection for their hosts are highly diverse and new impacts are regularly discovered. In filarial nematodes, these bacteria are mutualistic and required for the development and reproduction of their host. In arthropods, they have first been shown to alter their host reproduction through various mechanisms (cytoplasmic incompatibility, parthenogenesis, male-killing, feminization…) but a growing number of studies show that they also have some impacts on the immune response of their host (with some consequences for resistance against parasitoids, or on pathogen agents transmission in the case of arthropod vectors; [44]) or may even be nutritional mutualists in blood feeding bugs [20]. Previous reports of W. pipientis in ticks have only mentioned the known consequences of Wolbachia on nematodes or insects [33] or suggested their possible role as mutualistic endosymbionts of ticks [45] or as pathogenic agents [35]. Our finding that Wolbachia is associated with I. hookeri rather than with I. ricinus per se and that Wolbachia is thus not (or only very rarely) vertically transmitted to tick offspring (otherwise, the prevalence of Wolbachia in ticks would be much higher; anyway, most ticks parasitized by I. hookeri and thus containing W. pipientis are expected to die, once they will engorge) suggests that any consequences of Wolbachia infection on I. ricinus reproduction are without object. Additionnal investigations must be conducted to identify the consequences of Wolbachia on I. hookeri. Although the presence of Wolbachia in ticks may be considered a “dead-end” for the bacteria, it may have had some consequences on other microorganisms harbored by ticks. For example, a Wolbachia gene has been identified by Ishmael et al. within a plasmid of a Rickettsia species found in Ixodes scapularis (the “Rickettsia endosymbionts of I. scapularis” identified by Nodae et al. [45] and Benson et al.[46] and reported in 100% of the engorged females studied by [47]). The authors suggest that lateral gene transfer between these two obligate intracellular species has occurred. The 44 Wolbachia traces found in the I. scapularis genome [48] were finally due to the presence of these Rickettsia endosymbionts within the I. scapularis trace analysed [49].
Beside W. pipientis, a negative interaction between some pathogen agents (i.e. the Spirochaete Borrelia burgdorferi and the Apicomplexa Babesia microti) and I. hookeri in north American populations of I. scapularis has been reported [50]. Although this study was conducted prior to the development of molecular techniques to detect those micro-organisms within ticks, the authors observed that the prevalence of those human pathogens in host-seeking ticks collected in wasp-infested sites is nearly 40% lower than that found in other sites and that spirochaetes never infected wasp-infected ticks and few wasp-infected ticks were concurently infected by B. microti. The authors considered that “wasps may render the tick inhospitable to both pathogens. Our finding that I. hookeri harbors W. pipientis could provide an explanation for those observations : the bacteria may induce an immune reaction in the ticks that could have a negative impact on other micro-organism hosted by ticks (see for example [44]).
To conclude, in prevision to the growing number of studies that will use a metagenomic approach to identify micro-organisms within ticks [15], we would like to highlight the fact that ticks are also the hosts of macroparasites (including wasps such as I. hookeri, but also nematodes such as Cercopithafilaria rugosicauda associated with I. ricinus) that can harbor their own microbiome. Thus, the study of tick-borne diseases involves more than a tripartite system involving vertebrates, arthropod vectors and pathogen agents but also involves other organisms, pleading for a community ecology approach on these complex systems.
Materials and Methods
Tick and insect populations studied
Ixodes ricinus
The tick Ixodes ricinus is the most common european tick species, found mainly in forests, but also hedges or moors. Its 2–4 year lifecycle includes three stages (larva, nymph and adults), with each stage needing to take a blood meal on a vertebrate host (a mammal, bird or lizard) for its development. After each blood meal, the engorged ticks fall from the host to the ground, where they will metamorphose to the next stage. Questing (host-seeking) ticks will then waiting for a host by staying on the top of the vegetation.
Natural populations of Ixodes ricinus were collected from 5 different locations in France, either by collecting engorged tick nymphs directly on roe-deer (Réserve Biologique Intégrale de Chizé - 46° 7′18.89″N, 0°25′3.72″O -, Trois Fontaines - 48°41′46.14″N, 4°55′30.31″E - and Gardouch - 43° 23′ 27.6″ N, 01° 40′ 52.0″ E), or by collecting unfed questing tick nymphs on the vegetation using the standard “flagging method” (Gardouch and Fougère - 47°36′25.99″N, 0° 5′54.84″O -) from 2007 to 2010. Additional tick nymphs from our laboratory strain (originally collected in Belle-Ile; [51]) were used as a negative control.
Ixodiphagus hookeri
With 8 species described [52], Ixodiphagus (Hymenoptera, Chalcidoidea, Encyrtidae) is the single parasitoid genus of 80 000 parasitoid species described [53] known to parasitize ticks. In Europe, only I. hookeri has been reported, where it is mainly associated with Ixodes ricinus. Although the lifecycle of this parasitoid is not completely known (especially the stages during winter time; [40]), the parasitoids are only known to emerge from engorged nymphs. The females of I. hookeri oviposit 5–10 eggs into tick nymphs (or eventually in tick larvae). The parasitoid does not kill its host during oviposition (corresponding to the koinobiontic strategy) and the tick nymphs look healthy until their engorgement. Once the tick is engorged, the diapause of the wasp eggs is broken and the wasp larvae begin their development within the engorged tick. The tick will finally die and the adult parasitoids dig a hole at the posterior end of the tick to emerge, 1–2 months after oviposition. The adult wasps then mate and the females look for ticks to lay their eggs before to die 2 or 3 days after emergence.
Estimation of the parasitism rates due to I. hookeri in natural populations of I. ricinus
Parasitism rates due to I. hookeri in natural populations of I. ricinus were estimated both from engorged ticks collected on roe-deer and from questing ticks collected on the vegetation. Unfed tick nymphs were engorged on gerbils, after having been laid on the fur with a paintbrush and recovered 3 to 7 days after. All engorged tick nymphs were then placed in individual tubes and kept at a humidity of 80%. Emergence of parasitoids from engorged nymphs was checked weekly. The adults obtained were sexed under a steromicroscope using the key provided in Quaraishi 1958 [54]. Parasitism rates were calculated by taking into account only engorged nymphs producing either adult ticks or parasitoids (engorged nymphs that die without producing adult ticks or wasps were not included).
Exposition of I. ricinus to the parasitism by I. hookeri in laboratory experiments
Unfed nymph ticks from our laboratory rearing (without I. hookeri and shown by Wsp PCR to be Wolbachia-free), were exposed to parasitoids in sets of 25 (2 flasks), 50 (2 flasks)or 100 (3 flasks) individuals in a tissue culture cell flask (25 cm3) with filter screw cap (TRP). Several freshly emerged adult parasitoids (both females and males; from 4 to 10; mean of 7 wasps per flask) were added to each flask for 2 or 3 days until parasitoid death. In other experiments, tick nymphs or larvae were exposed to parasitoids while they were gorging on gerbils. To that aim, several freshly emerged wasps (from 4 to 7, mean of 5,8 per cage) were introduced to the cage containing the gerbil and the ticks that were deposited on the gerbil 4 days before.
DNA extraction
Individual ticks or wasps, killed at −80°C or conserved in alcohol, were put in one well (in 2 ml Deepwell plate; NUNC) with 2 pre-sterilised glass beads of 3 mm diameter and one of 5 mm. After 15 minutes at −80°C, they were mechanically crushed by using the Qiagen Tissue Lyser for 3 minutes at a frequency of 15 Hz. DNA was extracted using the NucleoMag 96 Tissue kit (Macherey-Nagel). DNA was eluted with 50 µl buffer for ticks and 25 µl for wasps and conserved at 4°C until PCR was performed.
PCR amplification and sequencing
To amplify the wsp gene of Wolbachia, we used the wsp-81F and wsp-691R primers [55] amplifying a DNA fragment of 590-632 pb. PCRs were conducted in 20 µl reaction volumes with 7,8 µl H2O (EPPI sterile), 4 µl 5X Buffer, 1 µl forward and reverse primer (10 µM), 2 µl dNTP (2mM), 0,2 µl of Phusion High-Fidelity Taq (New England Biolabs) and 4 µl of DNA extract. The amplification program is the following: 98°C for 30 seconds, then 29 cycles at 98°C for 45 seconds, 55°C for 45 seconds and 72°C for 60 seconds and final elongation at 72°C for 10 minutes. The PCR product obtained was sequenced using PCR primers as sequencing primers (accession number : JQ315223).
To amplify the ftsZ gene of Wolbachia, we used the ftsZf1 and ftsZr1 primers [56]. They amplified a DNA fragment of 1043–1055 pb. PCRs were conducted in 25 µl reaction volumes with 19,3 µl H2O (EPPI sterile), 2,5 µl 10X Buffer, 0,350 µl forward and reverse primer (20 µM), 0,5 µl dNTP (10mM), 0,75 µl MgCl2, 0,25 µl of Eurobio Taq and 1 µl of DNA extract. The amplification program is the following: 94°C for 60 seconds, 55°C for 60 seconds, 72°C for 3 minutes, then 35 cycles at 94°C for 15 seconds, 55°C for 60 seconds and 72°C for 3 minutes and final elongation with a cycle at 94°C for 15 seconds, 55°C for 60 seconds, 72°C for 10 minutes. The PCR product obtained was sequenced using PCR primers as sequencing primers (accession number : JQ315224).
To design specific primers for the CO1 gene of I. hookeri (that do not amplify I. ricinus DNA), we first used the primers 1775-Co1-F, and 2773-Co1-R, already described for other Chalcidoidea [57]. From the I. hookeri sequence obtained (accession number JQ315225), we designed two new primers 401-F (5′-TTTAGAATATTTATTGATTCAGGGACT-3′) and 44-R (5′-CTCCTGCTAAAACTGGTAAAGATAAT-3′) that amplify a 268 bp fragment. The non-amplification of I. ricinus with those primers specific to I. hookeri was confirmed by PCR.
PCRs were conducted in 20 µl reaction volumes with 9,2 µl H2O (EPPI sterile), 2 µl 10X Buffer (Eurobio), 1 µl forward and reverse primer (10 µM), 2 µl dNTP (2mM) and 0,6 µl MgCl2 (50 mM, Eurobio), 0,2 µl of Eurobio Taq and 4 µl of DNA. The amplification program is a touchdown program : 92°C for 2 minutes, then 92°C for 45 seconds, 53°C to 47°C for 45 seconds and 72°C for 60 seconds, then 29 cycles at 92°C for 45 seconds, 47°C for 45 seconds and 72°C for 60 seconds and final elongation at 72°C for 10 minutes.
Alignment and Phylogenetic analysis
To identify the Wolbachia supergroup of the Wolbachia sequence associated to I. hookeri, we downloaded Ftsz sequences from the Wolbachia supergroups A, B, C, D, E, F [58] to which we have added several Ftsz sequences with high query coverage and percentage of identity using BLAST. The sequences of Anaplasma phagocytophilum, Rickettsia rickettsi and Ehrlichia chaffeeensis were also added as outgroups and to illustrate the divergence between those well-known pathogen agents transmitted by ticks and W. pipientis. The alignment of the Ftsz sequences was conducted with CLUSTAL W2 [59]. Phylogenetic analyses were conducted with MEGA5 [60]. A Neighbor-Joining tree was built with the Maximum Composite Likelihood distance [61], and a complete deletion option. Bootstrap values were estimated using 10,000 replications.
Ethical Statements
All experiments were carried out at Oniris (Ecole Nationale Vétérinaire, Agroalimentaire, et de l′Alimentation Nantes Atlantique), adhering to local (Pays de la Loire Ethics Committee) and national guidelines and laws of experimental work with laboratory animals who has approved the protocol used in this study (permit number n° 44266).
Acknowledgments
We express our gratitude to N. Cebe (CEFS) and L. Malandrin (BioEpAR) for providing some ticks, the CEFS lab, the ONCFS (Office National de la Chasse et de la Faune Sauvage) and the ONF (Office National des Forêts) from the “Réserve biologique domaniale intégrale de la Sylve d'Argenson (Chizé)” and Trois-Fontaines for their help in the capture and manipulation of roe-deer, and B. Chaubet (INRA, UMR Bio3P, Rennes) for providing the picture of I. hookeri (cf figure 1A). We are extremely grateful to G. Stone (IEB, Edinburgh) and three anonymous reviewers for reading and commenting on an earlier draft of this paper. The authors also thank the “Tick and Tick-Borne Diseases” group (REID- Réseau Ecologie des Interactions Durables) for stimulating discussions. The Biogenouest plateform is acknowledged for providing access to sequencing facilities.
Footnotes
Competing Interests: The authors have declared that no competing interests exist.
Funding: This work was supported by "Jeune Equipe INRA Varibabix". Action Incitative Programmé du Centre International pour la Recherche Agronomique et le Développement (CIRAD) et de l'Institut National de la Recherche Agronomique (INRA) "Dynamique et diversité génétique des tiques et pathogènes transmis" INRA, Animal Health Division. http://www.inra.fr/sante_animale/ The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Parola P, Didier R. Ticks and tickborne bacterial diseases in humans: An emerging infectious threat. Clinical Infectious Diseases. 2001;32:897–928. doi: 10.1086/319347. [DOI] [PubMed] [Google Scholar]
- 2.Jongejan F, Uilenberg G. The global importance of ticks. Parasitology. 2004;129:S3–S14. doi: 10.1017/s0031182004005967. [DOI] [PubMed] [Google Scholar]
- 3.Sonenshine DE. Biology of ticks: Oxford University Press; 1991. [Google Scholar]
- 4.Randolph SE. Tick ecology: processes and patterns behind the epidemiological risk posed by ixodid ticks as vectors. Parasitology. 2004;129:S37–S65. doi: 10.1017/s0031182004004925. [DOI] [PubMed] [Google Scholar]
- 5.Parola P, Paddock CD, Raoult D. Tick-borne rickettsioses around the world: Emerging diseases challenging old concepts. Clinical Microbiology Reviews. 2005;18:719-+. doi: 10.1128/CMR.18.4.719-756.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Weinert LA, Werren JH, Aebi A, Stone GN, Jiggins FM. Evolution and diversity of Rickettsia bacteria. Bmc Biology. 2009;7 doi: 10.1186/1741-7007-7-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Clay K, Klyachko O, Grindle N, Civitello D, Oleske D, et al. Microbial communities and interactions in the lone star tick, Amblyomma americanum. Mol Ecol. 2008;17:4371–4381. doi: 10.1111/j.1365-294x.2008.03914.x. [DOI] [PubMed] [Google Scholar]
- 8.Zhong JM, Jasinskas A, Barbour AG. Antibiotic Treatment of the Tick Vector Amblyomma americanum Reduced Reproductive Fitness. Plos One. 2007;2 doi: 10.1371/journal.pone.0000405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lo N, Beninati T, Sassera D, Bouman EAP, Santagati S, et al. Widespread distribution and high prevalence of an alpha-proteobacterial symbiont in the tick Ixodes ricinus. Environmental Microbiology. 2006;8:1280–1287. doi: 10.1111/j.1462-2920.2006.01024.x. [DOI] [PubMed] [Google Scholar]
- 10.Epis S, Sassera D, Beninati T, Lo N, Beati L, et al. Midichloria mitochondrii is widespread in hard ticks (Ixodidae) and resides in the mitochondria of phylogenetically diverse species. Parasitology. 2008;135:485–494. doi: 10.1017/S0031182007004052. [DOI] [PubMed] [Google Scholar]
- 11.Sassera D, Beninati T, Bandi C, Bouman EA, Sacchi L, et al. ‘Candidatus Midichloria mitochondrii’, an endosymbiont of the tick Ixodes ricinus with a unique intramitochondrial lifestyle. Int J Syst Evol Microbiol. 2006;56:2535–2540. doi: 10.1099/ijs.0.64386-0. [DOI] [PubMed] [Google Scholar]
- 12.Sacchi L, Bighardi E, Corona S, Beninati T, Lo N, et al. A symbiont of the tick Ixodes ricinus invades and consumes mitochondria in a mode similar to that of the parasitic bacterium Bdellovibrio bacteriovorus. Tissue & Cell. 2004;36:43–53. doi: 10.1016/j.tice.2003.08.004. [DOI] [PubMed] [Google Scholar]
- 13.Sassera D, Lo N, Epis S, D'Auria G, Montagna M, et al. Phylogenomic evidence for the presence of a flagellum and cbb3 oxidase in the free-living mitochondrial ancestor. Molecular Biology and Evolution. 2011. [DOI] [PubMed]
- 14.Mediannikov O, Sekeyova Z, Birg M-L, Raoult D. A Novel Obligate Intracellular Gamma-Proteobacterium Associated with Ixodid Ticks Diplorickettsia massiliensis, Gen. Nov., Sp. Nov. PLoS ONE. 2010;5:e11478. doi: 10.1371/journal.pone.0011478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Andreotti R, de Leon AAP, Dowd SE, Guerrero FD, Bendele KG, et al. Assessment of bacterial diversity in the cattle tick Rhipicephalus (Boophilus) microplus through tag-encoded pyrosequencing. Bmc Microbiology. 2011;11 doi: 10.1186/1471-2180-11-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Carpi G, Cagnacci F, Wittekindt NE, Zhao F, Qi J, et al. Metagenomic Profile of the Bacterial Communities Associated with Ixodes ricinus Ticks. PLoS ONE. 2011;6:e25604. doi: 10.1371/journal.pone.0025604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Telfer S, Lambin X, Birtles R, Beldomenico P, Burthe S, et al. Species Interactions in a Parasite Community Drive Infection Risk in a Wildlife Population. Science. 2010;330:243–246. doi: 10.1126/science.1190333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dib L, Bitam I, Tahri M, Bensouilah M, De Meeus T. Competitive exclusion between piroplasmosis and anaplasmosis agents within cattle. PLoS Pathog. 2008;4:e7. doi: 10.1371/journal.ppat.0040007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kirkness EF, Haas BJ, Sun WL, Braig HR, Perotti MA, et al. Genome sequences of the human body louse and its primary endosymbiont provide insights into the permanent parasitic lifestyle. Proceedings of the National Academy of Sciences of the United States of America. 2010;107:12168–12173. doi: 10.1073/pnas.1003379107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hosokawa T, Koga R, Kikuchi Y, Meng X-Y, Fukatsu T. Wolbachia as a bacteriocyte-associated nutritional mutualist. Proceedings of the National Academy of Sciences. 2010;107:769–774. doi: 10.1073/pnas.0911476107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cirimotich CM, Dong Y, Clayton AM, Sandiford SL, Souza-Neto JA, et al. Natural Microbe-Mediated Refractoriness to Plasmodium Infection in Anopheles gambiae. Science. 2011;332:855–858. doi: 10.1126/science.1201618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mousson L, Martin E, Zouache K, Madec Y, Mavingui P, et al. Wolbachia modulates Chikungunya replication in Aedes albopictus. Molecular Ecology. 2010;19:1953–1964. doi: 10.1111/j.1365-294X.2010.04606.x. [DOI] [PubMed] [Google Scholar]
- 23.Bian GW, Xu Y, Lu P, Xie Y, Xi ZY. The endosymbiotic bacterium Wolbachia induces resistance to dengue virus in Aedes aegypti. PLoS Pathogens. 2010. e1000833. [DOI] [PMC free article] [PubMed]
- 24.Hedges LM, Brownlie JC, O′Neill SL, Johnson KN. Wolbachia and Virus Protection in Insects. Science. 2008;322:702. doi: 10.1126/science.1162418. [DOI] [PubMed] [Google Scholar]
- 25.Werren JH. Biology of Wolbachia. Annual Review of Entomology. 1997;42:587–609. doi: 10.1146/annurev.ento.42.1.587. [DOI] [PubMed] [Google Scholar]
- 26.Raychoudhury R, Grillenberger BK, Gadau J, Bijlsma R, van de Zande L, et al. Phylogeography of Nasonia vitripennis (Hymenoptera) indicates a mitochondrial-Wolbachia sweep in North America. Heredity. 2010;104:318–326. doi: 10.1038/hdy.2009.160. [DOI] [PubMed] [Google Scholar]
- 27.Engelstädter J, Hurst GDD. The Impact of Male-Killing Bacteria on Host Evolutionary Processes. Genetics. 2007;175:245–254. doi: 10.1534/genetics.106.060921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Reis C, Cote M, Paul REL, Bonnet S. Questing Ticks in Suburban Forest Are Infected by at Least Six Tick-Borne Pathogens. Vector-Borne and Zoonotic Diseases. 2011;11 doi: 10.1089/vbz.2010.0103. [DOI] [PubMed] [Google Scholar]
- 29.Duron O, Bouchon D, Boutin S, Bellamy L, Zhou L, et al. The diversity of reproductive parasites among arthropods: Wolbachia do not walk alone. BMC Biol. 2008;6:27. doi: 10.1186/1741-7007-6-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cooley RA, Kohls GM. Egg laying of Ixodiphagus caucurtei Du Buysson in larval ticks. Science. 1919;68:656. doi: 10.1126/science.67.1748.656. [DOI] [PubMed] [Google Scholar]
- 31.Brumpt E. Parasitisme latent de l′Ixodiphagus caucurtei chez les larves gorgées et les nymphes à jeun de divers ixodines (Ixodes ricinus et Rhipicephalus sanguineus). Comptes Rendus de l′Académie des Sciences de Paris. 1930;191:1085–1087. [Google Scholar]
- 32.Baldo L, Bordenstein S, Wernegreen JJ, Werren JH. Widespread Recombination Throughout Wolbachia Genomes. Molecular Biology and Evolution. 2006;23:437–449. doi: 10.1093/molbev/msj049. [DOI] [PubMed] [Google Scholar]
- 33.Hartelt K, Oehme R, Frank H, Brockmann SO, Hassler D, et al. Pathogens and symbionts in ticks: prevalence of Anaplasma phagocytophilum (Ehrlichia sp.), Wolbachia sp., Rickettsia sp., and Babesia sp. in Southern Germany. Int J Med Microbiol. 2004;293(Suppl 37):86–92. doi: 10.1016/s1433-1128(04)80013-5. [DOI] [PubMed] [Google Scholar]
- 34.van Overbeek L, Gassner F, van der Plas CL, Kastelein P, Rocha UND, et al. Diversity of Ixodes ricinus tick-associated bacterial communities from different forests. Fems Microbiology Ecology. 2008;66:72–84. doi: 10.1111/j.1574-6941.2008.00468.x. [DOI] [PubMed] [Google Scholar]
- 35.Sarih MH, M′Ghirbi Y, Bouattour A, Gern L, Baranton G, et al. Detection and Identification of Ehrlichia spp. in Ticks Collected in Tunisia and Morocco. J Clin Microbiol. 2005;43:1127–1132. doi: 10.1128/JCM.43.3.1127-1132.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hu R, Hyland KE, Oliver JH., Jr A review on the use of Ixodiphagus wasps (Hymenoptera: Encyrtidae) as natural enemies for the control of ticks (Acari: Ixodidae). Systematic and Applied Acarology. 1998;3:1362–1971. [Google Scholar]
- 37.Wood HP. Notes on the life history of the tick parasite Hunterellus hookeri Howard. Journal of Economic Entomology. 1911;4:425–431. [Google Scholar]
- 38.Niebylski ML, Peacock MG, Fischer ER, Porcella SF, Schwan TG. Characterization of an endosymbiont infecting wood ticks, Dermacentor andersoni, as a member of the genus Francisella. Applied and Environmental Microbiology. 1997;63:3933–3940. doi: 10.1128/aem.63.10.3933-3940.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hu RJ, Hyland KE. Effects of the feeding process of Ixodes scapularis (Acari : Ixodidae) on embryonic development of its parasitoid, Ixodiphagus hookeri (Hymenoptera : Encyrtidae). Journal of Medical Entomology. 1998;35:1050–1053. doi: 10.1093/jmedent/35.6.1050. [DOI] [PubMed] [Google Scholar]
- 40.Collatz J, Selzer P, Fuhrmann A, Oehme RM, Mackenstedt U, et al. A hidden beneficial: biology of the tick-wasp Ixodiphagus hookeri in Germany. Journal of Applied Entomology. 2011;135:351–358. [Google Scholar]
- 41.Breeuwer JAJ, Werren JH. Cytoplasmic Incompatibility and Bacterial Density in Nasonia vitripennis. Genetics. 1993;135:565–574. doi: 10.1093/genetics/135.2.565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Brumpt ME. Utilisation des insectes auxiliaires – Entomophages dans la lutte contre les insectes pathogenes. La Presse Medicale. 1913;36:359–361. [Google Scholar]
- 43.Stafford KC, Denicola AJ, Kilpatrick HJ. Reduced abundance of Ixodes scapularis (Acari : Ixodidae) and the tick parasitoid Ixodiphagus hookeri (Hymenoptera : Encyrtidae) with reduction of white-tailed deer. Journal of Medical Entomology. 2003;40:642–652. doi: 10.1603/0022-2585-40.5.642. [DOI] [PubMed] [Google Scholar]
- 44.Haine ER. Symbiont-mediated protection. Proceedings of the Royal Society B-Biological Sciences. 2008;275:353–361. doi: 10.1098/rspb.2007.1211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Noda H, Munderloh UG, Kurtti TJ. Endosymbionts of ticks and their relationship to Wolbachia spp. and tick-borne pathogens of humans and animals. Applied and Environmental Microbiology. 1997;63:3926–3932. doi: 10.1128/aem.63.10.3926-3932.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Benson MJ, Gawronski JD, Eveleigh DE, Benson DR. Intracellular symbionts and other bacteria associated with deer ticks (Ixodes scapularis) from Nantucket and Wellfleet, Cape Cod, Massachusetts. Applied and Environmental Microbiology. 2004;70:616–620. doi: 10.1128/AEM.70.1.616-620.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Moreno CX, Moy F, Daniels TJ, Godfrey HP, Cabello FC. Molecular analysis of microbial communities identified in different developmental stages of Ixodes scapularis ticks from Westchester and Dutchess Counties, New York. Environmental Microbiology. 2006;8:761–772. doi: 10.1111/j.1462-2920.2005.00955.x. [DOI] [PubMed] [Google Scholar]
- 48.Hotopp JC, Clark ME, Oliveira DC, Foster JM, Fischer P, et al. Widespread lateral gene transfer from intracellular bacteria to multicellular eukaryotes. Science. 2007;317:1753–1756. doi: 10.1126/science.1142490. [DOI] [PubMed] [Google Scholar]
- 49.Ishmael N, Hotopp JC, Ioannidis P, Biber S, Sakamoto J, et al. Extensive genomic diversity of closely related Wolbachia strains. Microbiology. 2009;155:2211–2222. doi: 10.1099/mic.0.027581-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mather TN, Piesman J, Spielman A. Absence of spirochaetes (Borrelia burgdorferi) and piroplasms (Babesia microti) in deer ticks (Ixodes dammini) parasitized by chalcid wasps (Hunterellus hookeri). Medical and Veterinary Entomology. 1987;1:3–8. doi: 10.1111/j.1365-2915.1987.tb00317.x. [DOI] [PubMed] [Google Scholar]
- 51.Bonnet S, Jouglin M, Malandrin L, Becker C, Agoulon A, et al. Transstadial and transovarial persistence of Babesia divergens DNA in Ixodes ricinus ticks fed on infected blood in a new skin-feeding technique. Parasitology. 2007;134:197–207. doi: 10.1017/S0031182006001545. [DOI] [PubMed] [Google Scholar]
- 52.Heath ACG, Cane RP. A new species of Ixodiphagus (Hymenoptera: Chalcidoidea: Encyrtidae) parasitizing seabird ticks in New Zealand. New Zealand Journal of Zoology. 2010;37:147–155. [Google Scholar]
- 53.Eggleton P, Belshaw R. Insect Parasitoids: An Evolutionary Overview. Philosophical Transactions of the Royal Society of London Series B: Biological Sciences. 1992;337:1–20. [Google Scholar]
- 54.Quaraishi MS. Morphology of two chalcidoid parasites of ticks, Hunterellus hookeri Howard, 1908, and Ixodiphagus texanus Howard, 1907. The American Midland Naturalist. 1958;59:489. [Google Scholar]
- 55.Braig HR, Zhou W, Dobson SL, O′Neill SL. Cloning and Characterization of a Gene Encoding the Major Surface Protein of the Bacterial Endosymbiont Wolbachia pipientis. J Bacteriol. 1998;180:2373–2378. doi: 10.1128/jb.180.9.2373-2378.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Werren JH, Zhang W, Guo LR. Evolution and Phylogeny of Wolbachia - reproductive parasites of arthropods. Proceedings of the Royal Society of London Series B-Biological Sciences. 1995;261:55–63. doi: 10.1098/rspb.1995.0117. [DOI] [PubMed] [Google Scholar]
- 57.Scheffer SJ, Grissell EE. Tracing the geographical origin of Megastigmus transvaalensis (Hymenoptera : Torymidae): an African wasp feeding on a South American plant in North America. Molecular Ecology. 2003;12:415–421. doi: 10.1046/j.1365-294x.2003.01725.x. [DOI] [PubMed] [Google Scholar]
- 58.Casiraghi M, Bain O, Guerrero R, Martin C, Pocacqua C, et al. Mapping the presence of Wolbachia pipientis on the phylogeny of filarial nematodes: evidence for symbiont loss during evolution. International Journal for Parasitology. 2004;34:191–203. doi: 10.1016/j.ijpara.2003.10.004. [DOI] [PubMed] [Google Scholar]
- 59.Goujon M, McWilliam H, Li W, Valentin F, Squizzato S, et al. A new bioinformatics analysis tools framework at EMBL-EBI. Nucleic Acids Research. 2010;38:W695–W699. doi: 10.1093/nar/gkq313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Tamura K, Peterson D, Peterson N, Stecher G, Nei M, et al. MEGA5: Molecular Evolutionary Genetics Analysis using Maximum Likelihood, Evolutionary Distance, and Maximum Parsimony Methods. Molecular Biology and Evolution. 2011. [DOI] [PMC free article] [PubMed]
- 61.Tamura K, Nei M, Kumar S. Prospects for inferring very large phylogenies by using the neighbor-joining method. Proceedings of the National Academy of Sciences of the United States of America. 2004;101:11030–11035. doi: 10.1073/pnas.0404206101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Inokuma H, Raoult D, Brouqui P. Detection of Ehrlichia platys DNA in brown dog ticks (Rhipicephalus sanguineus) in Okinawa Island, Japan. Journal of Clinical Microbiology. 2000;38:4219–4221. doi: 10.1128/jcm.38.11.4219-4221.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]