Abstract
Kidneys can be divided into four components: glomeruli, tubules, interstitium and blood vessels. The renal glomerulus consists of a network of capillaries covered with epithelial cells called podocytes. The entire glomerular tuft is structurally supported by mesangial cells which are contractile in nature and resemble vascular smooth muscle cells. Mesangial cells are secretory, producing growth factors and matrix proteins which have a role in both normal glomerular development and in pathologic states. They have also been shown to take the role of macrophages. The importance of mesangial cell contraction to glomerular physiology remains debated. It is postulated that mesangial cell contraction can attenuate the glomerular filtration rate by decreasing the renal ultrafiltration coefficient through a decrease in capillary surface area and capillary permeability. The physiology of mesangial cell contraction has been studied primarily utilizing cultured cells. The physiological status of receptors and ion channels may be doubtful, however, given the phenotypic changes cells are known to acquire in culture conditions. The contractility of renal glomeruli has been less well studied. In this report, we review the available data regarding the contractility of mesangial cell and of renal glomeruli. Moreover, we suggest newer techniques that can be used with whole glomeruli, thereby improving upon the data collected using previous techniques and cultured cells.
Keywords: Renal glomerulus, Mesangial cells, Contractility, Glomerular filtration rate, Confocal microscopy
1. INTRODUCTION
Morphologically, kidneys can be divided into four components: glomeruli, tubules, interstitium and blood vessels. The glomeruli, which function as filtering units, are concentrated in the cortical region of the kidneys. There are as many as a million glomeruli in each of the kidneys [1]. Glomeruli are multicellular in nature, consisting primarily of a network of capillaries that originate from a wider afferent arteriole while leaving via a narrower efferent arteriole. These capillary loops are wrapped in visceral epithelium, also called podocytes, while parietal epithelium lines the inside of the Bowman’s capsule/urinary space. The entire glomerular tuft is supported by mesangial cells (MC) which resemble vascular smooth muscle cells in phenotype and responsiveness to different stimuli. The major function of glomeruli is to filter plasma and solutes into the tubules, while retaining macromolecules such as albumin [2]. The plasma ultrafiltrate movement, or the glomerular filtration rate (GFR), is dependent on the permeability of the capillary membrane and on the difference between the hydraulic (in glomerular capillaries, Pgc and Bowman’s space, Pbs) and oncotic pressure (in plasma entering the glomerulus and Bowman’s space) gradients. In addition to these factors, GFR is also influenced by the renal plasma flow (RPF) rate. Glomerular capillary pressure (Pgc) is determined by the systemic pressure and by the resistances at the afferent and efferent arterioles which interpose the glomerular capillaries. GFR and RPF are both regulated simultaneously by tubuloglomerular feedback and myogenic responsiveness (autoregulation). The former process involves flow-dependent changes in salt concentration sensed at the macula densa and results in the altered tone of the nearby afferent arteriole [3]. The myogenic response involves direct constriction and relaxation of the resistance afferent arterioles [4]. This process, although not completely understood, involves the constriction of afferent arterioles in response to a rise in systemic blood pressure (BP), thus reducing the Pgc and GFR, while a situation with reduced BP leads to dilation of the afferent arteriole and thus a resultant increase in Pgc and GFR. Conversely, constriction of the efferent arteriole leads to increased Pgc and GFR while dilation results in the opposite effect.
Years of research have still not been sufficient to definitively prove a (patho)physiologic role for the contractility of MCs and glomeruli. The reasons for this include the multicellular nature of the glomerulus, the difficulty in specifically targeting a particular cell type in the glomerular structure [5,6] and the fact that most previous studies examining contractility have been done using cultured cells. In this communication, we will review the literature pertaining to glomerular and MC contraction and present some preliminary findings collected in our lab using intact native glomerular preparations which demonstrate the phenomenon of glomerular contractility and the contribution of [Ca2+]i to this process.
2. GLOMERULAR MCs AND FILTRATION
MCs are known to be secretory and to produce growth factors for normal cell turnover. They are targets of numerous inflammatory mediators, produce matrix proteins for structural support of capillaries, and sometimes take the role of macrophages [7]. It is also believed that MCs play a role in glomerular contraction, modulating the ultrafiltration coefficient (Kf) and filtration surface area, thus helping to regulate GFR [8,9]. Effective filtration surface area is regulated by constriction of capillary loops that results in reduction of capillary diameter or by segmental flow obstruction through glomerular microcirculation [10]. MC contraction, as can be affected by release of vasoactive hormones, may lead to a decreased capillary surface area and hence attenuation of Kf with a resultant decrease in GFR [11,12]. As there is no smooth muscle lining in the capillaries, MCs which connect to capillary loops and glomerular basement membrane are believed to be the primary regulators of capillary diameter and to enable overall glomerular contraction [13]. Conversely, MC stretch and distension may contribute to pathophysiology, provoking the release of cytokines, particularly that of transforming growth factor-beta. This results in the accumulation of extracellular matrix and leads to glomerulosclerosis and renal insufficiency [14,15]. Reduced glomerular contractility, as has been seen in early diabetes, may also lead to significant glomerular enlargement and hyperfiltration due to altered protein kinase C function, dysregulation of the polyol pathway [16,17] and impaired Ca2+ signalling, and all of these likely contribute to loss of MC contractile responsiveness [18].
3. STUDIES ON GLOMERULAR CONTRACTION
Studies of glomeruli contraction have been limited, examining ex vivo or in situ glomerular contraction, glomerular volume changes, changes in the glomerular filtration surface area and ultrafiltration coefficient as well as capillary blood flow. However, a wide variety of techniques are used and data are conflicting. Micropuncture studies are not ideal for assessing glomerular contraction as the agonists used can also constrict the arterioles. In fact, it is difficult to remove the effects of arterioles on RPF in studying only MC contraction. There have been direct morphological studies (e.g., time-lapse cinematography) reporting the effect of vasoconstrictors on the glomerular capillaries [19]. Bernic [20] and Hornych et al. [21] reported the contractile behaviour of isolated human glomeruli and glomerular capillaries respectively in response to angiotensin-II (Ang-II). When fluorescein-tagged erythrocytes were used in rats to determine glomerular capillary blood flow, Ang-II reduced the fluid flow and velocity without any change in the capillary diameter [22] while light and scanning electron microscopy [23] have also shown a similar inhibitory effect of Ang-II on glomerular volume and capillary diameter respectively. Some research groups in Europe have recently successfully used isolated intact glomeruli to study the effects on contractility of different nephrotoxic agents such as gentamicin, cisplatin, cadmium and cyclosporine [9,24,25]. This approach helps to quantitatively analyze the extent of glomerular contraction by assessing the change in glomerular cross-sectional area before and after exposing the glomeruli to the toxins. The effect of various stimuli has also been successfully assessed based on changes in GFR measured by [3H]inulin. Here, the quantity of [3H]inulin retained by the glomeruli is reflective of their intracapillary volume (glomerular inulin space) [26,27]. With such a technique, ATP [28] has been reported to have a dose-dependent contractile effect on glomeruli.
There is still much controversy regarding the putative contractile nature of the glomerulus. While some have been able to show contractility in response to agonists such as Ang-II [9,24,25] others have not [22,29]. If at all a definitive conclusion is drawn regarding this function of glomeruli, another concern is the identification of which resident glomerular cell type is responsible for overall contractility. Some of the studies reporting isotonic contractions in response to Ang-II in isolated glomeruli stand inconclusive as they did not show the influence of MC on these contractions rather other mechanisms were cited to be involved for such an effect [13,30]. Many recent studies as well fail to show the impact of MC on contractions induced with different agonists in isolated glomeruli [9,24,25]. Apart from MCs, podocytes are also one of the main components of the glomerular filtration barrier and are considered to be the most differentiated cell type within the glomerulus [31]. Podocytes are not only involved mechanically in stabilizing the glomerular basement membrane, but it has been reported that the contractile foot of podocytes, known to be responsive to endogenous vasoactive mediators such as Ang-II, is also involved in regulation of GFR through its effects on the filtration surface area and Kf [31,32].
4. IN VITRO STUDIES ON MC CONTRACTION
It has been almost three decades since the first in vitro report of MC contraction [33–35]. Since then, several agonists have been reported to constrict glomerular mesangium, including Ang-II, arginine vasopressin, endothelin-1, bradykinin, histamine, adenosine, serotonin and thrombin [6,12,36]. A number of ion channels have also been identified as being necessary for MC contraction, including Ca2+-activated K+ channels and store operated Ca2+ channels [6,12,36,37]. However, the physiological status of receptors and ion channels may be doubtful in cultured MC [38] as it is in cultured smooth muscle cells [39,40]. Ouardani et al. [5] have documented the phenotypic modulations in MC as they were cultured and passaged under various conditions. In another study, the medium used for growing MCs contained 30 mM glucose, thus mimicking a diabetic environment rather than a normal physiological milieu [6], and likely resulting in altered expression and function of various signalling molecules. The validity of extrapolating results obtained with cultured cells is thus questionable.
The ability of MC to contract was conjectured following electron microscopy studies of native MC which identified bundles of microfilaments similar to those seen in vascular smooth muscle cells [41,42]. Subsequently, different contractile proteins have been identified in cultured MC including myosin, tropomyosin, actin, α-actinin [43] and dystropin [5]. It was later reported, however, that smooth muscle actin, present in high amounts in cultured MC, is absent in native (i.e., non-cultured) cells [12,44]. Likewise, Johnson et al.[45] failed to find filamentous actin and desmin in glomerular cells in vivo. The myosin isoform found in MC is also different from that found in smooth muscle cells [46,47]. Furthermore, these contractile filaments in MC have been shown to change phenotypically under different culture conditions [48,49]. In response to many of the initial studies done on cultured cells, Kriz et al. [50] undertook a biomechanical interpretation of the structure of glomerular mesangium and suggested that the mesangium has more of a static than a dynamic function based on the geometric composition of its contractile machinery. The authors also suggested that if MCs were truly contractile, the overall impact of their contraction on glomerular capillary diameter and filtration area would be minimal.
5. STRATERGIES FOR THE FUTURE
Despite decades of research on glomeruli and their constituent cells and structures, many questions still remain, some of them very fundamental in nature. This is in part because of the complicated multicellular structure of the glomerulus, which complicates the designing of conclusive and decisive experiments. Despite the many reductionist morphological, microscopical, glomerular blood flow-based and culture-based studies done on renal glomeruli, there is still a need to study intact kidney tissues to look at the cellular interactions at the organ level. MC and other glomerular cells might be observed to contract in culture, where they possibly lack their typical functional and morphological features, but it stills needs to be determined whether they can contract in vivo or influence the overall renal capillary surface area and thus impact GFR. Approaches otherwise used for studies at the cellular or even molecular levels must be adapted for use at these macroscopic levels. For example, Pavenstadt[51] adapted a patch clamp method in which they sealed their electrode onto the vascular pole of an isolated glomerulus and measured the membrane voltage and conductance of the podocytes: although the procedure was quite difficult to carry out (the author reported a success rate of only 3%), it surely gives impetus to more in situ studies on intact glomerular structures.
Recently, in a method which we have adapted and modified from our work with lung tissue cross-sections, we have begun to apply confocal fluorescence microscopy to the study of isolated rat renal glomerular contractility. The merit of using isolated intact glomeruli lies in the preservation of their anatomy and cell-cell contacts while being free of vascular, nervous and humoral connections from other regions of the kidney. For the purpose of obtaining intact glomeruli, a graded sieving method[24] was modified and used to produce a relatively pure glomerular suspension. The acquired glomeruli were then resuspended in Hanks’ balanced salt solution (HBSS), loaded with the fluorescent dye fluo-4, placed on the microscope platform and perfused with HBSS. Different contractile agonists such as Ang-II and endothelin-1 were administered. Figure 1 shows a typical response of an isolated glomerulus to endothelin-1 (100 nM). Administration of the agonist evoked a generalized increase in fluorescence as well as distinct bright spots in many parts of the glomerulus, signifying changes in cytosolic [Ca2+]i concentration (FIG. 1). An intensity profile of a slice through the glomerulus shows two distinct peaks (FIG. 1D) which were absent in the control (FIG. 1C). Figure 2 shows a typical tracing for the change in [Ca2+]i in glomerular cells represented in Fig. 1.
FIGURE 1.
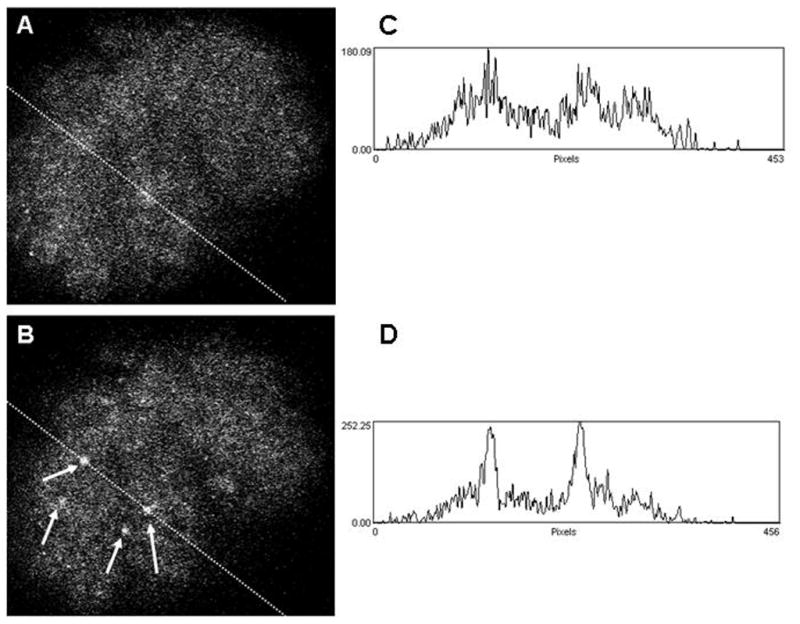
Confocal fluorimetric image of an isolated rat glomerulus loaded with fluo-4 before [A] and after [B] administering endothelin-1 (100 nM). Arrows in B indicate cells which have responded to the applied agonist. Corresponding plots of pixel intensities along a slice through the glomerulus are given in C and D.
FIGURE 2.
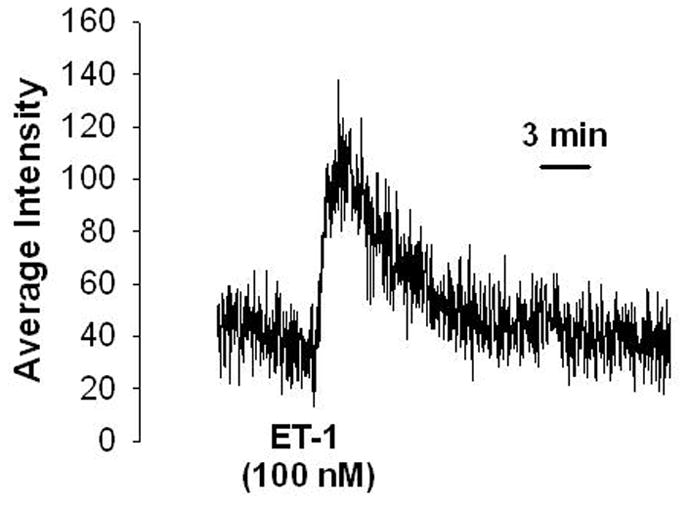
Typical tracing showing change in [Ca2+]i concentrations as increase in average fluorescence intensity in response to endothelin-1 (ET-1) in the glomerular cells. Fluorescence intensities were saved and plotted againsttime. An increase in fluorescence was interpreted as an increase in [Ca2+]i.
Unfortunately, there are some limitations of this method: the processing involved for isolation of glomeruli may lead to hypoxia, hypothermia and ischemia, and definitive identification of which resident glomerular cell type is showing these calcium changes is challenging. Further work needs to be done to address the latter. In attempts to overcome the former limitation, as well as to enable studies of glomerular contractility in situ, we have recently developed a microscopical method to study intact cortical slices from mouse kidneys (preliminary findings have already been presented) [52,53]. This extends the work of earlier reports of the use of confocal laser scanning microscopy to analyse glomerular hemodynamics in vivo in rats [54]. This method would enable us to examine the glomerular apparatus in situ, with all the cellular structures and intercellular communications intact.
6. CONCLUSION
While a great deal of work has been done in an attempt to understand the role and importance of MC and glomerular contraction in renal physiology and pathophysiology, much of the knowledge gained thus far has been from in vitro studies of cultured cells. These are limited by the phenotypic changes known to take place. In comparison, studies using ex vivo and in vivo models are more challenging. Fortunately, however, novel methods are emerging to overcome these concerns. In addition to confocal fluorescence microscopy, we suggest using other advanced techniques such as two-photon excitation fluorescence imaging which can provide detailed information on glomerular structure and function [55]. These applications may be applied in conjunction with genetically modified animals to assist in determining the physiological significance of many of the receptors and ion channels believed to be of importance in MC contraction. In this way, many of the unsolved questions may be answered while older concepts can simultaneously be reevaluated.
Acknowledgments
MN Ghayur, LJ Janssen and JC Krepinsky are supported by the Canadian Institutes of Health Research (CIHR).
References
- 1.Ballinger A, Patchett S. Clinical Medicine. Edinburgh: Saunders; 2004. pp. 309–363. [Google Scholar]
- 2.Tryggvason K, Wartiovaara J. How does the kidney filter plasma. Physiology. 2004;20:96–101. doi: 10.1152/physiol.00045.2004. [DOI] [PubMed] [Google Scholar]
- 3.Castrop H, Huang Y, Hashimoto S, Mizel D, Hansen P, Theilig F, Bachmann S, Deng C, Briggs J, Schnermann J. Impairment of tubuloglomerular feedback regulation of GFR in ecto-5′-nucleotidase/CD73-deficient mice. J Clin Invest. 2004;114:634–642. doi: 10.1172/JCI21851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Aukland K. Myogenic mechanisms in the kidney. J Hypertens. 1989;7:S71–S76. [PubMed] [Google Scholar]
- 5.Ouardani M, Travo P, Bastie MJ, Mornet D, Neff S, Leung-Tack J. Loss of differences in mesangial cell phenotype between diabetic and normal rats: role of culture passages. Biol Cell. 1996;86:127–133. doi: 10.1016/0248-4900(96)84775-7. [DOI] [PubMed] [Google Scholar]
- 6.Ma R, Pluznick J, Sansom SC. Ion channels in mesangial cells: function, malfunction, or fiction. Physiology. 2004;20:102–111. doi: 10.1152/physiol.00050.2004. [DOI] [PubMed] [Google Scholar]
- 7.Floege J, Eng E, Young BA, Johnson RJ. Factors involved in the regulation of mesangial cell proliferation in vitro and in vivo. Kidney Int. 1993;39:S47–S54. [PubMed] [Google Scholar]
- 8.Mene P, Simonson MS, Dunn MJ. Physiology of the mesangial cell. Physiol Rev. 1989;69:1347–1424. doi: 10.1152/physrev.1989.69.4.1347. [DOI] [PubMed] [Google Scholar]
- 9.Rodriguez-Barbero A, L’Azou B, Cambar J, Lopez-Novoa JM. Potential use of isolated glomeruli and cultured mesangial cells as in vitro models to assess nephrotoxicity. Cell Biol Toxicol. 2000;16:145–153. doi: 10.1023/a:1007683320660. [DOI] [PubMed] [Google Scholar]
- 10.Brenner BM, Dworkin LD, Ichikawa I. Glomerular ultrafiltration. In: Brenner BM, Rector FC, editors. The Kidney. Philadelphia: Saunders; 1986. pp. 124–144. [Google Scholar]
- 11.Pfeilschifter J. Cross-talk between transmembrane signalling systems: a prerequisite for the delicate regulation of glomerular hemodynamics by mesangial cells. Eur J Clin Invest. 1989;19:347–361. doi: 10.1111/j.1365-2362.1989.tb00241.x. [DOI] [PubMed] [Google Scholar]
- 12.Henderson RM, Barber RD. Ion channels in renal glomerular mesangial cells. J Membr Biol. 1998;163:1–8. doi: 10.1007/s002329900364. [DOI] [PubMed] [Google Scholar]
- 13.Scharschmidt LA, Douglas JG, Dunn MJ. Angiotensin II and eicosanoids in the control of glomerular size in the rat and human. Am J Physiol. 1986;250:F348–F56. doi: 10.1152/ajprenal.1986.250.2.F348. [DOI] [PubMed] [Google Scholar]
- 14.Cortes P, Riser BL, Zhao X, Narins RG. Glomerular volume expansion and mesangial cell mechanical strain: mediators of glomerular pressure injury. Kidney Int. 1994;45:S11–S16. [PubMed] [Google Scholar]
- 15.Riser BL, Cortes P, Heilig C, Grondin J, Ladson-Wofford S, Patterson D, Narins RG. Cyclic stretching force selectively up-regulates transforming growth factor-beta isoforms in cultured rat mesangial cells. Am J Pathol. 1996;148:1915–1923. [PMC free article] [PubMed] [Google Scholar]
- 16.Derylo B, Babazono T, Glogowski E, Kapor-Drezgic J, Hohman T, Whiteside C. High glucose-induced mesangial cell altered contractility: role of the polyol pathway. Diabetologia. 1998;41:507–515. doi: 10.1007/s001250050939. [DOI] [PubMed] [Google Scholar]
- 17.Kapor-Drezgic J, Zhou X, Babazono T, Dlugosz JA, Hohman T, Whiteside C. Effect of high glucose on mesangial cell protein kinase C-delta and -epsilon is polyol pathway-dependent. J Am Soc Nephrol. 1999;10:1193–1203. doi: 10.1681/ASN.V1061193. [DOI] [PubMed] [Google Scholar]
- 18.Frecker H, Munk S, Wang H, Whiteside C. Mesangial cell-reduced Ca2+ signaling in high glucose is due to inactivation of phospholipase C-beta3 by protein kinase C. Am J Physiol. 2005;289:F1078–F1087. doi: 10.1152/ajprenal.00434.2004. [DOI] [PubMed] [Google Scholar]
- 19.Elias H, Hossmann A, Barth IB, Solmar A. Blood flow in the renal glomerulus. J Urol. 1960;83:790–798. doi: 10.1016/S0022-5347(17)65799-9. [DOI] [PubMed] [Google Scholar]
- 20.Bernik MB. Contractile activity of human glomeruli in culture. Nephron. 1969;6:1–10. doi: 10.1159/000179708. [DOI] [PubMed] [Google Scholar]
- 21.Hornych H, Beaufils M, Richet G. The effect of exogenous angiotensin on superficial and deep glomeruli in the rat kidney. Kidney Int. 1972;2:336–343. doi: 10.1038/ki.1972.117. [DOI] [PubMed] [Google Scholar]
- 22.Zimmerhackl B, Parekh N, Kucherer H, Steinhausen M. Influence of systemically applied angiotensin II on the microcirculation of glomerular capillaries in the rat. Kidney Int. 1985;27:17–24. doi: 10.1038/ki.1985.4. [DOI] [PubMed] [Google Scholar]
- 23.Haley DP, Sarrafian M, Bulger RE, Dobyan DC, Eknoyan G. Structural and functional correlates of effects of angiotensin-induced changes in rat glomerulus. Am J Physiol. 1987;253:F111–F119. doi: 10.1152/ajprenal.1987.253.1.F111. [DOI] [PubMed] [Google Scholar]
- 24.Potier M, Wolf A, Cambar J. Comparative study of cyclosporin A, cyclosporin G, and the novel cyclosporin derivative IMM 125 in isolated glomeruli and cultured rat mesangial cells: a morphometric analysis. Nephrol Dial Transplant. 1998;13:1406–1411. doi: 10.1093/ndt/13.6.1406. [DOI] [PubMed] [Google Scholar]
- 25.L’Azou B, Medina J, Frieauff W, Cordier A, Cambar J, Wolf A. In vitro models to study mechanisms involved in cyclosporine A-mediated glomerular contraction. Arch Toxicol. 1999;73:337–345. doi: 10.1007/s002040050627. [DOI] [PubMed] [Google Scholar]
- 26.Fujiwara Y, Kitamura E, Ueda N, Fukunaga M, Orita Y, Kamada T. Mechanism of action of angiotensin II on isolated rat glomeruli. Kidney Int. 1989;36:985–991. doi: 10.1038/ki.1989.291. [DOI] [PubMed] [Google Scholar]
- 27.Jankowski M, Szczepanska-Konkel M, Kalinowski L, Angielski S. Cyclic GMP-dependent relaxation of isolated rat renal glomeruli induced by extracellular ATP. J Physiol. 2001;530:123–130. doi: 10.1111/j.1469-7793.2001.0123m.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jankowski M, Szczepanska-Konkel K, Kalinowski L, Angielski S. Involvement of Rho-kinase in P2Y-receptor-mediated contraction of renal glomeruli. Biochem Biophys Res Commun. 2003;302:855–859. doi: 10.1016/s0006-291x(03)00272-9. [DOI] [PubMed] [Google Scholar]
- 29.Steinhausen M, Endlich K, Wiegman DL. Glomerular blood flow. Kidney Int. 1990;38:769–784. doi: 10.1038/ki.1990.271. [DOI] [PubMed] [Google Scholar]
- 30.Savin VJ. In vitro effects of angiotensin II on glomerular function. Am J Physiol. 1986;251:F627–F634. doi: 10.1152/ajprenal.1986.251.4.F627. [DOI] [PubMed] [Google Scholar]
- 31.Gloy J, Henger A, Fischer KG, Nitschke R, Mundel P, Bleich M, Schollmeyer P, Greger R, Pavenstadt H. Angiotensin II depolarizes podocytes in the intact glomerulus of the Rat. J Clin Invest. 1997;99:2772–2781. doi: 10.1172/JCI119467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kriz W, Elger M, Nagata M, Kretzler M, Uiker S, Koeppen-Hageman I, Tenschert S, Lemley KV. The role of podocytes in the development of glomerular sclerosis. Kidney Int. 1994;45:S64–S72. [PubMed] [Google Scholar]
- 33.Ausiello DA, Kreisberg JI, Roy C, Karnovsky MJ. Contraction of cultured rat glomerular cells of apparent mesangial origin after stimulation with angiotensin II and arginine vasopressin. J Clin Invest. 1980;65:754–760. doi: 10.1172/JCI109723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Foidart J, Sraer J, Delarue F, Mahieu P, Ardaillou R. Evidence for mesangial glomerular receptors for angiotensin II linked to mesangial cell contractility. FEBS Lett. 1980;121:333–339. doi: 10.1016/0014-5793(80)80375-9. [DOI] [PubMed] [Google Scholar]
- 35.Mahieu PR, Foidart JB, Dubois CH, Dechenne CA, Deheneffe J. Tissue culture of normal rat glomeruli: contractile activity of the cultured mesangial cells. Invest Cell Pathol. 1980;3:121–128. [PubMed] [Google Scholar]
- 36.Stockand JD, Sansom SC. Glomerular mesangial cells: electrophysiology and regulation of contraction. Physiol Rev. 1998;78:723–744. doi: 10.1152/physrev.1998.78.3.723. [DOI] [PubMed] [Google Scholar]
- 37.Kim S, Iwao H. Molecular and cellular mechanisms of angiotensin II-mediated cardiovascular and renal diseases. Pharmacol Rev. 2000;52:11–34. [PubMed] [Google Scholar]
- 38.Floege J, Radeke HR, Johnson RJ. Glomerular cells in vitro versus the glomerulus in vivo. Kidney Int. 1994;45:360–368. doi: 10.1038/ki.1994.46. [DOI] [PubMed] [Google Scholar]
- 39.Chamley-Campbell J, Campbell GR, Ross R. The smooth muscle cell in culture. Physiol Rev. 1979;59:1–61. doi: 10.1152/physrev.1979.59.1.1. [DOI] [PubMed] [Google Scholar]
- 40.Chamley-Campbell JH, Campbell GR, Ross R. Phenotype-dependent response of cultured aortic smooth muscle to serum mitogens. J Cell Biol. 1981;89:379–383. doi: 10.1083/jcb.89.2.379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Latta H, Maunsbach AB, Madden SC. The centrolobular region of the renal glomerulus studied by electron microscopy. J Ultrastruct Res. 1960;4:455–472. doi: 10.1016/s0022-5320(60)80033-0. [DOI] [PubMed] [Google Scholar]
- 42.Andrews PM, Coffey AK. Cytoplasmic contractile elements in glomerular cells. Fed Proc. 1983;42:3046–3052. [PubMed] [Google Scholar]
- 43.Drenckhahn D, Franke RP. Ultrastructural organization of contractile and cytoskeletal proteins in glomerular podocytes of chicken, rat, and man. Lab Invest. 1988;59:673–682. [PubMed] [Google Scholar]
- 44.Elger M, Drenckhahn D, Nobiling R, Mundel P, Kriz W. Cultured rat mesangial cells contain smooth muscle alpha-actin not found in vivo. Am J Pathol. 1993;142:497–509. [PMC free article] [PubMed] [Google Scholar]
- 45.Johnson RJ, Floege J, Yoshimura A, Iida H, Couser WG, Alpers CE. The activated mesangial cell: A glomerular “myofibroblast”? J Am Soc Nephrol. 1992;2:S190–S197. doi: 10.1681/ASN.V210s190. [DOI] [PubMed] [Google Scholar]
- 46.Ishino T, Kobayashi R, Wakui H, Fukushima Y, Nakamoto Y, Miura AB. Biochemical characterization of contractile proteins of rat cultured mesangial cells. Kidney Int. 1991;39:1118–1124. doi: 10.1038/ki.1991.142. [DOI] [PubMed] [Google Scholar]
- 47.Nakai K, Ito C, Yumura W, Horita S, Nihei H, Sugino N, Nagai R. Difference of myosin heavy chain expression between mesangial cells and vascular smooth muscles. Nippon Jinzo Gakkai Shi. 1995;37:428–435. [PubMed] [Google Scholar]
- 48.Kreisberg JI, Hassid A. Functional properties of glomerular cells in culture. Miner Electrolyte Metab. 1986;12:25–31. [PubMed] [Google Scholar]
- 49.Hiraoka-Yoshimoto M, Higashida K, Takeda M, Kawamoto S, Ichikawa I, Hoover RL. Characterization of myosin heavy and light chains in cultured mesangial cells. Kidney Int. 1991;40:1013–1019. doi: 10.1038/ki.1991.309. [DOI] [PubMed] [Google Scholar]
- 50.Kriz W, Elger M, Lemley K, Sakai T. Structure of the glomerular mesangium: a biomechanical interpretation. Kidney Int. 1990;30:S2–S9. [PubMed] [Google Scholar]
- 51.Pavenstadt H. Roles of the podocyte in glomerular function. Am J Physiol. 2000;278:F173–F179. doi: 10.1152/ajprenal.2000.278.2.F173. [DOI] [PubMed] [Google Scholar]
- 52.Ghayur MN, Krepinsky JC, Janssen LJ. Real-time imaging of Ca2+-handling in glomeruli using murine whole kidney slices. Abst. Prevention in Renal Disease: 5th Annual Conference; 15–16th Sep; Toronto, Canada. 2006. p. 10. [Google Scholar]
- 53.Ghayur MN, Janssen LJ, Krepinsky JC. Studying intact renal glomeruli for Ca2+ handling by using a confocal microscope. Abst. 14th Annual North East Smooth Muscle Society Meeting; 19–20th Oct; Boston, USA. 2006. p. 22. [Google Scholar]
- 54.Li B, Yao J, Kawamura K, Oyanagi-Tanaka Y, Hoshiyama M, Morioka T, Gejyo F, Uchiyama M, Oite T. Real-time observation of glomerular hemodynamic changes in diabetic rats: effects of insulin and ARB. Kidney Int. 2004;66:1939–1948. doi: 10.1111/j.1523-1755.2004.00979.x. [DOI] [PubMed] [Google Scholar]
- 55.Helmchen F, Denk W. Deep tissue two-photon microscopy. Nat Methods. 2005;2:932–940. doi: 10.1038/nmeth818. [DOI] [PubMed] [Google Scholar]


