Abstract
Rationale
Enhanced sensitivity to the euphoric and locomotor-activating effects of psychostimulants may influence an individual's predisposition to drug abuse and addiction. While drug-induced behaviors are mediated by the actions of several neurotransmitter systems, past research revealed that the corticotropin-releasing factor (CRF) system is important in driving the acute locomotor response to psychostimulants.
Objectives
We previously reported that genetic deletion of the CRF type-2 receptor (CRF-R2), but not the CRF type-1 receptor (CRF-R1) dampened the acute locomotor stimulant response to methamphetamine (1 mg/kg). These results contrasted with previous studies implicating CRF-R1 in the locomotor effects of psychostimulants. Since the majority of previous studies focused on cocaine, rather than methamphetamine, we set out to test the hypothesis that these drugs differentially engage CRF-R1 and CRF-R2.
Methods
We expanded our earlier findings by first replicating our previous experiments at a higher dose of methamphetamine (2 mg/kg), and by assessing the effects of the CRF-R1-selective antagonist CP-376,395 (10 mg/kg) on methamphetamine-induced locomotor activity. Next, we used both genetic and pharmacological tools to examine the specific components of the CRF system underlying the acute locomotor response to cocaine (5–10 mg/kg). Results While genetic deletion of CRF-R2 dampened the locomotor response to methamphetamine (but not cocaine), genetic deletion and pharmacological blockade of CRF-R1 dampened the locomotor response to cocaine (but not methamphetamine).
Conclusions
These findings highlight the differential involvement of CRF receptors in acute sensitivity to two different stimulant drugs of abuse, providing an intriguing basis for the development of more targeted therapeutics for psychostimulant addiction.
Keywords: Corticotropin, Urocortin, CRF, Methamphetamine, Cocaine, Psychostimulant, Behavior, Locomotor activity, Stress, Addiction
Introduction
Addiction to psychostimulant drugs of abuse is maintained by repeated cycles of attempted abstinence followed by relapse, and stress-related neuropeptide systems are hypothesized to play a key role in precipitating relapse to compulsive drug-seeking (Boutrel 2008; Koob 2008; Shalev et al. 2010). In addition, susceptibility to psychostimulant addiction may depend on variations in neurotransmitter receptor signaling that determine acute sensitivity to drug effects. Importantly, psychostimulant exposure activates several stress-related neuropeptide systems that are involved in the acute drug-induced behavioral response, including the corticotropin-releasing factor (CRF) system (Sarnyai 1998).
The CRF system has been implicated in sensitivity to locomotor effects of cocaine and amphetamines (Cador et al. 1993a; Cole et al. 1990; Sarnyai et al. 1992), as well as in stress-induced reinstatement of operant cocaine self-administration (Erb et al. 2001; Wang et al. 2007), demonstrating its involvement not only in the initial behavioral response to psychostimulants, but also in the neuroadaptations that potentiate drug-seeking via heightened susceptibility to stress-induced relapse. Furthermore, the Gallagher and Bonci groups have demonstrated the involvement of specific CRF system components in electrophysiological phenomena observed following exposure to psychostimulants (Hahn et al. 2009; Krishnan et al. 2010; Liu et al. 2005; Orozco-Cabal et al. 2008; Ungless et al. 2003). Together, these prior studies suggest a common involvement of the CRF system in both the acute and long-term neural changes that result from psychostimulant use. In order to clarify the mechanisms by which acute psychostimulant exposure co-opts stress-related neuropeptidergic circuitry, we examined the specific components of the CRF system underlying the initial behavioral response to two widely abused psychostimulants: methamphetamine (MA) and cocaine (COC).
Comprised of two receptor subtypes (CRF-R1, CRFR2), four peptide ligands (CRF and the urocortins, Ucn1, Ucn2, and Ucn3), and a binding protein (CRF-BP), the CRF system initiates the neuroendocrine response to stress via the hypothalamic–pituitary–adrenal (HPA) axis, and coordinates diverse behaviors via actions on extra-HPA neural substrates. The HPA-axis is activated specifically by release of CRF from the hypothalamus onto CRF-R1 in the pituitary. This process results in secretion of adrenocortico-tropic hormone from the pituitary gland, and release of glucocorticoids from the adrenal glands. Indeed, while the effects of the CRF system on some addiction-relevant behaviors are dependent on HPA-mediated glucocorticoid release (DeVries et al. 1998; Graf et al. 2011), CRF and the urocortin peptides also contribute to psychostimulant-induced neural activity and behavior through their effects on extra-HPA loci (Krishnan et al. 2010; Liu et al. 2005; Orozco-Cabal et al. 2008; Spangler et al. 2009; Ungless et al. 2003; Wang et al. 2007; Vuong et al. 2010).
While the direct actions of MA and COC result in increased synaptic concentrations of monoamine neuro-transmitters, the behavioral effects of these drugs also rely on more indirect, or downstream, pharmacological actions that remain to be described in detail. In a previous examination of downstream neuropeptide systems underlying MA-induced locomotor activity, we identified a role for CRF-R2, but not CRF, Ucn1, or CRF-R1 (Giardino et al. 2011). Specifically, relative to wild-type littermates, mice deficient for CRF-R2 demonstrated an attenuated locomotor response to 1 mg/kg MA that was associated with decreased neuronal activity within the basolateral and central nuclei of the amygdala. Furthermore, mice deficient for CRF-R1 did not differ from wild-type littermates in the locomotor response to 1 mg/kg MA, and the selective CRFR1 antagonist CP-154,526 was unable to attenuate the sensitization of locomotor activity that was observed in DBA/2J mice treated repeatedly with MA.
Our previous results were surprising in light of an abundance of literature identifying a role for the HPA-axis in psychostimulant-induced behavior (Cador et al. 1993b; Deroche et al. 1992; Marinelli et al. 1997; Piazza et al. 1994; Rivet et al. 1989), and specifically, a role for CRF-R1 in the locomotor response to COC (Lu et al. 2003; Przegalinski et al. 2005). This led to the intriguing possibility that specific CRF receptor subtypes are differentially involved in behavioral response to distinct classes of psychostimulants. Thus, in the current study, we sought to (1) replicate our previous findings in CRF receptor-deficient mice using a higher dose of MA, (2) examine the effects of CRF receptor deficiency on the acute locomotor response to COC —a psychostimulant with a mechanism of action distinct from that of MA, and (3) confirm our experiments in mutant mice by evaluating the effects of the novel water-soluble, brain-penetrable, CRF-R1-selective antagonist CP-376,395 (Chen et al. 2008) on the acute stimulant locomotor responses produced by MA and COC.
Materials and methods
Animals
For experiments in knockout (KO) and wild-type (WT) littermate mice, we used single-gene mutant mice created from embryonic stem cells that underwent targeted gene inactivation. CRF-R1 KO mice generated on a 129P2/OlaHsd×CD1 background contained a deletion of exons 4– 7 of the Crhr1 gene (Timpl et al. 1998), and CRF-R2 KO mice generated on a 129X1/SvJ×C57BL/6J (B6) background contained a deletion of exons 3–4 of the Crhr2 gene (Coste et al. 2000). Each KO was backcrossed onto a B6 genetic background for eight to ten generations. KO and WT mice were littermates, generated by heterozygous matings. Mice were weaned at 28–32 days of age, isosexually housed, and tested at 8–16 weeks of age. For experiments using the selective CRF-R1 antagonist CP-376,395, we used male B6 mice (Jackson Laboratories, housed four per cage) that arrived in our colony at 8– 10 weeks of age and were allowed to habituate to conditions in the colony for 1–2 weeks before testing. Animals were maintained on a 12-h light/dark cycle (lights on at 06:00 h) in a temperature- and humidity-controlled environment, and allowed ad libitum access to food (LabDiet 5001; LabDiet, Richmond, IN, USA) and water. All protocols were approved by the Oregon Health & Science University animal care and use committee, and performed within the National Institutes of Health Guide lines for the Care and Use of Laboratory Animals, as well as the Guidelines for the Care and Use of Mammals in Neuroscience and Behavioral Research.
Drugs
Methamphetamine HCl (The Research Triangle Institute; Research Triangle Park, NC, USA) was dissolved in 0.9% saline at 0.20 mg/mL, cocaine HCl (The Research Triangle Institute; Research Triangle Park, NC, USA) was dissolved in 0.9% saline at either 0.50, 0.75, or 1.0 mg/mL, and CP-376,395 (Tocris; Ellisville, MO, USA) was dissolved in 0.9% saline at 1.0 mg/mL. All injections were given intraperitoneally (i.p.) at a volume of 10 mL/kg, for doses of 2, 5, 7.5, 10, and 10 mg/kg, respectively. The 2 mg/kg dose of MA was chosen specifically to examine acute stimulation in the absence of stereotypy, and to compare the current studies with our previous experiments using a lower dose of MA (1 mg/kg). In addition, the 2 mg/kg dose of MAwas ideal for comparing to the 5 and 7.5 mg/kg doses of COC, which produced roughly equivalent levels of locomotor stimulation.
Genetic deletion of CRF receptors and acute psychostimulant sensitivity
Male and female KO and WT littermate mice from the CRF-R1 and CRF-R2 lines were assessed for acute locomotor sensitivity in a previously established protocol in which a single administration of MA or COC induces a reliable stimulant response in B6 and other mouse genotypes (Kamens et al. 2005; Phillips et al. 1994). Following 2 days of saline administration to allow habituation (day 1) and examine baseline activity levels (day 2), mice received an injection of MA (2 mg/kg) or COC (5 mg/kg) on day 3. Horizontal locomotor activity was measured for 15 min immediately following the injection on each of the days.
CRF-R1 blockade and acute psychostimulant sensitivity
Male B6 mice received two injections per day, separated by 30 min on each day. During the 30 min in between injections, mice remained in their home cages. On days 1 and 2, mice were treated with vehicle (saline), followed 30 min later by a second injection of saline. On day 3, mice received either vehicle (saline) or the selective CRF-R1 antagonist CP-376,395 (10 mg/kg) followed 30 min later by either saline or psychostimulant treatment (either 2 mg/kg MA or 5, 7.5, or 10 mg/kg COC). Horizontal locomotor activity was measured for 15 min immediately following the second injection on each of the days.
Previous studies established that CP-376,395 binds with significantly higher affinity to CRF-R1 relative to CRF-R2 (Ki of 12 nM vs. >10,000 nM), and that systemic doses of CP-376,395 comparable to those used in the present studies significantly blocked HPA-axis activity and CRF-induced behavior (Chen et al. 2008). The pretreatment time for CP- 376,395 (30 min) was determined by previous experiments demonstrating significant effects of this manipulation on anxiety-related behavior in rats (Myers and Greenwood-Van Meerveld 2010).
Locomotor test apparatus
Horizontal locomotor activity was detected by interruption of a 10×12 array of photocell beams equally spaced at a height of 1 cm along the walls of a 21×25×18 cm enclosure with a steel bar grid floor (San Diego Instruments; San Diego, CA, USA). This chamber resided within a larger sound-attenuating box containing a fan and houselight. Horizontal activity was defined as the total number of photocell beam breaks during the 15-min tests.
Statistical analysis
For studies in male and female KO and WT mice, baseline locomotor activity scores across repeated saline trials (days 1 and 2) were analyzed by repeated measures ANOVA, with sex and genotype as between-subjects factors, and day as the repeated measure. The acute stimulant response to MA or COC was defined as the difference between locomotor activity scores on the saline baseline day and the day of the initial drug exposure (i.e., day 3 activity score minus day 2 activity score), and was subjected to two-way ANOVA with between-subjects factors of sex and genotype. For studies using the CRF-R1 antagonist CP-376,395 in male B6 mice, baseline locomotor activity scores across repeated saline trials (days 1 and 2) were analyzed by repeated measures ANOVA, with psychostimulant treatment (saline vs. either MA or COC) and CP-376,395 treatment (vehicle vs. 10 mg/kg) as between-subjects factors, and day as the repeated measure. The acute stimulant response to MA or COC (day 3 minus day 2 activity scores) was analyzed by two-way ANOVA with between-subjects factors of psychostimulant treatment and CP-376,395 treatment. Significant effects were followed by either simple main effect analyses (MA study) or Bonferroni post-hoc comparisons (COC study). Significance level was set at α<0.05.
Results
CRF-R1 and methamphetamine sensitivity
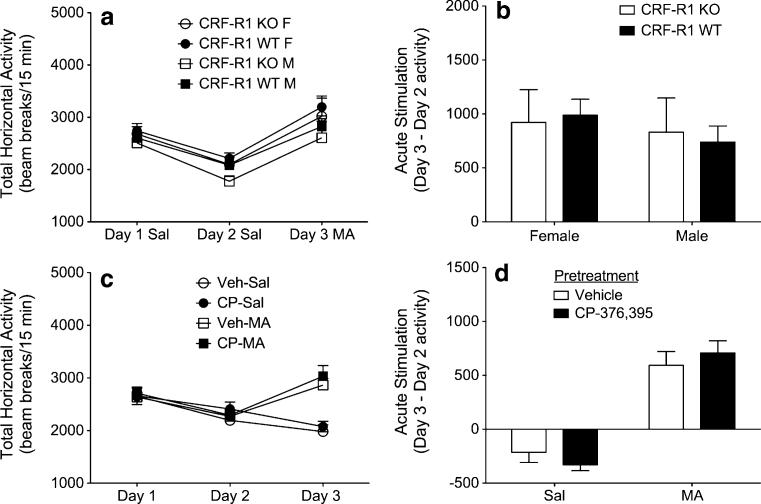
First, we sought to examine the acute locomotor response to 2 mg/kg MA in CRF-R1 KO and WT mice. Analysis of baseline locomotor activity levels (Fig. 1a) failed to identify any significant main or interacting effects. Relative to baseline activity levels on day 2, treatment with MA on day 3 produced a stimulant response that did not vary between sexes or genotypes (Fig. 1b).
Fig. 1.
Genetic deletion or pharmacological blockade of CRF-R1 does not alter acute MA sensitivity. a Locomotor activity counts (mean+ SEM) from male and female CRF-R1 KO and WT mice following administration of saline on days 1–2, and 2 mg/kg MA on day 3 (n=5–10 per sex, per genotype). b Acute MA stimulation scores (day 3 activity minus day 2 activity; mean+SEM) from male and female CRF-R1 KO and WT mice. c Locomotor activity counts (mean+SEM) from male B6 mice following administration of vehicle followed by saline on days 1–2, and either vehicle or 10 mg/kg CP-376,395 (CP) followed by either saline or 2 mg/kg MA on day 3 (n=8 per group). d Acute MA stimulation scores (day 3 activity minus day 2 activity; mean+SEM) from male B6 mice
To verify our results from genetic mutant mice, additional experiments evaluated the effects of the selective CRF-R1 antagonist CP-376,395 on acute MA-induced locomotor stimulation. Again, analysis of baseline locomotor activity levels identified no significant main or interacting effects (Fig. 1c). On day 3, MA exposure produced an acute stimulant response (main effect of MA treatment; F1,28=82.8; p<0.0001) that did not interact significantly with CP-376,395 treatment (p=0.264) (Fig. 1d). Together, these results demonstrate that ablation of CRF-R1 signaling, either by genetic or pharmacological means, is incapable of altering acute sensitivity to MA.
CRF-R2 and methamphetamine sensitivity
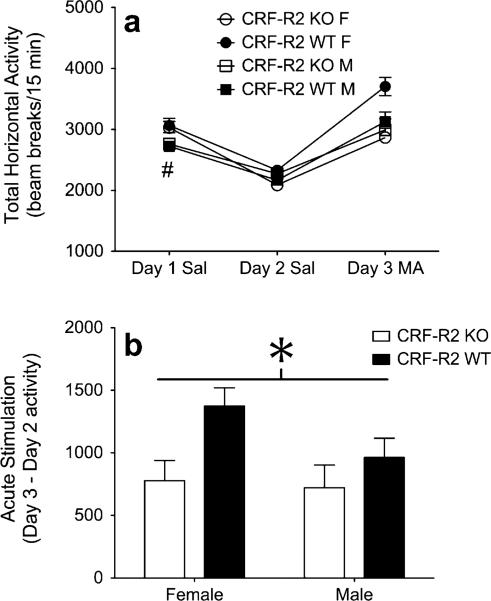
Next, we sought to compare the acute locomotor response to 2 mg/kg MA between CRF-R2 KO and WT mice. Preliminary examination of baseline locomotor activity levels identified a significant interaction between day and sex (F1,41=12.0; p=0.001). Follow-up analyses revealed that relative to male mice, female mice demonstrated greater locomotor activity scores on day 1 (p<0.005), but not on day 2 (p=0.932) (Fig. 2a). Importantly, the effect of sex on day 1 did not interact significantly with genotype (p=0.724), confirming that CRF-R2 KO and WT mice displayed equivalent baseline locomotor activity scores.
Fig. 2.
Genetic deletion of CRF-R2 dampens acute MA sensitivity. a Locomotor activity counts (mean+SEM) from male and female CRFR2 KO and WT mice following administration of saline on days 1–2, and 2 mg/kg MA on day 3 (n=9–15 per sex, per genotype). b Acute MA stimulation scores (day 3 activity minus day 2 activity; mean+SEM) from male and female CRF-R2 KO and WT mice. Number sign indicates main effect of sex on day 1 (p<0.005); asterisk indicates main effect of genotype (p<0.05)
Treatment with MA on day 3 produced an acute stimulant response that was significantly dampened by deletion of CRF-R2 (main effect of genotype; F1,41=6.3; p<0.05) (Fig. 2b). Although it appeared that the effect of CRF-R2 deletion on MA-induced stimulation was stronger in female than male mice, this effect was far from reaching significance (sex×genotype interaction; F1,41=1.1; p=0.295). These results confirm our previous finding that intact CRF-R2 signaling underlies the acute locomotor stimulant response to 1 mg/kg MA (Giardino et al. 2011).
CRF-R1 and cocaine sensitivity
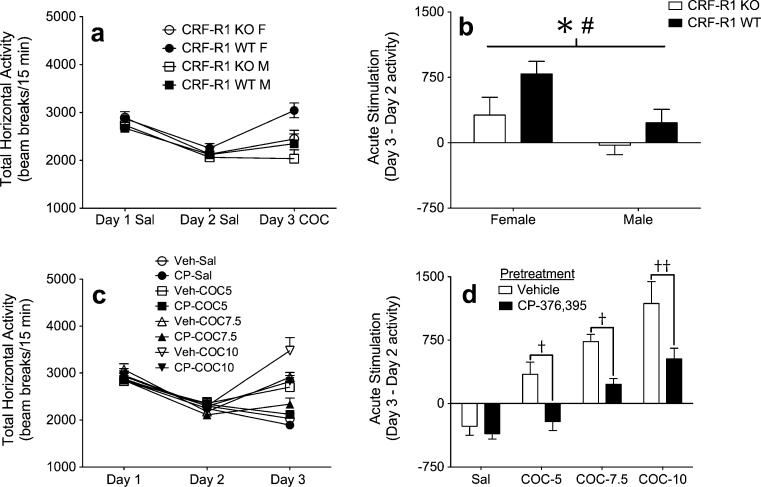
Analysis of baseline locomotor activity levels in CRF-R1 KO and WT littermate mice did not identify any significant main or interacting effects (Fig. 3a). Treatment with COC on day 3 produced an acute stimulant response that was significantly dampened by deletion of CRF-R1 (main effect of genotype; F1,33=4.7; p<0.05) (Fig. 3b). COC treatment also resulted in an acute stimulant response that was greater among female mice, relative to male mice (main effect of sex; F1,33=7.2; p<0.05). However, this sex effect did not interact significantly with genotype (p=0.531), indicating that deletion of CRF-R1 impacted COC sensitivity equivalently across both sexes.
Fig. 3.
Genetic deletion and pharmacological blockade of CRF-R1 dampens acute COC sensitivity. a Locomotor activity counts (mean+SEM) from male and female CRF-R1 KO and WT mice following administration of saline on days 1–2, and 5 mg/kg COC on day 3 (n=7–12 per sex, per genotype). b Acute COC stimulation scores (day 3 activity minus day 2 activity; mean+SEM) from male and female CRF-R1 KO and WT mice. c Locomotor activity counts (mean+SEM) from male B6 mice following administration of vehicle followed by saline on days 1–2, and either vehicle or 10 mg/kg CP- 376,395 (CP) followed by either saline or 5, 7.5, or 10 mg/kg COC on day 3 (n=7–11 per group). d Acute COC stimulation scores (day 3 activity minus day 2 activity; mean+SEM) from male B6 mice. Asterisk indicates main effect of genotype (p<0.05); number sign indicates main effect of sex (p<0.05); single dagger denotes significant difference between vehicle-COC and CP-COC groups (p<0.05); double dagger denotes significant difference between vehicle-COC and CP-COC groups (p<0.01)
To verify our results from genetic mutant mice, additional experiments evaluated the effects of the selective CRF-R1 antagonist CP-376,395 on acute COC-induced locomotor stimulation. Again, analysis of baseline locomotor activity levels identified no significant main or interacting effects (Fig. 3c). However, administration of COC on day 3 produced an acute stimulant response that was dependent on CP-376,395 treatment (main effect of COC treatment; F3,58=34.2; p<0.0001, main effect of CP treatment; F1,58=24.6; p<0.0001, COC treatment×CP treatment interaction; F3,58=2.3; p=0.088). Follow-up analyses confirmed that 10 mg/kg of CP-376,395 selectively dampened locomotor activity scores in mice treated on day 3 with COC (all p<0.05), but not in mice treated on day 3 with saline (p>0.05) (Fig. 3d). Together, these results indicate that CRF-R1 signaling is critical for the acute locomotor response to COC.
CRF-R2 and cocaine sensitivity
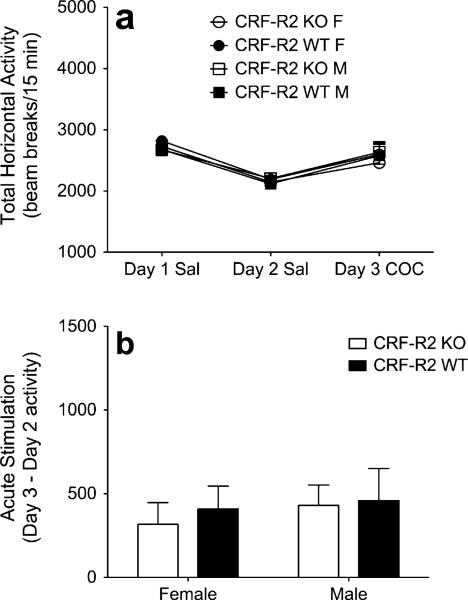
Analysis of baseline locomotor activity levels in CRF-R2 KO and WT littermate mice failed to identify any significant main or interacting effects (Fig. 4a). Treatment with COC on day 3 produced an acute stimulant response that did not vary between sexes or genotypes (Fig. 4b).
Fig. 4.
Genetic deletion of CRF-R2 does not alter acute COC sensitivity. a Locomotor activity counts (mean+SEM) from male and female CRF-R2 KO and WT mice following administration of saline on days 1–2, and 5 mg/kg COC on day 3 (n=10–11 per sex, per genotype). b Acute COC stimulation scores (day 3 activity minus day 2 activity; mean+SEM) from male and female CRF-R2 KO and WT mice
Discussion
The present study demonstrates that while both genetic deletion and pharmacological blockade of CRF-R1 were ineffective in altering sensitivity to locomotor effects of MA, genetic deletion of CRF-R2 was effective. Conversely, while genetic deletion of CRF-R2 was ineffective in altering sensitivity to locomotor effects of COC, either genetic deletion or pharmacological blockade of CRF-R1 was effective. Together, by demonstrating that MA and COC rely on alternate receptor subtypes within the CRF system to mediate their respective locomotor-activating effects, these findings significantly advance our understanding of the neural stress systems underlying behavioral sensitivity to psychostimulant exposure.
The current data complement our previous findings by using genetic and pharmacological manipulations to reveal that the locomotor response to MA is fully maintained in the absence of CRF-R1 signaling. These effects cannot be explained by dosing issues, as CRF-R1 KO and WT mice do not differ in the locomotor response to MA at either 1 (Giardino et al. 2011) or 2 mg/kg (Fig. 1b). Furthermore, it is important to note that the dose of COC at which CRF-R1 KO mice showed a dampened response (5 mg/kg) is one that produces a level of stimulation similar in magnitude to those produced by 1 and 2 mg/kg MA.
These findings cannot be explained by differences in stereotypic behaviors, which only occur in response to psychostimulant treatment at doses higher than those used here. In addition, the current results cannot be accounted for by sex differences, because analyses of CRF-R1 and CRFR2 KO and WT mice yielded no statistically significant interactions between sex and genotype. Finally, our data cannot be explained by pre-existing differences in activity levels, because analyses of baseline activity scores failed to identify any significant differences between genotypes or drug treatment groups.
While developmental compensations may complicate the interpretation of behavioral studies performed in genetically engineered mice, this is also an unlikely explanation for our results. In a series of pharmacological studies intended to complement our experiments in KO mice, two different CRF-R1-selective antagonists (CP-154,526 and CP-376,395), were incapable of altering MA-induced psychomotor sensitization (Giardino et al. 2011), as well as acute MA-induced locomotor stimulation (Fig. 2d). Interestingly, this lack of contribution of CRF-R1 to MA-induced behavior is independent of the genetic background, because the experiments using CP-154,526 were performed in DBA/2J mice, a strain known for its robust locomotor response to MA (Phillips et al. 1994).
Although the present data appear contradictory to previous reports that psychostimulant-induced locomotor activation relies on glucocorticoid release (Cador et al. 1993b; Deroche et al. 1992; Marinelli et al. 1997; Piazza et al. 1994; Rivet et al. 1989) and CRF-R signaling (Cador et al. 1993a; Erb and Brown 2006; Erb et al. 2003; Lu et al. 2003; Przegalinski et al. 2005; Sarnyai et al. 1992), our examination of the differential involvement of CRF-R subtypes in sensitivity to MA vs. COC provides an intriguing explanation for these apparent inconsistencies.
Unlike the response to MA, acute locomotor stimulation following COC was significantly dampened by both genetic deletion (Fig. 3b) and pharmacological blockade (Fig. 3d) of CRF-R1. These data are consistent with previous studies in which a selective CRF-R1 antagonist attenuated COC-induced locomotor activity (Lu et al. 2003; Przegalinski et al. 2005). In addition, our data suggest that CRF-R1 is the specific CRF receptor subtype responsible for the bidirectional effects of CRF (Erb et al. 2003; Sarnyai et al. 1992) and a non-selective CRF-R antagonist (Erb and Brown 2006) on the locomotor response to COC. Furthermore, these findings are consistent with the aforementioned importance of glucocorticoid signaling in COC sensitivity (Marinelli et al. 1997; Piazza et al. 1994), thereby implicating a role for CRFR1-mediated activation of the HPA-axis in the locomotor response to COC.
Our results in CRF-R2 KO mice are also in agreement with pharmacological studies, which have shown that CRF-R2 signaling is not involved in sensitivity to locomotor effects of COC (Lu et al. 2003). Furthermore, the current study replicates our previous findings by showing that genetic deletion of CRF-R2 significantly dampens MA-induced locomotor activation (Giardino et al. 2011). Despite the apparent involvement of CRF systems in amphetamine-induced locomotor activity (Cador et al. 1993a), neither genetic deletion, nor pharmacological blockade of CRF-R1 prevented MA-induced locomotor stimulation (Fig. 1b, d). Our studies have used both genetic and pharmacological techniques to demonstrate that this involvement is mediated by CRF-R2, and not CRF-R1.
Because activation of the HPA-axis and subsequent release of glucocorticoids are directly initiated by CRF-R1, but not by CRF-R2, our findings implicating CRF-R2 in the response to MA lie in contrast to previous data identifying a role for glucocorticoid signaling in amphetamine-induced locomotor activity (Cador et al. 1993b; Deroche et al. 1992; Rivet et al. 1989). It is important to note that while these previous studies highlighted the importance of glucocorticoid release, they did not provide direct evidence for CRF-R1 involvement, and therefore, fell short of elucidating a functional mechanism. In this case, it is reasonable to hypothesize that MA upregulates HPA-axis activity independently of CRF and CRF-R1. Because vasopressin (AVP) released from the paraventricular nucleus of the hypothalamus (PVN) is also capable of activating the HPA-axis via the AVP type-1b receptor (V1b-R) (Jard et al. 1986), one possibility is that amphetamines activate the HPA-axis in an AVP-dependent manner, initiating V1b-R signaling in the pituitary and causing release of glucocorticoids through this alternate pathway. Intriguingly, intracranial administration of Ucn3 (an endogenous CRF-R2-selective ligand) increased mRNA expression of AVP (but not CRF) in the rat PVN (Jamieson et al. 2006), and metabolites of MA initiated massive release of AVP from rat hypothalamic explants (Forsling et al. 2002). Both of these findings raise the possibility that MA-induced increases in CRF-R2 signaling cause release of AVP from the PVN, initiating subsequent HPA-axis activation via V1b-R.
When examining possibilities for the differential involvement of CRF-R subtypes in the behavioral response to MA vs. COC, one feasible explanation lies at the divergent mechanisms of actions of the two drugs. For example, MA can stimulate release of monoamines from vesicular stores in addition to blocking monoamine reuptake transport, while COC functions as an inhibitor of monoamine reuptake transporters without directly altering release of monoamines from vesicular stores (Fleckenstein et al. 2007). Furthermore, in a series of studies examining psychostimulant binding to mouse monoamine transporters, MA displayed significantly higher affinity for the norepinephrine transporter (NET) compared to the dopamine transporter (DAT), whereas COC bound the DAT and NET with similarly high affinity (Han and Gu 2006). Given the divergent actions of these two drugs, the behavioral effects of MA and COC would be expected to rely on a unique complement of downstream neurotransmitter systems. Therefore, it is possible that the actions of MA occur preferentially at the site of NE terminals within CRF-R2-containing circuits, whereas the actions of COC occur equally at both NE and DA terminals within CRF-R1-containing circuits.
A previous examination of the neural substrates underlying CRF-R2-dependent responses to MA identified a role for the basolateral and central nuclei of the amygdala, in which CRF-R2-deficient mice were resistant to MA-induced increases in neuronal activity, relative to their wild-type littermates (Giardino et al. 2011). Because the amygdala receives projections from noradrenergic and dopaminergic nuclei, this region may be critically involved in CRF-R-dependent psychostimulant sensitivity.
Despite weak affinity of MA and COC for the serotonin transporter (SERT; Han and Gu 2006), it was recently reported that CRF-induced serotonin (5-HT) release in the amygdala was potentiated by amphetamine treatment (Scholl et al. 2010). Such a result suggests the involvement of 5-HT in MA-induced neural activity within CRF-R2-containing amygdala circuits. Indeed, the contribution of urocortin peptides within 5-HT pathways to anxiety-like behavior was recently established (Neufeld-Cohen et al. 2010a, b), and CRF-R2 signaling within serotonergic nuclei is critically involved in amphetamine withdrawal-induced anxiety-like behavior (Vuong et al. 2010). Together, these findings suggest the importance of limbic CRF-R2/5-HT systems in mediating behaviors that lie at the intersection of stress responses and psychostimulant actions.
Overall, our data add to the growing number of recent studies implicating central urocortin peptides and CRF-R2 signaling in anxiety- and depressive-like behaviors associated with the neural processes underlying addiction (Kozicz et al. 2008; Kuperman et al. 2010; Neufeld-Cohen et al. 2010a, b; Vuong et al. 2010). In order to develop more targeted therapeutics for the treatment of neuropsychiatric disorders related to both stress and addiction, future studies will need to address the precise contributions of endogenous CRF, Ucn1, Ucn2, and Ucn3 to the many addiction-relevant behavioral and synaptic adaptations that rely on central CRF-R1 and CRF-R2 signaling (Giardino et al. 2011; Liu et al. 2005; Orozco-Cabal et al. 2008; Ungless et al. 2003; Wang et al. 2007; Vuong et al. 2010).
Acknowledgments
This research was supported by the National Institutes of Health [Grants P50DA018165 (AER, GPM), RO3DA02854 (AER, GPM) and T32DA007262 (WJG, via Kim A. Neve)]. We thank Ju Li and Dawn M. Cote for their excellent technical assistance. These experiments were performed in compliance with current laws designated by the USA. The authors have no conflicts of interest to report.
Contributor Information
William J. Giardino, Department of Behavioral Neuroscience and Methamphetamine Abuse Research Center, Oregon Health & Science University, 3181 SW Sam Jackson Park Road, Portland, OR 97239, USA
Gregory P. Mark, Department of Behavioral Neuroscience and Methamphetamine Abuse Research Center, Oregon Health & Science University, 3181 SW Sam Jackson Park Road, Portland, OR 97239, USA
Mary P. Stenzel-Poore, Department of Molecular Microbiology and Immunology, Oregon Health & Science University, 3181 SW Sam Jackson Park Road, Portland, OR 97239, USA
Andrey E. Ryabinin, Department of Behavioral Neuroscience and Methamphetamine Abuse Research Center, Oregon Health & Science University, 3181 SW Sam Jackson Park Road, Portland, OR 97239, USA
References
- Boutrel B. A neuropeptide-centric view of psychostimulant addiction. Br J Pharmacol. 2008;154:343–357. doi: 10.1038/bjp.2008.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cador M, Cole BJ, Koob GF, Stinus L, Le Moal M. Central administration of corticotropin releasing factor induces long-term sensitization to D-amphetamine. Brain Res. 1993a;606:181–186. doi: 10.1016/0006-8993(93)90982-s. [DOI] [PubMed] [Google Scholar]
- Cador M, Dulluc J, Mormede P. Modulation of the locomotor response to amphetamine by corticosterone. Neuroscience. 1993b;56:981–988. doi: 10.1016/0306-4522(93)90144-5. [DOI] [PubMed] [Google Scholar]
- Chen YL, Obach RS, Braselton J, Corman ML, Forman J, Freeman J, Gallaschun RJ, Mansbach R, Schmidt AW, Sprouse JS, Tingley Iii FD, Winston E, Schulz DW. 2-aryloxy-4-alkylamino-pyridines: discovery of novel corticotropin-releasing factor 1 antagonists. J Med Chem. 2008;51:1385–1392. doi: 10.1021/jm070579c. [DOI] [PubMed] [Google Scholar]
- Cole BJ, Cador M, Stinus L, Rivier C, Rivier J, Vale W, Le Moal M, Koob GF. Critical role of the hypothalamic pituitary adrenal axis in amphetamine-induced sensitization of behavior. Life Sci. 1990;47:1715–1720. doi: 10.1016/0024-3205(90)90344-q. [DOI] [PubMed] [Google Scholar]
- Coste SC, Kesterson RA, Heldwein KA, Stevens SL, Heard AD, Hollis JH, Murray SE, Hill JK, Pantely GA, Hohimer AR, Hatton DC, Phillips TJ, Finn DA, Low MJ, Rittenberg MB, Stenzel P, Stenzel-Poore MP. Abnormal adaptations to stress and impaired cardiovascular function in mice lacking corticotropin-releasing hormone receptor-2. Nat Genet. 2000;24:403–409. doi: 10.1038/74255. [DOI] [PubMed] [Google Scholar]
- Deroche V, Piazza PV, Maccari S, Le Moal M, Simon H. Repeated corticosterone administration sensitizes the locomotor response to amphetamine. Brain Res. 1992;584:309–313. doi: 10.1016/0006-8993(92)90911-r. [DOI] [PubMed] [Google Scholar]
- DeVries AC, Taymans SE, Sundstrom JM, Pert A. Conditioned release of corticosterone by contextual stimuli associated with cocaine is mediated by corticotropin-releasing factor. Brain Res. 1998;786:39–46. doi: 10.1016/s0006-8993(97)01328-0. [DOI] [PubMed] [Google Scholar]
- Erb S, Brown ZJ. A role for corticotropin-releasing factor in the long-term expression of behavioral sensitization to cocaine. Behav Brain Res. 2006;172:360–364. doi: 10.1016/j.bbr.2006.05.024. [DOI] [PubMed] [Google Scholar]
- Erb S, Salmaso N, Rodaros D, Stewart J. A role for the CRF-containing pathway from central nucleus of the amygdala to bed nucleus of the stria terminalis in the stress-induced reinstatement of cocaine seeking in rats. Psychopharmacology (Berl) 2001;158:360–365. doi: 10.1007/s002130000642. [DOI] [PubMed] [Google Scholar]
- Erb S, Funk D, Le AD. Prior, repeated exposure to cocaine potentiates locomotor responsivity to central injections of corticotropin-releasing factor (CRF) in rats. Psychopharmacology (Berl) 2003;170:383–389. doi: 10.1007/s00213-003-1556-1. [DOI] [PubMed] [Google Scholar]
- Fleckenstein AE, Volz TJ, Riddle EL, Gibb JW, Hanson GR. New insights into the mechanism of action of amphetamines. Annu Rev Pharmacol Toxicol. 2007;47:681–698. doi: 10.1146/annurev.pharmtox.47.120505.105140. [DOI] [PubMed] [Google Scholar]
- Forsling ML, Fallon JK, Shah D, Tilbrook GS, Cowan DA, Kicman AT, Hutt AJ. The effect of 3,4-methylenedioxymethamphetamine (MDMA, ‘ecstasy’) and its metabolites on neurohypophysial hormone release from the isolated rat hypothalamus. Br J Pharmacol. 2002;135:649–656. doi: 10.1038/sj.bjp.0704502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giardino WJ, Pastor R, Anacker AM, Spangler E, Cote DM, Li J, Stenzel-Poore MP, Phillips TJ, Ryabinin AE. Dissection of corticotropin-releasing factor system involvement in locomotor sensitivity to methamphetamine. Genes Brain Behav. 2011;10:78–89. doi: 10.1111/j.1601-183X.2010.00641.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graf EN, Hoks MA, Baumgardner J, Sierra J, Vranjkovic O, Bohr C, Baker DA, Mantsch JR. Adrenal activity during repeated long-access cocaine self-administration is required for later CRF-induced and CRF-dependent stressor-induced reinstatement in rats. Neuropsychopharmacology. 2011;36:1444–1454. doi: 10.1038/npp.2011.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hahn J, Hopf FW, Bonci A. Chronic cocaine enhances corticotropin-releasing factor-dependent potentiation of excitatory transmission in ventral tegmental area dopamine neurons. J Neurosci. 2009;29:6535–6544. doi: 10.1523/JNEUROSCI.4773-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han DD, Gu HH. Comparison of the monoamine transporters from human and mouse in their sensitivities to psychostimulant drugs. BMC Pharmacol. 2006;6:6. doi: 10.1186/1471-2210-6-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jamieson PM, Li C, Kukura C, Vaughan J, Vale W. Urocortin 3 modulates the neuroendocrine stress response and is regulated in rat amygdala and hypothalamus by stress and glucocorticoids. Endocrinology. 2006;147:4578–4588. doi: 10.1210/en.2006-0545. [DOI] [PubMed] [Google Scholar]
- Jard S, Gaillard RC, Guillon G, Marie J, Schoenenberg P, Muller AF, Manning M, Sawyer WH. Vasopressin antagonists allow demonstration of a novel type of vasopressin receptor in the rat adenohypophysis. Mol Pharmacol. 1986;30:171–177. [PubMed] [Google Scholar]
- Kamens HM, Burkhart-Kasch S, McKinnon CS, Li N, Reed C, Phillips TJ. Sensitivity to psychostimulants in mice bred for high and low stimulation to methamphetamine. Genes Brain Behav. 2005;4:110–125. doi: 10.1111/j.1601-183X.2004.00101.x. [DOI] [PubMed] [Google Scholar]
- Koob GF. A role for brain stress systems in addiction. Neuron. 2008;59:11–34. doi: 10.1016/j.neuron.2008.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozicz T, Tilburg-Ouwens D, Faludi G, Palkovits M, Roubos E. Gender-related urocortin 1 and brain-derived neurotrophic factor expression in the adult human midbrain of suicide victims with major depression. Neuroscience. 2008;152:1015–1023. doi: 10.1016/j.neuroscience.2007.12.050. [DOI] [PubMed] [Google Scholar]
- Krishnan B, Centeno M, Pollandt S, Fu Y, Genzer K, Liu J, Gallagher JP, Shinnick-Gallagher P. Dopamine receptor mechanisms mediate corticotropin-releasing factor-induced long-term potentiation in the rat amygdala following cocaine withdrawal. Eur J Neurosci. 2010;31:1027–1042. doi: 10.1111/j.1460-9568.2010.07148.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuperman Y, Issler O, Regev L, Musseri I, Navon I, Neufeld-Cohen A, Gil S, Chen A. Perifornical urocortin-3 mediates the link between stress-induced anxiety and energy homeostasis. Proc Natl Acad Sci U S A. 2010;107:8393–8398. doi: 10.1073/pnas.1003969107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J, Yu B, Orozco-Cabal L, Grigoriadis DE, Rivier J, Vale WW, Shinnick-Gallagher P, Gallagher JP. Chronic cocaine administration switches corticotropin-releasing factor2 receptor-mediated depression to facilitation of glutamatergic transmission in the lateral septum. J Neurosci. 2005;25:577–583. doi: 10.1523/JNEUROSCI.4196-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu L, Liu Z, Huang M, Zhang Z. Dopamine-dependent responses to cocaine depend on corticotropin-releasing factor receptor subtypes. J Neurochem. 2003;84:1378–1386. doi: 10.1046/j.1471-4159.2003.01635.x. [DOI] [PubMed] [Google Scholar]
- Marinelli M, Rouge-Pont F, De Jesus-Oliveira C, Le Moal M, Piazza PV. Acute blockade of corticosterone secretion decreases the psychomotor stimulant effects of cocaine. Neuropsychopharmacology. 1997;16:156–161. doi: 10.1016/S0893-133X(96)00169-8. [DOI] [PubMed] [Google Scholar]
- Myers B, Greenwood-Van Meerveld B. Elevated corticosterone in the amygdala leads to persistent increases in anxiety-like behavior and pain sensitivity. Behav Brain Res. 2010;214:465–469. doi: 10.1016/j.bbr.2010.05.049. [DOI] [PubMed] [Google Scholar]
- Neufeld-Cohen A, Evans AK, Getselter D, Spyroglou A, Hill A, Gil S, Tsoory M, Beuschlein F, Lowry CA, Vale W, Chen A. Urocortin-1 and −2 double-deficient mice show robust anxiolytic phenotype and modified serotonergic activity in anxiety circuits. Mol Psychiatry. 2010a;15(426–41):339. doi: 10.1038/mp.2009.115. [DOI] [PubMed] [Google Scholar]
- Neufeld-Cohen A, Tsoory MM, Evans AK, Getselter D, Gil S, Lowry CA, Vale WW, Chen A. A triple urocortin knockout mouse model reveals an essential role for urocortins in stress recovery. Proc Natl Acad Sci U S A. 2010b;107:19020–19025. doi: 10.1073/pnas.1013761107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orozco-Cabal L, Liu J, Pollandt S, Schmidt K, Shinnick-Gallagher P, Gallagher JP. Dopamine and corticotropin-releasing factor synergistically alter basolateral amygdala-to-medial prefrontal cortex synaptic transmission: functional switch after chronic cocaine administration. J Neurosci. 2008;28:529–542. doi: 10.1523/JNEUROSCI.2666-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips TJ, Dickinson S, Burkhart-Kasch S. Behavioral sensitization to drug stimulant effects in C57BL/6J and DBA/2J inbred mice. Behav Neurosci. 1994;108:789–803. doi: 10.1037//0735-7044.108.4.789. [DOI] [PubMed] [Google Scholar]
- Piazza PV, Marinelli M, Jodogne C, Deroche V, Rouge-Pont F, Maccari S, Le Moal M, Simon H. Inhibition of corticosterone synthesis by metyrapone decreases cocaine-induced locomotion and relapse of cocaine self-administration. Brain Res. 1994;658:259–264. doi: 10.1016/s0006-8993(09)90034-8. [DOI] [PubMed] [Google Scholar]
- Przegalinski E, Filip M, Frankowska M, Zaniewska M, Papla I. Effects of CP 154,526, a CRF1 receptor antagonist, on behavioral responses to cocaine in rats. Neuropeptides. 2005;39:525–533. doi: 10.1016/j.npep.2005.07.002. [DOI] [PubMed] [Google Scholar]
- Rivet JM, Stinus L, LeMoal M, Mormede P. Behavioral sensitization to amphetamine is dependent on corticosteroid receptor activation. Brain Res. 1989;498:149–153. doi: 10.1016/0006-8993(89)90411-3. [DOI] [PubMed] [Google Scholar]
- Sarnyai Z. Neurobiology of stress and cocaine addiction. Studies on corticotropin-releasing factor in rats, monkeys, and humans. Ann N YAcad Sci. 1998;851:371–387. doi: 10.1111/j.1749-6632.1998.tb09011.x. [DOI] [PubMed] [Google Scholar]
- Sarnyai Z, Hohn J, Szabo G, Penke B. Critical role of endogenous corticotropin-releasing factor (CRF) in the mediation of the behavioral action of cocaine in rats. Life Sci. 1992;51:2019–2024. doi: 10.1016/0024-3205(92)90151-e. [DOI] [PubMed] [Google Scholar]
- Scholl JL, Vuong SM, Forster GL. Chronic amphetamine treatment enhances corticotropin-releasing factor-induced serotonin release in the amygdala. Eur J Pharmacol. 2010;644:80–87. doi: 10.1016/j.ejphar.2010.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shalev U, Erb S, Shaham Y. Role of CRF and other neuropeptides in stress-induced reinstatement of drug-seeking. Brain Res. 2010;16:15–28. doi: 10.1016/j.brainres.2009.07.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spangler E, Cote DM, Anacker AM, Mark GP, Ryabinin AE. Differential sensitivity of the perioculomotor urocortin-containing neurons to ethanol, psychostimulants and stress in mice and rats. Neuroscience. 2009;160:115–125. doi: 10.1016/j.neuroscience.2009.02.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Timpl P, Spanagel R, Sillaber I, Kresse A, Reul JM, Stalla GK, Blanquet V, Steckler T, Holsboer F, Wurst W. Impaired stress response and reduced anxiety in mice lacking a functional corticotropin-releasing hormone receptor 1. Nat Genet. 1998;19:162–166. doi: 10.1038/520. [DOI] [PubMed] [Google Scholar]
- Ungless MA, Singh V, Crowder TL, Yaka R, Ron D, Bonci A. Corticotropin-releasing factor requires CRF binding protein to potentiate NMDA receptors via CRF receptor 2 in dopamine neurons. Neuron. 2003;39:401–407. doi: 10.1016/s0896-6273(03)00461-6. [DOI] [PubMed] [Google Scholar]
- Vuong SM, Oliver HA, Scholl JL, Oliver KM, Forster GL. Increased anxiety-like behavior of rats during amphetamine withdrawal is reversed by CRF2 receptor antagonism. Behav Brain Res. 2010;208:278–281. doi: 10.1016/j.bbr.2009.11.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang B, You ZB, Rice KC, Wise RA. Stress-induced relapse to cocaine seeking: roles for the CRF(2) receptor and CRF-binding protein in the ventral tegmental area of the rat. Psychopharmacology (Berl) 2007;193:283–294. doi: 10.1007/s00213-007-0782-3. [DOI] [PubMed] [Google Scholar]