Abstract
Many of the countries in the Asia Pacific Region, particularly those with depressed and developing economies, are just initiating newborn screening programs for selected metabolic and other congenital disorders. The cultural, geographic, language, and economic differences that exist throughout the region add to the challenges of developing sustainable newborn screening systems. There are currently more developing programs than developed programs within the region. Newborn screening activities in the Asia Pacific Region are particularly important since births there account for approximately half of the world’s births. To date, there have been two workshops to facilitate formation of the Asia Pacific Newborn Screening Collaboratives. The 1st Workshop on Consolidating Newborn Screening Efforts in the Asia Pacific Region occurred in Cebu, Philippines, on March 30–April 1, 2008, as a satellite meeting to the 7th Asia Pacific Conference on Human Genetics. The second workshop was held on June 4–5, 2010, in Manila, Philippines. Workshop participants included key policy-makers, service providers, researchers, and consumer advocates from 11 countries with 50% or less newborn screening coverage. Expert lectures included experiences in the United States and the Netherlands, international quality assurance activities and ongoing and potential research activities. Additional meeting support was provided by the U.S. National Institutes of Health, the Centers for Disease Control and Prevention, the U.S. National Newborn Screening and Genetics Resource Center, the International Society for Neonatal Screening, and the March of Dimes. As part of both meeting activities, participants shared individual experiences in program implementation with formal updates of screening information for each country. This report reviews the activities and country reports from two Workshops on Consolidating Newborn Screening Efforts in the Asia Pacific Region with emphasis on the second workshop. It also updates the literature on screening activities and implementation/expansion challenges in the participating countries.
Keywords: Newborn screening, Asia Pacific, Cebu Declaration, Manila Declaration
Introduction
Many of the countries in the Asia Pacific Region, particularly those with depressed and developing economies are just initiating newborn screening programs for selected metabolic and other congenital disorders (Padilla and Therrell 2007). Vast cultural, geographic, language, and economic differences exist throughout the region adding to the challenges of developing sustainable newborn screening systems. There are currently more developing programs than developed programs within the region and the status of screening activities in a few countries still remains unclear or unknown. Newborn screening activities in the Asia Pacific region are particularly important since births there account for approximately half (68.5 million) of the 136.7 million babies born in the world. Of these, about 85% are born in five countries (China, India, Indonesia, Bangladesh, Pakistan) (UNICEF. The State of the World’s Children 2011), which do not yet have organized newborn screening for half or more of their newborn population.
Newborn dried bloodspot screening (NDBS) as a public health improvement strategy has existed in some countries in the Asia Pacific since the 1960s (Australia, Japan, New Zealand), and newborn cord blood screening (NCBS) has a lengthy history in others (Singapore, Hong Kong). Despite attempts over time to begin organized newborn screening in various countries in the region, implementation has been slow (primarily for economic reasons) (Padilla and Therrell 2007). In recent years, extensive efforts in Korea and Thailand, partially aided by support from the International Atomic Energy Agency (IAEA), have led to implementation of universal NBDS at the national level, and now both countries have NDBS programs that reach essentially all newborns. Most other countries in the region, however, have only begun NDBS implementation efforts during the past decade. Many of these also received partial support from the IAEA (Solanki 2007); however, direct funding support of this type is no longer available. Despite the unavailability of outside funding from the IAEA, the fledgling NDBS programs in the region have continued their growth and development through self determination.
Building on an informal network of Asia Pacific NDBS collaborators and experts who existed as part of the IAEA Regional Project, newborn screening innovators in the Asia Pacific region have initiated a collaborative network of local newborn screening pioneers. Establishment of a collaborative communications network is intended to facilitate and improve local NDBS program implementation and foster related research collaborations. To ensure up-to-date information exchange, to provide expert advice and training, and to assist in additional networking, interactions with more developed NDBS programs outside the region have been an essential part of the collaborations.
To date, there have been two workshops to facilitate formation of the Asia Pacific Newborn Screening Collaboratives. The 1st Workshop on Consolidating Newborn Screening Efforts in the Asia Pacific Region occurred in Cebu, Philippines, on March 30–April 1, 2008, as a satellite meeting to the 7th Asia Pacific Conference on Human Genetics. The second workshop was held on June 4–5, 2010, in Manila Philippines. Both workshops were hosted by the Philippine Newborn Screening Reference Center (NSRC). Workshop participants included key policy-makers, service providers, researchers, and consumer advocates from 11 countries with less than 50% newborn screening coverage (Bangladesh, China, India, Indonesia, Laos, Mongolia, Pakistan, Palau, Philippines, Sri Lanka, and Vietnam). Expert lectures included NDBS experiences in the United States and the Netherlands, international quality assurance activities and ongoing and potential NDBS-related research activities. Additional meeting support was provided by the U.S. National Institutes of Health (NIH), the Centers for Disease Control and Prevention (CDC), the U.S. National Newborn Screening and Genetics Resource Center (NNSGRC), the International Society for Neonatal Screening (ISNS), and the March of Dimes.
As part of both meeting activities, participants shared individual experiences in NDBS program implementation with formal updates of screening information for each country. In order to develop strategies and supporting activities, participants were also divided into working groups with facilitated discussions as part of the agenda. This report reviews the activities and country reports from the Workshops on Consolidating Newborn Screening Efforts in the Asia Pacific Region with emphasis on the second workshop. It also updates the literature on screening activities and implementation/expansion challenges in each of the participating countries.
Method — conference descriptions
The two workshops now completed have both focused on building a working network of NDBS collaborators within the Asia Pacific region. The network has evolved as an activity to improve the health of newborns by implementing, refining and expanding newborn screening systems. These activities continue the international focus on newborn screening and are similar to activities in the Middle East and North Africa region previously reported (Krotoski et al. 2009). They include discussions of the issues and challenges that routinely face developing programs and shared solutions (Padilla 2008; Padilla et al. 2010). Given that approximately half of all births worldwide occur in the Asia Pacific region, the potential for improved newborn health and the societal benefits from successful newborn screening here is extremely important.
Initially, a limited number of key policy-makers and screening champions were identified from previous participation in IAEA Newborn Screening Project meetings. Others were added to the group as they were identified through their involvement in various NDBS screening activities in the Region. A total of 39 participants including representatives from 11 countries (Bangladesh, China, India, Indonesia, Laos, Mongolia, Pakistan, Palau, Philippines, Sri Lanka, and Vietnam) attended the first workshop in Cebu. Because this meeting was a satellite to the Asia Pacific Society of Human Genetics (APSHG) meeting, several experts were also present representing the U.S. National Institutes of Health, the Centers for Disease Control and Prevention, and NDBS programs in both the U.S. and the Netherlands.
As an outcome of the Cebu meeting, and in order to improve the chances of successful implementation of a sustainable NDBS system within each country, representatives of the various Ministries of Health were invited to participate (along with their country NDBS champion) in the second workshop in Manila. This workshop included 41 participants including representatives from ten of the original 11 countries (a representative from Palau could not attend), including several representatives of health ministries. An expert representing developed U.S. newborn screening programs was also present to discuss: (1) current processes for selection of disorders for screening; (2) indicators for measuring quality throughout the newborn screening system; and (3) issues currently being faced in developed programs relative to the storage and use of residual NDBS specimens.
The stated goals of the Manila workshop were to:
Review the current status of newborn screening and related research within Southeast Asia and the Western Pacific Regions.
Explore national and regional health care delivery and research infrastructure needs for maximizing research/service collaborations in newborn screening.
Identify strategies for continuing a regional collaborative research group focused on improving newborn screening throughout the Asia Pacific region.
Define research and other activities emanating from newborn screening that can complement and integrate with existing collaborations.
Assess resource needs, identify currently available resources, and develop information about other possible resources.
Developing NDBS programs in the Asia Pacific region may be broadly separated into two groups. At the first (Cebu) workshop, participants were divided into two working groups having similar experiences and issues based on time previously spent in developing a national NDBS screening program. Group 1 included countries with NDBS screening efforts ongoing for 5 or more years (the Philippines, China, and Indonesia). Group 2 included all other countries (programs in development for less than 5 years — Bangladesh, India, Laos, Mongolia, Pakistan, Palau, Sri Lanka, and Vietnam). These groupings allowed general comparisons of program implementation and provided peer countries an opportunity to exchange ideas and set future goals based on similar experiences.
At the second (Manila) workshop, participants were divided into three working groups to more efficiently identify and address issues of importance in continuing to build sustainable national NDBS programs. Group 1 focused on identifying strategies for increasing awareness and participation of health ministries in implementing and sustaining NDBS screening. Group 2 focused on identifying regional activities that might positively affect the expansion and improvement of NDBS screening. Group 3 focused on identifying activities within individual countries that could improve NDBS screening efficiency and assist in ensuring sustainability.
Activities and discussions at both workshops resulted in a shared vision among participants for improving screening activities within the region. Participants at the Manila meeting agreed to request the APSHG to recognize and support their activities by formalizing a Working Group on Consolidating Newborn Screening Efforts in the Asia Pacific Region within the Society. Meeting participants also renewed their support for the ideals expressed in the Cebu Declaration (Appendix 1) through their unanimous support of a new meeting output, the “Manila Declaration” (Appendix 2). Wording in this declaration approaches more strongly the work group participation of representatives of Ministries of Health in the various countries. While NDBS screening can exist without Ministry of Health support, this support is obligatory if the program is to become national, universal, and sustainable.
Results — current status of NDBS in the Asia Pacific region
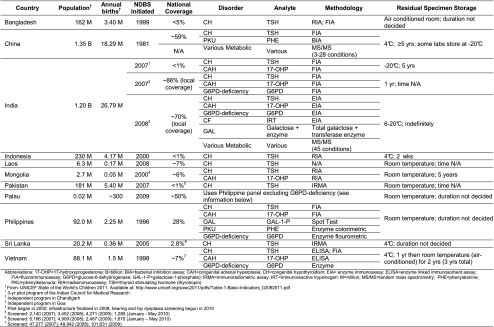
Table 1 lists the demographics of the countries involved, including their screening population and approximate national coverage. Table 2 contains an individualized summary of barriers to screening implementation and future short-term goals for program improvement. All 11 Asia Pacific countries report continuing NDBS progress despite significant barriers in some instances. A basic NDBS infrastructure including professional and parent education, screening laboratory, specimen transport, and result follow-up, exists within all participating countries, although this infrastructure is often limited in scope (i.e., not national) (Padilla et al. 2010). Functions within program infrastructures (such as testing and follow-up) are modeled after those of developed programs and suffer from some of the same challenges (locating patients, reliable and speedy specimen transport, etc.). All programs offer NDBS for congenital hypothyroidism (CH) and some include other disorders, including limited availability of tandem mass spectrometry (MS/MS) testing for metabolic disorders. There is a tendency to include congenital adrenal hyperplasia (CAH) in programs with more developed infrastructures (since fast result turnaround/patient follow-up is necessary). In most countries, this inclusion relates to initial funding assistance from the IAEA, which focused on screening methodologies that used nuclear techniques (such as radioimmunoassay). There is also an apparent (unexplained) high incidence of CAH (relative to other screenable conditions) in some Asian countries. Laws requiring NDBS or its offering now exist in some of the countries represented and significant efforts are being made throughout the region to include coverage of NDBS in national health insurance maternity benefits packages.
Table 1.
Summation of screening statistics reported by participants at the 2nd Conference on Consolidating Newborn Screening Efforts
Table 2.
Summation of current challenges and future goals reported by participants at the 2nd Conference on Consolidating Newborn Screening Efforts in the Asia Pacific Region
| Country | Barriers | Future Goals | Successes | |
|---|---|---|---|---|
| Bangladesh | • Health ministry not directly involved | • Prepare a new project proposal for continuation of the program after December 2011 | • Screening is free; increased public awareness | |
| • No priority in national healthcare system | • Integrate health ministry into the next expansion | • Continuing support from government, professionals, NGOs and international organizations | ||
| • Variable social economic pattern in population | • To implement the screening policy formulated by health ministry | • Improved coverage; some hospitals screen >90% of births | ||
| • No funds allocated after December 2011 | • Recall system established; more regular case follow-up | |||
| • Few health professionals directly involved | • New laboratory equipment; program reports published | |||
| • Policy makers agreed in principle to continue the program | ||||
| • 6 new screening labs proposed for national expansion | ||||
| China | • Imbalance in coverage geographically, likely resulting from economic variations | • Create provincial NDBS network across China; start in 2012 and complete by 2015 | • Some provinces are increasing the number of conditions screened beyond government recommendations | |
| • Early treatment and long-term follow-up not optimal | • To improve screening rates with two goals: | • The screening coverage as increased considerably over the past 10 years | ||
| 2012 | 2015 | |||
| • A screening fee exists which may inhibit screening in poorer areas in the West and Central Regions | East – 90% | East – 95% | • There are various research studies ongoing PAH deficiency and BH4 deficiency | |
| • Variability in MS/MS screening availability | Central – 50% | Central – 80% | ||
| West – 40% | West – 60% | |||
| • Upgrade the NDBS information system | ||||
| • Create national/provincial medical training centers | ||||
| • Professional information exchange through meetings, workshops and published studies have improved both diagnostic and research capabilities | ||||
| India | • Millennium Development Goals have not been attained; focus on NDBS not yet established | • To complete the Indian Council for Medical Research (ICMR) project on schedule | • NDBS project for CH and CAH funded by ICMR | |
| • Huge population; significant healthcare variation | • To approach the government for support with incidence and feasibility data following project data analysis | • 5 centers enrolled in CDC NSQAP; part of ICMR project | ||
| • State programs likely necessary before national due to healthcare variations | • To initiate a model screening program in selected states with low infant/neonatal mortality rate | • Consent begun; program currently considered as research | ||
| • Competition with health priorities; infant mortality | • Process to release results by 1 week (maximum of 2 weeks) after samples arrive in the laboratory is in place | |||
| • Political will not established; awareness promotion difficult | • G6PD high incidence confirmed in Delhi (19/1,000 births) | |||
| • Adequate financing not established | • Free NDBS since 2007 in Chandigarh (CH, CAH, G6PD) | |||
| • Screening began n 2008 in Goa [metabolics (MS/MS), CAH, CH, G6PD, CF, and GAL) | ||||
| Indonesia | • No government NDBS policy; lack of knowledge | • Implement NDBS in 10 provinces | • CH pilot studies conducted in two centers | |
| • Mobile population continually seeking better jobs | • Improve provincial capacity and capability | • NDBS Reference Center established under Ministries of Health and National Education | ||
| • High number of home births | • Include NDBS as a health insurance benefit | • Fee mechanism established | ||
| • No program infrastructure exists | • Expand to include newborn hearing screening | |||
| • Inadequate number of health care professionals | ||||
| • Screening fee exists; ~40% cannot afford screen | ||||
| • Limited confirmatory testing; only in urban areas | ||||
| Laos | • No local screening laboratory; using Germany | • Establish NDBS laboratory in the country | • A proposal for a pilot project screening 10,000 babies in Germany was approved and initiated | |
| • Lack of familiarity with NDBS processes by hospital staff; specimens collected too early | • To implement nationwide NDBS; coalition with private companies for public awareness (Note: Process approved and is beginning in 2011) | • A NDBS workshop was held; began the education process | ||
| • Screening card is in German language | • Shipping schedule initiated; specimens sent to Germany on Fridays by FedEx — abnormal results within 4–5 days | |||
| Mongolia | • Education/awareness lacking — health workers, parents/public, policy makers; policy-maker workshops have been poorly attended) | • To establish financial and legislative support | • Plan have been made to send samples to Germany for CH, CAH, BIO, GAL, and PKU via MS/MS; awaiting government approved | |
| • Remote pockets of population | • To install an integrated infrastructure | • Expanded screening planned; CH, CAH and CMV testing (~US$10), hearing screening(no charge) and hip dislocation (no charge) | ||
| • Model patient management strategies (counseling, treatment monitoring, long-term follow-up) not yet established | • To provide guidelines, policies, procedures, and evaluation techniques | • Hearing screening test guidelines have been developed and health professionals have been trained | ||
| • Some medications difficult to access, particularly for conditions like CAH | • To optimize the diagnostic and treatment capabilities in Ulaanbaatar | |||
| • To educate parents and train healthcare providers, policy-makers | ||||
| • To increase community awareness | ||||
| Pakistan | • Government support uncertain | • Standardize consent, sampling, follow-up and counseling procedures across facilities | • Screening laboratory participates in the CDC NSQAP | |
| • NDBS fee~US$2.35; confirmatory testing, free | • Establish more NDBS screening centers within the country | • CH cases have been detected and confirmed | ||
| • Universal lack of awareness | • Sampling and quality control procedures appear to be satisfactory; results are released quickly for both screening (4 days after collection) and confirmation (24 h) | |||
| • Screening coverage is very limited nationally | • Consent forms discontinued because parents felt that consent implied a potential for harm to the baby | |||
| • Standardized screening procedures are lacking | ||||
| • No consensus on treatment/follow-up strategies | ||||
| • Topical experts are lacking | ||||
| • High home births (65%) and consanguinity (60%) | ||||
| • One dedicated screening laboratory (Lahore) | ||||
| Philippines | • Funding is major problem for those without insurance; NDBS fee~US$12 for 5 tests (CH, GAL, PKU, G6PD, CAH); very low income society | • Increase coverage to 50% in 2009 and 85% in 2010 | • Screening costs remain low at US$12 [advocacy, screening (5 conditions) specimen transport, and recall] | |
| • Home deliveries are approximately 60% | • Include MSUD in the panel of disorders | • Four testing centers have been established and ~3000 hospitals are sending specimens | ||
| • Initiate a pilot study using MS/MS | • A midwifery training program is in place | |||
| • Numerous islands, mountains, and remote areas | • Notice sent that Department of Health will penalize hospitals not complying with the NDBS law | |||
| • High humidity and temperature presents challenge for some tests (e.g. CAH) | • Convince the National Health Insurance policy makers to cover everything including treatment (except confirmatory testing for G6PD deficiency due to high number of patients) | • Plans are being completed for a Master’s program in Genetic Counseling to begin in 2011 | ||
| • Lack of genetic counselors and specialists | • 10 G6PD confirmatory labs available; prices are controlled | |||
| • Produce one genetic counselor per province | ||||
| Sri Lanka | • Poor follow-up after initial tests in some areas | • Obtain health ministry approval for NDBS implementation | • Free screening exists in 2 government hospitals; and a charge of US$2 exists in private hospitals | |
| • Lack of government support has led to discouraged staff and decreased specimens | • Establish a laboratory network to provide testing for the entire country | • Selected hospitals in 2 provinces have local programs with all testing free of charge | ||
| • War has hampered progress | • Establish a Metabolic Screening Reference Laboratory in the private sector as an alternative (if no government support will be provided) | • The Ministries of Health and Higher Education are collaborating on the program | ||
| • Training lacking; specimen collection/submission | • Support from the IAEA was available from 2005 to 2009 | |||
| • Lack of awareness/support from some physicians | ||||
| • Changes in the health bureaucracy are slow | ||||
| • Manpower lacking; results released late (around 3–4 weeks); testing is once a week (Friday) | ||||
| • Issues on who should collect the specimens | ||||
| • 3 labs for country; transport issues persist | ||||
| Vietnam | • Early hospital discharge of newborns (<24 h) | • Increase awareness through television ads, pamphlets | • Develop NDBS Center in Hue to cover 7 provinces in center of country in 2009–2010 | |
| • Parents fear pain from heelstick for their baby | • Institutional workshops about NDBS | • National insurance plan to cover CH and G6PD | ||
| • Hot/humid weather negatively affects specimens | • Develop national plan for program consolidation | • Pilot study of MS/MS metabolic screening underway | ||
| • Specimen transport difficult – mountains, remote | • Develop government plan for national coverage by 3 centers – Hanoi, Ho Chi Minh City and Hue | |||
| • No fixed funding support | ||||
Significant strides have been made in China where approximately half of the newborn population now has access to NDBS for CH and phenylketonuria (PKU). This is important since China has the second largest number of births in the world. Screening is locally based with significant coverage in the Eastern China with increasing outreach to the West. Pilot testing with MS/MS is ongoing with recent findings in Shanghai suggesting a combined prevalence of 1:5,800 for all metabolic conditions detected by the MS/MS screen (Gu et al. 2008). The largest number of births by far occurs in India, where NDBS is still in its infancy. However, progress in India with formal pilot studies, a phased in approach, and increased government interest continue to increase screening availability. In India, there is also interest in expanded MS/MS screening in addition to expansion to other non-MS/MS screening tests and some laboratories are already offering MS/MS testing to private patients (Kapoor and Kabra 2010).
While most other countries in the region reported continual but slow progress, significant coverage increases were reported in the Philippines, where a national law now requires that NDBS be offered to all newborns (12th Congress of the Philippines. Republic Act 9288 – Newborn Screening Act of 2004). Four regional screening laboratories currently screen approximately half of the 2 million Philippine newborns (up from 30% reported at the 2010 Manila meeting). The regional screening center in Manila is also providing screening laboratory services for the small birth population in Palau, and it is likely that the Federated States of Micronesia will soon initiate a similar screening activity. Out-of-country laboratory services are a viable option in countries just beginning to screen or with small birth populations or limited technical capabilities. The Philippines initially used screening laboratory services from New South Wales, Australia, and pilot services from Hamburg, Germany, were reported to be currently ongoing in Laos and Mongolia.
Other challenges most often identified included geography (large land masses in China, India, and Mongolia; numerous islands in the Philippines, remote mountainous regions), cultural differences (religious, ethnic, regional, migratory), prioritization (versus infection, malnutrition, etc.), and education (professional, political and parental lack of program awareness). In some instances, competition from the private sector has led to difficulties in obtaining popular support. In at least one instance, government rules were reported to have been implemented to prevent fragmented testing in some hospitals that was disrupting national standardization efforts. While most developing programs recognized the need for organized data collection in order to demonstrate the value of NDBS activities, many noted that an automated centralized system of comprehensive data was not available and would be a worthwhile investment, should funding be available. All countries acknowledged and understood the need for good program quality control and most were willing participants in international laboratory proficiency testing programs. A formalized performance evaluation system was described in the Philippines (David-Padilla et al. 2009) modeled after a similar but more complex system in the United States (Therrell et al. 2010). Organized laboratory and program certification were identified as needs, and various examples of certification mechanisms within the region were discussed. These included an international technical review team combined with local health ministry certification in the Philippines and national laboratory proficiency testing in China among others.
Conclusion
A number of challenges appear to be universal in NDBS program implementation. Knowledgeable energetic leadership, financing, and health ministry support present the largest barriers uniformly identified within the region. Development of the regional screening network reported here has served to help identify and train champions for NDBS throughout the Region, but support of appropriate policy-makers within government and health ministry’s continues to be elusive in some settings. By inviting the appropriate government policy-makers at the health ministry level to the networking meetings, this challenge has begun to be overcome. The health ministry representatives who attended the Cebu and Manila meetings provided leadership in workgroup strategies and planning for continued involvement of their peers in ongoing NDBS activities.
The challenges of adequate and sustainable program financing remain unresolved in many programs. While NDBS service fees have been used for primary program support in China and the Philippines, dependence on government funding either directly or indirectly (through national insurance programs) appears to continue as a primary funding mechanism in some programs. Various models for financing were shared including local government loan programs and research grant support, but simple funding solutions were elusive. It is clear that in any of the models for success, awareness and inclusion/prioritization of NDBS in national health planning are essential for long term program stabilization and success.
While out-of-country laboratories can play a significant role in screening implementation, their activities have the potential to negatively impact a fledgling national program. This sometimes occurs when academic laboratories pursue newborn screening research agendas in particular locations (usually with a local academic institution) without proper attention to, or arrangements for, data sharing with an ongoing national implementation effort. The competitive environment thus created has the potential for slowing national progress in favor of local availability. Developed programs seeking to assist developing ones must be careful to create training activities that lead to infrastructure development so that services can be transitioned from the developed program to the developing. In this way, developing programs can take advantage of already ongoing developed screening efforts and more rapidly implement and expand their own programs.
Acknowledgements
The authors express special appreciation to members of the workshop planning and organizing committee for their support in helping to prepare this manuscript: Rommel Idmilao Sales, Joy Tumulak and Kathryn Ty. We also thank Vina Mendoza, Riza Suarez, Felix Alipasa, Jesus Sagun and Marian Reantaso for technical support before, during and after the meeting. We gratefully acknowledge the financial support of: The Institute of Human Genetics–National Institutes of Health–University of the Philippines Manila, the U.S. National Institutes of Health, the U.S. Centers for Disease Control and Prevention, Bio-Rad Southeast Asia, Lifeline Diagnostic Supplies Inc., GE Healthcare Pte Ltd, Perkin Elmer Southeast Asia. Other meeting participants included Wonlop Tunsura, Chad Ng, Joleen Seah, Marika Kase, Colin Hocking, Kok-Yiong Lam, Jasmine Torres and Wilkinson Tan.
*Members of the Working Group on Consolidating Newborn Screening Efforts in the Asia Pacific Region, Asia Pacific Society for Human Genetics:
Mizanul Hasan, Bangladesh; Tahmina Banu, Bangladesh; Faridul Ala, Bangladesh; Xuefan Gu, China; Madhulika Kabra, India; Seema Kapoor, India; Gurjit Kaur, India; Diet Sadiah Rustama, Indonesia; Mujaddid Djalal, Indonesia; Saysanasongkham Bounnack, Lao People’s Democratic Republic; Erdentuya Ganbaatar, Mongolia; Gerelmaa Zagd, Mongolia; Tariq Zafar, Pakistan; Anthony Calibo, Philippines; Lita Orbillo, Philippines; Eva Maia Cutiongco-de la Paz, Philippines; Sylvia Estrada, Philippines; J Edgar Winston Posecion, Philippines; Conchita Abarquez, Philippines; Maria Elouisa Reyes, Philippines; Damayanthi Nanayakkara, Sri Lanka; Le Anh Tuan, Vietnam; Ngo Toan Anh, Vietnam; Carmencita David-Padilla, Philippines; Bradford L. Therrell Jr., United States of America.
Glossary
- APSHG
Asia Pacific Society of Human Genetics
- ASEAN
Association of Southeast Asian Nations
- BH4
Tetrahydrobiopterin
- BIA
Bacterial immunoassay
- BIO
Biotinidase deficiency
- CAH
Congenital adrenal hyperplasia
- CDC
Centers for Disease Control and Prevention
- CF
Cystic fibrosis
- CH
Congenital hypothyroidism
- CMV
Cytomegalovirus
- ELISA
Enzyme-linked immunosorbent assay
- EPI
World Health Organization Expanded Program on Immunization
- FIA
Fluoro immunoassay
- FT4
Free thyroxin
- G6PD
Glucose-6-phosphate dehydrogenase
- GAL
Galactosemia
- GAL-1-P
Galactose-1-phosphate
- GALT
Galactose transferase deficiency
- IAEA
International Atomic Energy Agency
- ICMR
Indian Council for Medical Research
- IRMA
Immunoradiometric assay
- IRT
Immunoreactive trypsinogen
- ISNS
International Society for Neonatal Screening
- IYCF
World Health Organization Global Bank on Infant and Young Child Feeding
- JICA
Japanese International Cooperation Agency
- N/A
Not available
- NCBS
Newborn cord blood screening
- NDBS
Newborn dried bloodspot screening
- NIH
National Institutes of Health
- NNSGRC
National Newborn Screening and Genetics Resource Center (United States)
- NSQAP
Newborn Screening Quality Assurance Program
- NSRC
Newborn Screening Reference Center (Philippines)
- MS/MS
Tandem mass spectrometry
- MSUD
Maple syrup urine disease
- PAH
Phenylalanine hydroxylase
- PHE
Phenylalanine
- PKU
Phenylketonuria
- RIA
Radioimmunoassay
- SAARC
Southeast Asian Association for Regional Cooperation
- T4
Thyroxine
- TR-FIA
Time resolved fluoroimmunoassay
- TSH
Thyrotropin (thyroid-stimulating hormone)
- UNFPA
United Nations Fund for Population Activities (now called the UN Population Fund)
- UNICEF
United National International Children’s Emergency Fund (now called the UN Children’s Fund)
- USAID
United States Agency for International Development
- WHO
World Health Organization
Appendix 1
Cebu Declaration
01 April 2008
Cebu, Philippines
Preamble
In countries with depressed and developing economies, such as in Asia, newborn screening and infant screening is either not yet a priority or just emerging as a priority. A group of 11 countries (Bangladesh, China, India, Indonesia, Laos, Mongolia, Pakistan, Palau, Philippines, Sri Lanka, Vietnam) participated in a workshop called “Consolidating Newborn Screening Efforts in the Asia Pacific.” The participants of this meeting recognize that children’s health is a high priority for our countries. Newborn screening coverage in Asia and the Asia Pacific region remains low at 10% of babies born within the region. As an output of this workshop, the participants wish to put forward this Cebu Declaration.
Declaration
The international community has achieved important advances in infant survival and the reduction of neonatal mortality. As a consequence, in view of the United Nation’s Convention on the Rights of the Child (1989), governments must now focus increased attention on assuring our children’s optimal development and to put in place policies to ensure that tomorrow’s adults are as free as possible from disability that will limit achieving their potential. This is facilitated by early screening for congenital genetic disorders that are responsible for major disability; if not treated early, the costs of treatment of preventable disability will be prohibitive for society and the lives of children and their families will be tragically and unnecessarily limited. Systematic newborn screening for these genetic disorders is, thus, a necessity for public health programs based on the resources available.
Newborn screening is an important tool in the prevention of disease and disability in our children and thus should be a key part of a comprehensive public health system in all of our countries. Each country should prioritize the panel of screening disorders and system of care that is appropriate to their situation. Based on the meeting’s deliberations, the following recommendations have received high priority:
Encourage all countries to develop policies and provide necessary support to establish a systematic national newborn screening program within the context of a global national policy for children’s health that will provide access to all newborn infants in these countries and provide follow-up services. Such services should integrate both public and private health care delivery systems.
Screen nationally for at least one condition in all newborns and develop a national screening model program that takes into account all aspects for post-testing care.
Establish national research priorities around newborn screening, through culturally relevant and ethical strategies.
Reduce disability and death by assuring that the children identified as having screened positive for a genetic disorder have the opportunity to a good quality of life through access to medical treatment including behavioral, physical therapeutic interventions as well as assistive technology in order to preserve healthy development and improve autonomy and independence.
Develop population studies to determine the incidence of genetic disorders in the region and consider linking to national databases with standardized measurements. (Clearly, population genetic data needs to be accumulated country by country as it is anticipated that each country will have unique disorders related to their own population.)
Begin regionalization and cooperation among countries by sharing of expertise, information, and other resources.
Develop training programs that focus on role-specific activities that build the interdisciplinary teams needed for newborn screening systems of care.
Stimulate regional research capacity that addresses the specific conditions of priority to Asia and the Asia Pacific.
In view of all of the above recommendations, the attendees recognize the need to establish collaborative, cooperative networking to facilitate the development of newborn screening systems for all nations;
In order to develop such a collaborative network it would be of value to:
Hold periodic (e.g., every 18 months) meetings to assess country advances.
Develop smaller focused meetings on issues of particular importance (e.g., training).
Establish structures for increased communication across the region including a regional website and biennial regional meetings.
Establish an advisory committee to set up an agenda for addressing the recommendations identified above.
Establish working groups that can implement identified priorities.
Involve other professional groups interested in quality child health care.
Appendix 2
Manila Declaration
05 June 2010
Manila, Philippines
The participants of the workshop entitled “2nd Workshop on Consolidating Newborn Screening Efforts in the Asia Pacific Region” (composed of representatives from Bangladesh, China, India, Indonesia, Laos, Mongolia, Pakistan, Philippines, Sri Lanka, and Vietnam with expert assistance from the United States) held on June 4–5, 2010 in Manila, Philippines, reaffirm their commitment to the principles and provisions listed in the Cebu Declaration (Appendix 1).
In addition, the group resolves to continue their activities in implementing and refining newborn screening in the Asia Pacific Region through the following actions:
At the Ministry of Health level, to:
Develop national policies on newborn screening.
Develop health financing schemes for newborn screening.
Partner with local and international funding agencies [USAID (United States Agency for International Development), WHO (World Health Organization), UNICEF (United National International Children’s Emergency Fund — now called the UN Children’s Fund), UNFPA (United Nations Fund for Population Activities — now called the UN Population Fund), JICA (Japanese International Cooperation Agency), etc.].
Integrate newborn screening with existing child health programs [IYCF (WHO Global Bank on Infant and Young Child Feeding), EPI (WHO Expanded Program on Immunization), etc.].
At the country level, to:
Involve policy-makers in activities related to newborn screening.
Communicate with various stakeholders, i.e., policy-makers, non-government organizations (NGOs), newborn screening product vendors, parents.
Promote advocacy on newborn screening at all levels.
Integrate newborn screening training into undergraduate and postgraduate curricula.
Create a central newborn screening database in each country.
Organize regular monitoring and review of the newborn screening program.
Participate in external newborn screening laboratory proficiency testing programs.
Network with other countries and experts having similar newborn screening interests.
Begin to address the issues of storage and use of residual dried blood spots.
At the regional level, to:
Create a “Working Group on Consolidating Newborn Screening Efforts in the Asia Pacific Region” within the Asia Pacific Society on Human Genetics (APSHG) with every country represented by at least one newborn screening manager and one Ministry of Health representative.
Encourage ASEAN (Association of Southeast Asian Nations) and SAARC (Southeast Asian Association for Regional Cooperation) to prioritize newborn screening in their government health agenda.
Motivate existing regional professional societies and NGOs to promote newborn screening: ISNS (International Society for Neonatal Screening), APSHG, pediatric societies, obstetric societies, perinatal and neonatal societies, Asia Pacific endocrine societies, nuclear medicine societies, Asian Association of Pediatric Societies, etc.
Conference participants recognize and embrace the need to continue collaborative, cooperative networking to facilitate the development of a quality newborn screening system in all nations. In order to facilitate continuation of such a collaborative network, it is of value to:
Establish structures for increased communication across the region including a regional website and biennial regional meetings.
Establish a regional advisory committee to set up an agenda for addressing the recommendations identified above
Footnotes
Members of the Working Group the Asia Pacific Society for Human Genetics on Consolidating Newborn Screening Efforts in the Asia Pacific Region are listed at the end of the paper.
References
- 12th Congress of the Philippines. Republic Act 9288 – Newborn Screening Act of 2004. Available at: http://www.doh.gov.ph/ra/ra9288.html (Accessed September 1, 2011)
- David-Padilla C, Basilio JA, Therrell BL. A Performance Evaluation and Assessment Scheme (PEAS) for improving the Philippine Newborn Screening Program. Acta Medica Philipp. 2009;43(2):58–63. [Google Scholar]
- Gu X, Wang Z, Jun Y, Han L, Qiu W. Newborn screening in China: phenylketonuria, congenital hypothyroidism and expanded screening. Ann Acad Med Singap. 2008;37(Suppl 3):107–110. [PubMed] [Google Scholar]
- Kapoor S, Kabra M. Newborn screening in India: current perspectives. Indian Pediatr. 2010;47:219–223. doi: 10.1007/s13312-010-0043-0. [DOI] [PubMed] [Google Scholar]
- Krotoski D, Namaste S, Raouf RK, et al. Conference report: second conference of the Middle East and North Africa newborn screening initiative: partnerships for sustainable newborn screening infrastructure and research opportunities. Genet Med. 2009;11(9):663–668. doi: 10.1097/GIM.0b013e3181ab2277. [DOI] [PubMed] [Google Scholar]
- Padilla CD. Towards universal newborn screening in developing countries: obstacles and the way forward. Ann Acad Med Singap. 2008;37(Suppl 3):6–9. [PubMed] [Google Scholar]
- Padilla CD, Therrell BL. Newborn screening in the Asia Pacific Region. J Inherit Metab Dis. 2007;30:490–506. doi: 10.1007/s10545-007-0687-7. [DOI] [PubMed] [Google Scholar]
- Padilla CD, Krotoski D, Therrell BL. Newborn screening progress in developing countries – overcoming internal barriers. Semin Perinatol. 2010;34:145–155. doi: 10.1053/j.semperi.2009.12.007. [DOI] [PubMed] [Google Scholar]
- Solanki KK. Training programs for developing countries. J Inherit Metab Dis. 2007;30(4):596–599. doi: 10.1007/s10545-007-0680-1. [DOI] [PubMed] [Google Scholar]
- Therrell BL, Schwartz M, Southard C, Williams D, Hannon WH, Mann MY, et al. Newborn Screening Performance Evaluation Assessment Scheme (PEAS) Sem Perinatol. 2010;34(2):105–120. doi: 10.1053/j.semperi.2009.12.002. [DOI] [PubMed] [Google Scholar]
- UNICEF. The State of the World’s Children 2011. Statistical tables. United Nations Children’s Fund (UNICEF), February 2011, New York, USA pp. 88–91. Available at http://www.unicef.org/sowc2011/pdfs/SOWC-2011-Main-Report_EN_02092011.pdf