Background
Population-based screening for carrier status of genetic conditions is increasingly becoming available in developed countries with future directions including carrier testing for large numbers of genetic conditions in a single instance using next generation technologies (Bell et al. 2011). In Australia, population-based carrier screening is undertaken widely for haemoglobinopathies (Metcalfe et al. 2007) and is offered in some parts of the country for cystic fibrosis (CF) (Christie et al. 2006; Massie et al. 2009). In addition, screening is available in some states for conditions more prevalent in Ashkenazi Jewish populations, such as Tay Sachs disease (Gason et al. 2005; Barlow-Stewart et al. 2003). Fragile X syndrome (FXS) and spinal muscular atrophy have also been identified as conditions for which carrier screening could be offered (Delatycki 2008). Despite a population of approximately 21 million, there are no national screening programmes in Australia; genetics services and screening programmes vary from state to state (Metcalfe et al. 2009).
Although a range of factors are important to consider when developing population-based carrier screening programmes, a challenge is how to address the apparent lack of community awareness and knowledge about genetic conditions, in particular, the risk of being a carrier. Thus, it is recognised that education is an important component of population-based genetic screening programmes. Further, consideration of psychosocial aspects, such as decision making, attitudes, and understanding, should be incorporated into any evaluation of the feasibility and acceptability of such a programme (Godard et al. 2003).
In the state of Victoria, Australia, research investigating population-based carrier screening for CF and FXS has involved needs assessments incorporating exploration of a wide range of factors which may inform decisions about implementation of screening programmes for both of these conditions (Archibald et al. 2009; McClaren et al. 2008; Metcalfe et al. 2008).
Fragile X population-based carrier screening for non-pregnant women
FXS is the leading cause of inherited intellectual disability. The carrier frequency in females is estimated to be 1 in 178 (Hantash et al. 2011). There is a spectrum of clinical effects associated with FXS which includes learning, behavioural, emotional and medical problems, the most significant usually being cognitive disabilities and autistic-like behaviours. Female carriers of the premutation are at risk of having a child with FXS, may experience reduced fertility due to fragile X-associated primary ovarian insufficiency (Allingham-Hawkins et al. 1999) and are also at risk of fragile X-associated tremor ataxia syndrome (FXTAS), although the risk of FXTAS is greater for male carriers than for female carriers (Hagerman et al. 2001). Due to concern about the multiple phenotypes and complexity of fragile X-associated conditions, current guidelines state that population screening for FXS be offered as part of ‘well-defined clinical research protocols’ (McConkie-Rosell et al. 2005; Sherman et al. 2005). Recently, a pilot programme has offered fragile X carrier screening to non-pregnant women in Victoria (Metcalfe et al. 2008), and this research is ongoing.
Cystic fibrosis population-based carrier screening for couples planning pregnancy or in the early stages of pregnancy
In January 2006, a fee-for-service programme commenced offering population-based CF carrier screening to couples either planning a pregnancy or at ≤14 weeks gestation (Massie et al. 2007; Ioannou et al. 2010). Cystic fibrosis has a birth prevalence of 1 in 3,500 and a carrier frequency of 1 in 25 in Caucasians making it the most common severe recessive condition in children (Southern et al. 2007). Cystic fibrosis is characterised by chronic suppurative lung disease, pancreatic exocrine insufficiency and elevated sweat electrolytes (O’Sullivan and Freedman 2009). Due to improvements and advances in treatment, average life expectancy of people with cystic fibrosis has increased; there is a reported range of average life expectancy of around 30 years of age up to 50 years of age (O’Sullivan and Freedman 2009; Dodge et al. 2007). There is, however, no cure for cystic fibrosis. In addition to people with a family history of CF, guidelines recommend offering CF carrier screening to pregnant couples or couples planning a pregnancy (National Institutes of Health 1999; Human Genetics Society of Australasia Genetic Services Committee 2009).
In two recent studies conducted in Victoria evaluating psychosocial aspects of population-based carrier screening (Archibald et al. 2009; McClaren et al. 2008), similarities were observed with respect to decision making in individuals from the general population. Qualitative approaches were used in both studies as the research questions were exploratory in nature. The studies were conducted independently and aimed to describe and understand participants’ interpretations of the issues around population-based carrier screening, particularly focusing on views about screening, as well as barriers and facilitators to decision making. The FXS study involved interviews with 31 non-pregnant women who had been offered carrier screening as part of a pilot study (Archibald et al. 2009). The CF study sought participation, through interviews and focus groups, from 68 members of various ‘stakeholder’ groups of population-based carrier screening, including both male and female participants, none of whom were offered screening (McClaren et al. 2008). These were: pregnant women and their partners (n = 15), individuals and couples prior to pregnancy (n = 19), health professionals (n = 12) and people with a family history of the condition (n = 22). Further detail of recruitment of participants is described elsewhere (McClaren et al. 2008; Archibald et al. 2009). Participants in the CF study were not offered carrier screening; rather, they were asked to consider the issues hypothetically whereas the FXS study did offer carrier screening and participants were interviewed about their experiences of this offer.
In this commentary, we describe similarities in perceptions about decision making observed in these two studies, despite their differences in design, and discuss the implications for the development of population-based carrier screening programmes. Variation in the design of these studies, specifically hypothetical vs. actual consideration of carrier screening, must be acknowledged. However, the purpose of this commentary is not to directly compare findings or provide new evidence but rather to discuss in greater detail observations made as a result of previous qualitative research and to highlight their potential implications. In doing so, this commentary aims to promote further consideration and discussion of the role of prior experience in decision making with respect to carrier screening for genetic conditions.
Perceived relevance: the role of reproductive stage of life and health-related life experience in decision making
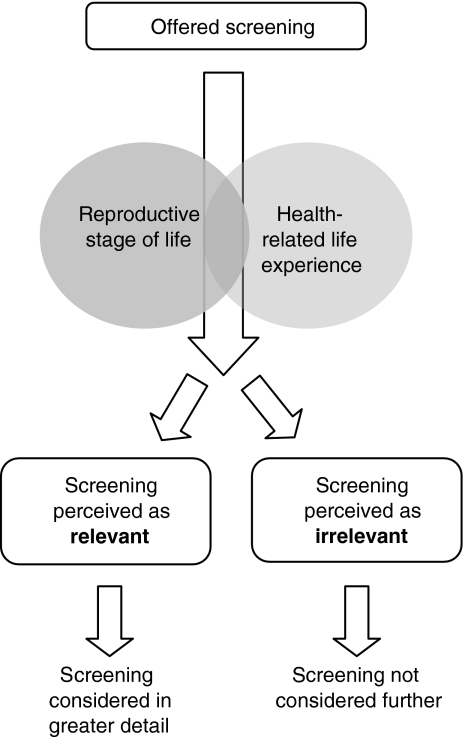
During analysis of dialogue from the focus group and interview data from the two studies described above, we observed that for people offered a genetic test, there may be multiple steps along the pathway to ultimately making a decision about whether or not to have the genetic test on offer. It was observed that participants commonly made an initial judgement regarding the relevance of the genetic test for them. This initial judgement of relevance centred on two key areas: the participant’s reproductive stage of life and the presence or absence of health-related life experiences. Based on their perception of relevance, participants would either consider screening in more detail or not consider screening further (Archibald et al. 2009). This staged approach to decision making is illustrated in Fig. 1, and future research is needed to explore stages of decision making in more detail.
Fig. 1.
Role of reproductive stage of life and health-related life experience in perceiving relevance of a genetic screening test. Adapted from Archibald et al. (2009)
Research indicates that personal experiences are key factors influencing screening decisions (Etchegary et al. 2008) and can play a greater role in decision making than technical and clinical information (d’Agincourt-Canning 2005; Tercyak et al. 2001; Etchegary et al. 2008). Further, the process of learning new information is grounded in experience, that is to say, the information that one takes in will be influenced by and interpreted based on prior relevant experiences (Kolb 1984). Personal experiences and experience gained through a connection with others who have personal experience, also described as experiential knowledge, play an important role in decision making about health (Ziebland and Herxheimer 2008; Etchegary et al. 2008). When considering prenatal diagnostic testing, it has been observed that women weave medical information with their own personal experiences, feelings and beliefs to negotiate decisions about testing (Lippman 1999). Experiential knowledge is both subjective and objective and is gained through personal and interpersonal experiences; in the present context, this includes experience with health-related matters, genetic conditions and disability. Abel and Browner (1998) describe two types of experiential knowledge: embodied and empathic. Embodied experiential knowledge is that originating from a personal experience, whereas empathic experiential knowledge is that which occurs from a connection with others who are experiencing an event (Abel and Browner 1998; Etchegary et al. 2008). When participants in both the FXS and CF studies were presented with the concept of genetic testing, they commonly drew on both embodied and empathic experiential knowledge to assist in determining whether the genetic test was salient. The use of embodied and empathic experiential knowledge observed in the two studies is illustrated in the examples provided in Table 1 and can be classified into the following five approaches to perceiving relevance of a genetic test offered in a population-based carrier screening programme (Table 2):
-
I know nothing about this topic; therefore, I think genetic testing is not relevant for me.
Absence of health-related life experiences may lead individuals to perceive a genetic screening test as irrelevant to them and individuals may dismiss the offer of the test with little consideration. This perspective is potentially the most problematic approach when offering population-based genetic screening. It is widely agreed that when offering genetic tests, it is important for individuals to make autonomous decisions, based on sufficient knowledge, that are consistent with their own beliefs and values and that there has been an opportunity for the individual to deliberate (van den Berg et al. 2006). However, if a person perceives that screening is not relevant to them before fully weighing up the pros and cons of having the test, they may have missed the opportunity to make an informed decision.
-
I know something about a genetic condition/a disability/a health problem; therefore, I think genetic testing might be relevant for me.
We observed that prior health-related life experiences appeared to spark a person’s interest in learning more about the genetic test, leading to them to consider screening in greater detail. However, unless that experiential knowledge is similar to the condition being screened, there would be potential for confusion and misinformed decisions. For example, as seen in the CF study, some women perceived CF carrier screening to be relevant to them because they incorrectly associated it with advanced maternal age and increased risk of a Down syndrome pregnancy. This might be expected if CF screening was offered in a prenatal setting, along with other tests including screening for Down syndrome; however, even in the context of discussing preconception CF carrier screening, participants drew a connection to advanced maternal age.
-
I know something about this genetic condition; therefore, I think genetic testing might be relevant for me.
Having knowledge about the specific genetic condition could be particularly useful in making a decision about the genetic test being offered and has the potential to lead to well-informed decisions. However, for many genetic conditions, including FXS and CF, there is substantial variation in severity, and therefore, peoples’ perceptions about the way in which a genetic condition affects an individual may vary.
-
I am pregnant/thinking of having children (soon); therefore, I think genetic testing might be relevant for me.
Our data indicated that participants, not unreasonably, associated genetic carrier tests for both FXS and CF with reproduction and those who were pregnant or planning to have children in the near future appeared to be more interested in contemplating screening. Although the genetic status of an individual does not change and thus genetic testing is applicable to an individual or couple regardless of their stage of life, it may be that this is not always grasped by members of the general population due to a lack of awareness of genetic testing and its implications. Thus, the timing of the offer of testing becomes an important consideration as it can impact on the extent to which an individual will perceive screening to be beneficial to them. If screening is offered before pregnancy, there are a range of reproductive options available to the individual/couple as well as time to consider those options and make decisions, whereas in pregnancy options are more limited and time for decision making is reduced. Although gaining knowledge of carrier status prior to pregnancy is ideal, this situation is less likely to occur at present due to limited awareness of carrier screening and lack of widespread availability of preconception screening. In order to offer screening at a time that is perceived to be relevant and beneficial to learn one’s carrier status, more research and resources are needed to determine whether to and how to approach offering carrier screening at multiple stages of life.
-
I am not thinking of having children (soon); therefore, I think genetic testing is not relevant for me.
As stated above, it was observed that individuals often perceive genetic carrier screening to be related to reproduction and consequently consider it with respect to their reproductive stage of life. Although those who decline screening due to a perceived lack of relevance may take up the offer of screening at a later date, this approach does not take into account the possibility of an unplanned pregnancy. Further, if an individual chooses to defer their consideration of carrier screening to a later date, perhaps when planning a pregnancy, strategies would need to be put in place to ensure these individuals know how to access the test when it becomes more relevant to them.
Table 1.
Examples of experiential knowledge which influenced consideration of carrier screening
| Examples from the FXS study (Archibald et al. 2009) | Examples from the CF study (McClaren et al. 2008) | |
|---|---|---|
| Health-related life experience | A woman who learnt she had haemochromatosis after a prolonged search for an explanation for her health problems was highly motivated to consider FXS carrier screening. | A man would not consider CF carrier screening because he has had relatively good health to date. |
| A woman who recalled feeling frustrated about having missed the opportunity to have first trimester screening for Down syndrome for her first pregnancy was eager to consider FXS carrier screening. | A woman from the CF study discussed her enthusiasm about the idea of being offered testing ‘for everything’, including CF carrier screening, because of prior experience of recurrent miscarriages. | |
| A woman who works with children with special needs spoke of her motivation to consider FXS carrier testing because of her perceptions of the impact of disability on families. | ||
| Reproductive stage of life | A woman who, at 23 years of age, did not consider having FXS carrier screening because she was not currently planning to have a family. | A woman who commented that she would be motivated to think about CF carrier screening because she was planning to have a child. |
Table 2.
Approaches to perceiving relevance of population carrier screening based on presence or absence of experiential knowledge
| Genetic carrier screening might be relevant for me | Genetic carrier screening is not relevant for me | |
|---|---|---|
| Health-related life experience | I know something about a genetic condition/a disability/a health problem… | I know nothing about this topic… |
| I know something about THIS genetic condition… | ||
| Reproductive stage of life | I am pregnant/thinking about having children (soon)… | I am not thinking about having children (soon)… |
Implications for population-based genetic carrier screening programmes
We observed that people may use a combination of experiential knowledge, embodied and empathic, as a way of perceiving relevance, or primarily use one or the other. It is therefore important to consider the use of such experiences when making the offer of carrier screening at a population level. Drawing on personal or interpersonal experiences may mean that two people of similar a priori risk make different decisions regarding carrier testing based on their own perceptions of relevance created through experiential knowledge, and in some cases, these perceptions may be misinformed. We believe it is crucial that the role of experiential knowledge in influencing individuals’ perceptions of relevance is incorporated into the development of educational materials and genetic counselling approaches in population-based genetic carrier screening programmes.
We suggest that experiential knowledge may play a role in subconscious filtering of information about screening occurring before a formal decision-making process involving weighing the pros and cons of screening. Factors influencing decision making about genetic carrier screening have been widely investigated, particularly in the context of carrier screening for CF. Research indicates that a variety of factors play a role in screening decisions such as perceptions of the value of knowing carrier status, particularly for reproductive planning; the extent to which individuals perceive themselves to be at risk of receiving a carrier result and the severity of the condition. Other factors also impacting on screening decisions include parity and intention to have children, views of healthcare providers and level of knowledge (Chen and Goodson 2007). Interestingly, perceptions of the severity of the genetic condition appear to influence decision making in population-based carrier screening to a lesser extent (Janz and Becker 1984) which may potentially be due to lack of personal experience of the condition (Sheeran and Abraham 1995). Additionally, perceptions of relevance influenced by experiential knowledge may underlie perceptions of risk.
It is often difficult to ascertain the proportion of people who choose not to participate in genetic screening research or in fact choose not to have a genetic test. There are many examples where uptake of screening has been demonstrated to be influenced by the presence or absence of barriers and the way such screening is offered, with active approaches yielding higher uptake than passive approaches (Bekker et al. 1993; Clayton et al. 1996; Hill et al. 2010; Archibald and Wilfond 2006; Metcalfe et al. 2008; Cronister et al. 2005; Henneman et al. 2003). Despite best efforts to target screening to populations for whom it is most relevant based on life stage or a priori risk, there is always a proportion of people who opt not to consider screening. It is likely that some of these individuals have perceived the offer of a genetic test as being irrelevant to them and therefore have chosen not to consider testing. Conversely, it is possible that those who have screening, even when offered using passive approaches, are those who most strongly draw on their life experience and/or life stage, no matter the context, and choose to have screening. For these people, their decision may be informed by factors that have little or no relevance to the context in which they are making the decision and therefore may not be considered to be an ‘informed decision’. Future research is necessary to ascertain the extent to which perceptions of relevance play a role in whether or not people consider genetic carrier screening for themselves.
These findings pose challenges for the implementation of screening programmes: how might ‘perceived relevance’ be incorporated into decision-making about screening? Can perceptions of relevance be influenced by providing personal accounts of genetic conditions to simulate experiential knowledge? How do we offer a screening programme that allows for changing stage of life with time? For example, people offered screening one year may perceive screening to be irrelevant, but in time, with changing circumstances, their perceptions may change. For those who perceive screening as relevant, how do we avoid misinformed decisions due to inadequate knowledge gained through prior experience? We believe perceptions of relevance based on experiential knowledge could be problematic for informed decision making because by perceiving the test as irrelevant, or by perceiving it as relevant but making a decision based on misinformation, the opportunity for informed decision making could be limited. We are not suggesting that people have genetic tests per se but rather that people are provided with the opportunity to make an informed decision about whether or not to have a test.
These observations illustrate challenges of population-based screening and suggest the need for educational and counselling approaches tailored to the general population. In order to facilitate informed decisions, the role of perceived relevance in decision making should be incorporated into genetic carrier screening programmes. Potential avenues for doing so are summarised in Box 1. Pre-test information should provide insight into the ‘lived experience’ of the condition so that those offered screening can formulate a perception of what a carrier result would mean for them and their families. Information about the condition could be presented using vignettes or videos of people with the condition and their families which may help personalise the information. Ziebland and Herxheimer (2008) discuss the use of a website (www.heatlhtalkonline.org) which provides narratives from people with a range of medical conditions. Other examples exist relating specifically to genetic conditions such as the ‘telling stories’ website (www.tellingstories.nhs.uk).
In developing this information for screening programmes, it is essential that it occurs in collaboration with patient organisations and support groups to assist in gathering stories and in ensuring that the information provided is an accurate reflection of the condition (Godard et al. 2003). In addition to providing avenues to create experiential knowledge, individuals could be encouraged to critically reflect on their prior experiences to consider how these might be influencing their perceptions of the carrier screening test. Critical reflection is a key component of adult learning and is a process whereby individuals explore the basis for assumptions and reframe preconceptions in the presence of new information (Brookfield 1996). Health professionals offering screening (including genetic counsellors) can be instrumental in supporting individuals to reflect on experiences of health-related matters (particularly, of disability) and how this impacts on perceptions of the genetic screening test and choices made (Hodgson and Weil 2011). In a population-based screening setting, experiential knowledge may be more challenging to incorporate into the offer of screening compared to a clinical setting. Some potential modes of doing so have been suggested in Box 1.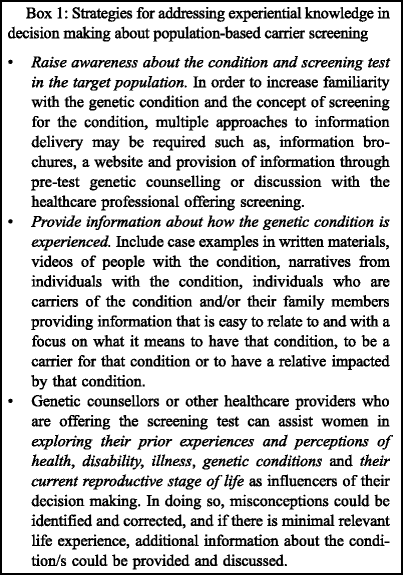
New approaches to testing will mean moving away from offering a single test for a single condition, to offering one test for many conditions (Bell et al. 2011). A particular challenge will be determining how to provide information for people to make a decision about testing, especially in the absence of relevant life experiences. Providing the ‘lived experience’ of each individual condition will not be possible. A recent approach in the newborn screening setting, in which testing for multiple conditions was offered, involved providing information on categories of conditions in the general terms ‘treatable’, ‘less treatable’ and ‘untreatable’ and gave one specific example for each category (Plass et al. 2010). Similarly, population-based carrier screening in which multiple conditions are tested for could involve providing information on the experience of a specific example within a category of conditions such as neuromuscular conditions (i.e. Duchenne muscular dystrophy or spinal muscular atrophy). In which case, information could focus on the pathway of delayed development, loss of previous function such as ambulation and the impact on daily life of the individual and the family. In addition to provision of information about the personal and family experience of the condition, those offered screening could be encouraged, through information materials, to consider the experience of receiving a carrier result.
Population-based carrier screening brings with it a number of complexities related to the lack of knowledge and awareness of genetic conditions in the general population. With advances in technology and the expanding scope of genetic carrier screening at the population level, it is unlikely that individuals from the general population offered screening will have much knowledge about the conditions for which they are being offered screening. Consequently, we suggest that prior experiences and personal situations may influence individuals’ perceptions of the relevance of screening and, if undergoing testing, their interpretation of their results. This could bring with it additional challenges for the large scale education that is needed if such tests are offered at a population level. In order to ensure screening is accessible and decision making is facilitated, screening programme development could include creative strategies to incorporate and address the role of experiential knowledge in the decision-making process.
Acknowledgements
The authors would like to express their appreciation to the participants in both studies for taking the time to share their views and perspectives with us. We would like to thank A/Prof Sylvia Metcalfe for her helpful comments on this manuscript and involvement in both the FXS and CF studies. We would also like to thank the following: Dr Samantha Wake, Dr Alice Jaques, and Dr Veronica Collins (FXS study) and Dr Mary Anne Aitken, Professor Martin Delatycki and Dr Veronica Collins (CF study). We thank Dr Jan Hodgson for her valuable feedback on an earlier version of this manuscript. These studies were supported by the Fragile X Alliance Inc., Murdoch Childrens Research Institute, the University of Melbourne and the Victorian Government’s Operational Infrastructure Support Program. While undertaking this work, Alison Archibald was supported by an Australian Postgraduate Award scholarship, and Belinda McClaren was supported by a Melbourne Research Scholarship.
Conflicts of interest The authors declare that they have no conflicts of interest.
Contributor Information
Alison D. Archibald, Phone: +61-3-83416331, FAX: +61-3-83416207, Email: alison.archibald@mcri.edu.au
Belinda J. McClaren, Email: belinda.mcclaren@mcri.edu.au
References
- Abel K, Browner C. Selective compliance with biomedical authority and the uses of experiential knowledge. In: Lock M, Kaufert P, editors. Pragmatic women and body politics. Cambridge: Cambridge University Press; 1998. pp. 310–326. [Google Scholar]
- Allingham-Hawkins DJ, Babul-Hirji R, Chitayat D, Holden JJ, Yang KT, Lee C, Hudson R, Gorwill H, Nolin SL, Glicksman A, Jenkins EC, Brown WT, Howard-Peebles PN, Becchi C, Cummings E, Fallon L, Seitz S, Black SH, Vianna-Morgante AM, Costa SS, Otto PA, Mingroni-Netto RC, Murray A, Webb J, MacSwinney F, Dennis N, Jacobs PA, Syrrou M, Georgiou I, Patsalis PC, Giovannucci U, Guarducci S, Lapi E, Cecconi A, Ricci U, Ricotti G, Biondi C, Scarselli B, Vieri F. Fragile X premutation is a significant risk factor for premature ovarian failure: the International Collaborative POF in Fragile X study—preliminary data. Am J Med Genet. 1999;83(4):322–325. doi: 10.1002/(SICI)1096-8628(19990402)83:4<322::AID-AJMG17>3.0.CO;2-B. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Archibald AD, Wilfond BS. Population carrier screening: psychological impact. New York: Wiley; 2006. [Google Scholar]
- Archibald AD, Jaques AM, Wake S, Collins VR, Cohen J, Metcalfe SA. “It’s something I need to consider”: decisions about carrier screening for fragile X syndrome in a population of non-pregnant women. Am J Med Genet A. 2009;149A(12):2731–2738. doi: 10.1002/ajmg.a.33122. [DOI] [PubMed] [Google Scholar]
- Barlow-Stewart K, Burnett L, Proos A, Howell V, Huq F, Lazarus R, Aizenberg H. A genetic screening programme for Tay–Sachs disease and cystic fibrosis for Australian Jewish high school students. J Med Genet. 2003;40(4):e45. doi: 10.1136/jmg.40.4.e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bekker H, Modell M, Denniss G, Silver A, Mathew C, Bobrow M, Marteau T. Uptake of cystic fibrosis testing in primary care: supply push or demand pull? Br Med J. 1993;306(6892):1584. doi: 10.1136/bmj.306.6892.1584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell CJ, Dinwiddie DL, Miller NA, Hateley SL, Ganusova EE, Mudge J, Langley RJ, Zhang L, Lee CC, Schilkey FD. Carrier testing for severe childhood recessive diseases by next-generation sequencing. Sci Transl Med. 2011;3(65):65ra64. doi: 10.1126/scitranslmed.3001756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brookfield SD. Adult learning: an overview. In: Tuijman AC, editor. International encyclopedia of education and training. 2. Tarrytown: Pergamon; 1996. [Google Scholar]
- Chen LS, Goodson P. Factors affecting decisions to accept or decline cystic fibrosis carrier testing/screening: a theory-guided systematic review. Genet Med. 2007;9(7):442. doi: 10.1097/GIM.0b013e3180986767. [DOI] [PubMed] [Google Scholar]
- Christie LM, Zilliacus EM, Ingrey AJ, Turner G. Screening couples for cystic fibrosis carrier status: why are we waiting? Med J Aust. 2006;184(9):477. doi: 10.5694/j.1326-5377.2006.tb00331.x. [DOI] [PubMed] [Google Scholar]
- Clayton EW, Hannig VL, Pfotenhauer JP, Parker RA, Campbell P. Lack of interest by nonpregnant couples in population-based cystic fibrosis carrier screening. Am J Hum Genet. 1996;58(3):617. [PMC free article] [PubMed] [Google Scholar]
- Cronister A, DiMaio M, Mahoney MJ, Donnenfeld AE, Hallam S. Fragile X syndrome carrier screening in the prenatal genetic counseling setting. Genet Med. 2005;7(4):246. doi: 10.1097/01.GIM.0000159898.90221.D3. [DOI] [PubMed] [Google Scholar]
- d’Agincourt-Canning L. The effect of experiential knowledge on construction of risk perception in hereditary breast/ovarian cancer. J Genet Couns. 2005;14(1):55–69. doi: 10.1007/s10897-005-1500-0. [DOI] [PubMed] [Google Scholar]
- Delatycki MB. Population screening for reproductive risk for single gene disorders in Australia: now and the future. Twin Res Hum Genet. 2008;11(4):422–430. doi: 10.1375/twin.11.4.422. [DOI] [PubMed] [Google Scholar]
- Dodge JA, Lewis PA, Stanton M, Wilsher J. Cystic fibrosis mortality and survival in the UK: 1947–2003. Eur Respir J. 2007;29(3):522–526. doi: 10.1183/09031936.00099506. [DOI] [PubMed] [Google Scholar]
- Etchegary H, Potter B, Howley H, Cappelli M, Coyle D, Graham I, Walker M, Wilson B. The influence of experiential knowledge on prenatal screening and testing decisions. Genet Test. 2008;12(1):115–124. doi: 10.1089/gte.2007.0057. [DOI] [PubMed] [Google Scholar]
- Gason AA, Metcalfe SA, Delatycki MB, Petrou V, Sheffield E, Bankier A, Aitken M. Tay Sachs disease carrier screening in schools: educational alternatives and cheekbrush sampling. Genet Med. 2005;7(9):626–632. doi: 10.1097/01.gim.0000187162.28070.a7. [DOI] [PubMed] [Google Scholar]
- Godard B, Kate L, Evers-Kiebooms G, Ayme S. Population genetic screening programmes: principles, techniques, practices, and policies. Eur J Hum Genet. 2003;11(Suppl 2):S49–S87. doi: 10.1038/sj.ejhg.5201113. [DOI] [PubMed] [Google Scholar]
- Hagerman RJ, Leehey M, Heinrichs W, Tassone F, Wilson R, Hills J, Grigsby J, Gage B, Hagerman PJ. Intention tremor, parkinsonism, and generalized brain atrophy in male carriers of fragile X. Neurology. 2001;57(1):127–130. doi: 10.1212/wnl.57.1.127. [DOI] [PubMed] [Google Scholar]
- Hantash FM, Goos DM, Crossley B, Anderson B, Zhang K, Sun W, Strom CM. FMR1 premutation carrier frequency in patients undergoing routine population-based carrier screening: Insights into the prevalence of fragile X syndrome, fragile X-associated tremor/ataxia syndrome, and fragile X-associated primary ovarian insufficiency in the United States. Genet Med. 2011;13(1):39. doi: 10.1097/GIM.0b013e3181fa9fad. [DOI] [PubMed] [Google Scholar]
- Henneman L, Bramsen I, Kempen L, Acker MB, Pals G, Horst HE, Adèr HJ, Ploeg HM, Kate LP. Offering preconceptional cystic fibrosis carrier couple screening in the absence of established preconceptional care services. Community Genetics. 2003;6(1):5–13. doi: 10.1159/000069540. [DOI] [PubMed] [Google Scholar]
- Hill MK, Archibald AD, Cohen J, Metcalfe SA. A systematic review of population screening for fragile X syndrome. Genet Med. 2010;12(7):396. doi: 10.1097/GIM.0b013e3181e38fb6. [DOI] [PubMed] [Google Scholar]
- Hodgson J, Weil J (2011) Talking about disability in prenatal genetic counseling: a report of two interactive workshops. J Genet Couns. doi:10.1007/s10897-011-9410-9. [DOI] [PubMed]
- Cystic fibrosis population screening position paper. Alexandria: Human Genetics Society of Australasia Genetic Services Committee; 2009. [Google Scholar]
- Ioannou L, Massie J, Collins V, McClaren B, Delatycki MB. Population-based genetic screening for cystic fibrosis: attitudes and outcomes. Public Health Genomics. 2010;13(7–8):449–456. doi: 10.1159/000276544. [DOI] [PubMed] [Google Scholar]
- Janz NK, Becker MH. The health belief model: a decade later. Health Educ Behav. 1984;11(1):1. doi: 10.1177/109019818401100101. [DOI] [PubMed] [Google Scholar]
- Kolb DA. Experiential learning: experience as the source of learning and development. Englewood Cliffs: Prentice Hall; 1984. [Google Scholar]
- Lippman A. Embodied knowledge and making sense of prenatal diagnosis. J Genet Couns. 1999;8(5):255–273. doi: 10.1023/A:1022901131305. [DOI] [PubMed] [Google Scholar]
- Massie J, Forbes R, Dusart D, Bankier A, Delatycki MB. Community-wide screening for cystic fibrosis carriers could replace newborn screening for the diagnosis of cystic fibrosis. J Paediatr Child Health. 2007;43(11):721–723. doi: 10.1111/j.1440-1754.2007.01224.x. [DOI] [PubMed] [Google Scholar]
- Massie J, Petrou V, Forbes R, Curnow L, Ioannou L, Dusart D, Bankier A, Delatycki M. Population-based carrier screening for cystic fibrosis in Victoria: the first three years experience. Aust N Z J Obstet Gynaecol. 2009;49(5):484–489. doi: 10.1111/j.1479-828X.2009.01045.x. [DOI] [PubMed] [Google Scholar]
- McClaren BJ, Delatycki MB, Collins V, Metcalfe SA, Aitken M. ‘It is not in my world’: an exploration of attitudes and influences associated with cystic fibrosis carrier screening. Eur J Hum Genet. 2008;16(4):435–444. doi: 10.1038/sj.ejhg.5201965. [DOI] [PubMed] [Google Scholar]
- McConkie-Rosell A, Finucane B, Cronister A, Abrams L, Bennett RL, Pettersen BJ. Genetic counseling for fragile X syndrome: updated recommendations of the national society of genetic counselors. J Genet Couns. 2005;14(4):249–270. doi: 10.1007/s10897-005-4802-x. [DOI] [PubMed] [Google Scholar]
- Metcalfe S, Barlow-Stewart K, Delatycki MB, Emery JD (2007) Population genetic screening. Aust Fam Physician 36(10):794–800 [PubMed]
- Metcalfe S, Jaques A, Archibald A, Burgess T, Collins V, Henry A, McNamee K, Sheffield L, Slater H, Wake S, Cohen J. A model for offering carrier screening for fragile X syndrome to nonpregnant women: results from a pilot study. Genet Med. 2008;10(7):525–535. doi: 10.1097/GIM.0b013e31817c036e. [DOI] [PubMed] [Google Scholar]
- Metcalfe SA, Bittles AH, O’Leary P, Emery J. Australia: public health genomics. Public Health Genomics. 2009;12(2):121–128. doi: 10.1159/000160666. [DOI] [PubMed] [Google Scholar]
- National Institutes of Health Consensus development conference statement on genetic testing for cystic fibrosis. Arch Intern Med. 1999;159(14):1529–1539. doi: 10.1001/archinte.159.14.1529. [DOI] [PubMed] [Google Scholar]
- O’Sullivan BP, Freedman SD. Cystic fibrosis. Lancet. 2009;373(9678):1891–1904. doi: 10.1016/S0140-6736(09)60327-5. [DOI] [PubMed] [Google Scholar]
- Plass AMC, El CG, Pieters T, Cornel MC. Neonatal screening for treatable and untreatable disorders: prospective parents’ opinions. Pediatrics. 2010;125(1):e99. doi: 10.1542/peds.2009-0269. [DOI] [PubMed] [Google Scholar]
- Sheeran P, Abraham C. The health belief model. In: Conner M, Norman P, editors. Predicting health behaviour. Buckingham: Open University Press; 1995. pp. 23–61. [Google Scholar]
- Sherman S, Pletcher BA, Driscoll DA. Fragile X syndrome: diagnostic and carrier testing. Genet Med. 2005;7(8):584–587. doi: 10.1097/01.GIM.0000182468.22666.dd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Southern KW, Munck A, Pollitt R, Travert G, Zanolla L, Dankert-Roelse J, Castellani C. A survey of newborn screening for cystic fibrosis in Europe. J Cyst Fibros. 2007;6(1):57–65. doi: 10.1016/j.jcf.2006.05.008. [DOI] [PubMed] [Google Scholar]
- Tercyak KP, Johnson SB, Roberts SF, Cruz AC. Psychological response to prenatal genetic counseling and amniocentesis. Patient Educ Couns. 2001;43(1):73–84. doi: 10.1016/S0738-3991(00)00146-4. [DOI] [PubMed] [Google Scholar]
- Berg M, Timmermans DR, Kate LP, Vugt JM, Wal G. Informed decision making in the context of prenatal screening. Patient Education and Counseling. 2006;63(1–2):110–117. doi: 10.1016/j.pec.2005.09.007. [DOI] [PubMed] [Google Scholar]
- Ziebland S, Herxheimer A. How patients’ experiences contribute to decision making: illustrations from DIPEx (personal experiences of health and illness) J Nurs Manag. 2008;16(4):433–439. doi: 10.1111/j.1365-2834.2008.00863.x. [DOI] [PubMed] [Google Scholar]