Abstract
Background
Early identification of physical impairment related to AD is increasingly identified as an important aspect of diagnosis and care. Clinically accessible tools for evaluating physical capacity and impairment in AD have been developed but require further characterization for their effective use.
Purpose
To assess the utility of the Physical Performance Test (PPT) for identifying functionally-limiting aerobic capacity in older adults with Alzheimer’s disease (AD) and without dementia.
Methods
Secondary analysis of a dataset of community dwelling older adults, 70 without dementia and 60 with early-stage AD. Participants were administered the PPT and performed a graded maximal exercise test. The clinical utility of two versions of the PPT was described by determining sensitivity and specificity to functionally-limiting aerobic capacity.
Results
The 9-item PPT is predictive of diminished aerobic capacity in older adults with AD. A score of 28 or less indicates likelihood of functionally-limiting aerobic capacity that would limit independent function with 67% sensitivity and 67% specificity. The 4-item mini-PPT demonstrates improved capability for identifying impaired functional aerobic capacity with 85% sensitivity and 62% specificity. The PPT was not useful for identifying impaired functional aerobic capacity in older adults without dementia.
Conclusions
The PPT, which incorporate basic and instrumental activities of daily living as test items, and the mini-PPT which focuses on basic activities of daily living and simple physical functions, are both clinically useful tool for the evaluation for individuals in the earliest stages of AD and both provide important information about functional performance. The mini-PPT additionally inform the clinician as to whether or not individual with early-stage AD is likely to have insufficient aerobic capacity to perform instrumental daily functions.
Keywords: oxygen uptake, dementia, functional fitness, older adults, clinical test
INTRODUCTION
Assessment of function in the rapidly aging population is an essential aspect to the administration of quality health care. Among the most prevalent diseases of age is Alzheimer’s disease (AD), now affecting nearly 1 in 8 adults over 65.1 Though, AD is typically associated with declines in episodic memory and cognition, decline in physical function is common.2 Early identification of physical impairment related to AD is increasingly identified as an important aspect of diagnosis and care.3 Clinically accessible tools for evaluating physical capacity and impairment in AD are necessary to identify those who may benefit from rehabilitative care.
The Physical Performance Test (PPT) is one tool with potential clinical utility for identifying physical and functional change in adults with AD.3, 4 The original PPT, tested primarily in community dwelling older adults, demonstrated high inter-rater reliability (Chronbach’s alpha=0.87) and concurrent validity with measures of activities of daily living (r=0.65 to 0.80). It also correlated with other performance-based measures of function and subjective report of physical role function, showing resistance to floor and ceiling effects.5, 6 Various versions of the PPT have been found to be predictive of need for assistance,7 future nursing home placement,8 fall risk,9, 10 and mortality.8 Recently, intra-rater, inter-rater and test-retest reliability were all established for older adults with dementia.11
Physical decline can have a negative impact on aerobic capacity and thus daily functional performance. Peak oxygen consumption (VO2peak) is a measure of aerobic capacity obtained during a graded exercise test. VO2 peak provides information regarding one’s ability to perform “external work”,12 and allows clinicians to evaluate functional health and capacity.13 For example, aerobic capacity is directly and independently associated with functional capacity for daily activities, along with muscular performance and flexibility.14 Individuals must maintain aerobic capacity sufficient to perform activities with varying intensities and durations. It has been previously suggested that basic activities of daily living (ADL) require 13 ml*kg−1*min−1. 15 Aerobic capacity of less than 20 ml*kg−1*min−1 is associated with increased functional limitation in older adults. For every milliliter decrease in peak oxygen capacity below this threshold, an individual is 8-times more likely to report functional limitations.16 Levels above this threshold represent additional capacity that can be drawn upon for activities requiring greater oxygen consumption or emergent situations.17, 18 We have previously demonstrated that individuals in the earliest stages of AD (very mild or mild dementia) have decreased aerobic capacity compared to peers without dementia.19 In these early stages of AD, individuals also have decreased independence in ADLs.20 Further, there are subtle, but systemic changes such as lower body mass, accelerating sarcopenia, fat mass reduction, altered brain glucose metabolism and mitochondrial dysfunction.21–28 These changes could affect aspects of physical function, including aerobic capacity, muscular performance and motor ability.
The PPT has already been identified as a useful, clinical screening tool for functional impairment associated with AD.4 Thus, the purpose of this study was to determine if this instrument could accurately identify those with functionally-limiting aerobic capacity in elderly individuals with AD and those without dementia. The PPT offers the advantage of objective assessment of performance on everyday tasks and is easy to administer in the clinic. A shorter, 4-item version of the PPT (mini-PPT) has also recently been shown to be useful for screening for early physical function change in those with AD.3 We hypothesized in those with and without dementia, the PPT and mini-PPT would predict peak oxygen consumption < 20 ml*kg−1*min−1, which is associated functionally-limiting aerobic capacity. To accomplish this, we examined the relationship of PPT and mini-PPT scores and an objective measure of aerobic capacity and function (VO2 peak).
METHODS
We performed a secondary analysis of participants from the larger Brain Aging Project cohort of community dwelling, age 60 and over at the University of Kansas Medical Center Alzheimer and Memory Program. Institutionally-approved, informed consent was obtained from all participants and their legal representatives as appropriate and the procedures followed were in accordance with the Helsinki Declaration. Inclusion and exclusion criteria and cross-sectional data from this cohort has been reported previously.19 Briefly, individuals were excluded from enrollment in the Brain Aging Project if they were diagnosed with neurological disorders other than AD that had the potential to impair cognition, had insulin-dependent diabetes, a recent history (< 2 years) of coronary artery disease, significant orthopedic issues or pulmonary disease that would limit their ability to perform exercise testing (including use of an assistive device for ambulation), or clinically significant depressive symptoms. For the present analysis we assessed a sample of individuals from the Brain Aging Project, 65 to 84 years of age who completed all assessments relevant to the present analysis.
Clinical Assessment
All participant underwent a clinical assessment included a semi-structured interview that included a collateral source knowledgeable about the participant. A comprehensive medical and personal history was collected from the informant. Dementia status of the participant was based on this clinical evaluation.29 National Institutes of Neurological and Communicative Disorders and Stroke – Alzheimer’s Disease and Related Disorders Association criteria diagnostic criteria for AD require the gradual onset and progression of impairment in memory and in at least one other cognitive and functional domain using.30 Based on this evaluation participants were identified by the Clinical Dementia Rating scale (CDR) as either having no dementia (CDR 0) or being in the earliest stages of dementia, i.e. having very mild (CDR 0.5) or mild dementia (CDR 1.0) related to AD.31 The Mini-Mental State Examination (MMSE) 32 was also administered as a common measure of cognitive impairment.
Physical Performance and Exercise Testing
There are multiple versions of the PPT, varying slightly in the number and type of items.4, 5, 33, 34 The battery used in the present analysis included writing a sentence, simulated eating, lifting a book onto a shelf above shoulder height, simulated dressing, picking up an item from the floor, walking 50 feet, turning in a circle, rising from a chair five times, and a progressive Rhomberg test of balance (i.e. standing with feet side-by-side, semi-tandem and tandem).4 This battery of common basic and instrumental activities of daily living is scored 0–4 based on performance time. The score range is 0–36 with higher scores indicating better performance.
We also calculated scores for the 4-item mini-PPT.3 The mini-PPT includes picking up an item from the floor, 50’ walk, chair rise, and the progressive Rhomberg test. Scores are summed for a maximum score of 16.
After the PPT, peak oxygen consumption (VO2 peak; ml*kg−1*min−1) was assessed during a symptom-limited, graded treadmill test using a modified Bruce protocol19, 35 and metabolic cart (Parvomedics, Sandy, UT) as described previously.36 Oxygen consumption was averaged over 15-second intervals. VO2 peak was considered the highest observed value during the exercise test.
Data Analysis
Group differences in demographics and our measures of interest were first assessed using parametric (ANOVA) and nonparametric statistics (Chi-square, Mann-Whitney U) as appropriate, α=0.05 (SPSS, Version 17). PPT, mini-PPT and VO2 peak were correlated using Pearson’s correlation. We then performed stepwise logistic regression analyses37 to explore the relationship of predictor variables on the dependent variable, functionally-limiting aerobic capacity (defined as VO2 peak<20 ml*kg−1*min−1; dummy coding below threshold =1, greater than or equal to threshold =0).16 PPT or mini-PPT, MMSE, age and gender (categorical dummy coding Female=1, Male=0) were used as predictor variables. The predictor variables were selected based on prior experience with potentially influential demographics in those with dementia and to ensure that cognitive confounding of physical and aerobic testing were not influencing our model. We entered our predictor variables in forward stepwise fashion using the likelihood ratio (α<0.05 to enter, α>0.1 to remove).
Diagnostic utility (sensitivity and specificity) of the PPT and mini-PPT to identify limited aerobic capacity was assessed using standard procedures.38 We then plotted a Receiver-Operator Characteristic (ROC) curve to determine optimal sensitivity and specificity for functionally-limiting aerobic capacity. ROC curves are a common way to assess the usefulness of a measure to categorize individuals. Sensitivity in the present case is the proportion of patients who have functionally-limiting aerobic capacity who would also be identified as such by the PPT or mini-PPT. Specificity here is the proportion of patients who are not aerobically impaired who are also ruled out by the PPT or mini-PPT. An ROC curve plots true positives (sensitivity) against false positives (1-specificity). This is done to identify a cutoff score that achieves the best sensitivity and specificity for classifying functionally-limiting aerobic capacity.
RESULTS
Of the 130 participants included in the analysis, 70 did not have dementia and 60 had very mild (CDR 0.5, n=50) or mild (CDR 1, n=11) dementia related to AD. Both groups were predominantly female (59%) with an average age of 74.7 (Range = 65.3–84.5, SD=5.4). The groups did not differ in age or gender distribution. PPT, mini-PPT and MMSE scores were significantly lower in the group with AD compared to the group without dementia (p ≤0.001). See Table 1 for group summaries of demographic and outcome measures.
Table 1.
Participant Characteristics
| Healthy Control (n= 70) | AD (n=60) | Sig. | |
|---|---|---|---|
| Age (Range) | 74.8 (65.3 – 84.3) | 74.5 (65.3–84.5) | 0.83 |
| Gender (% Female) | 40 (56%) | 38 (62%) | 0.59 |
| PPT (Range) | 30.6 (23–36) | 27.9 (17–33) | <0.001 |
| mini-PPT (Range) | 13.5 (7–16) | 12.6 (9–15) | 0.001 |
| MMSE (Range) | 29.4 (27 – 30) | 26.2 (15–30) | <0.001 |
| VO2 peak (Range) | 21.5 (12.6 – 44.6) | 19.4 (12.5 – 28.4) | 0.02 |
The groups also differed in VO2 peak (p=0.02), with mean of the group without dementia above the 20ml*kg−1*min−1 threshold sufficient for functional performance and the mean of the group with AD falling slightly below this threshold. Based on this threshold, 31 individuals (24 females, mean age 77.0) in the group without dementia and, 39 individuals in the group with AD (31 females, mean age 75.6) were identified as having an functionally-limiting aerobic capacity.. functionally-limiting aerobic capacity.
The average total PPT score in the group without dementia was 30.4 (SD 2.8), compared to 27.5 (SD 3.2) in the group with AD. The average mini-PPT score in the group without dementia was 13.5 (SD 1.6), compared to 12.6 (SD 1.4) in the group with AD. Comparison to previous reports is difficult as some have used different scales or enrolled participants who are much older.4, 39
In the group without dementia, the PPT was moderately correlated with VO2 peak (r=0.33, p=0.006). The mini-PPT was weakly correlated with VO2 peak (r=0.25, p=0.04). In the group with AD, the PPT (r=0.35, p=0.006) and the mini-PPT (r=0.49, p<0.001) were both moderately correlated with VO2 peak, with the mini-PPT having a stronger relationship to aerobic capacity in this group.
We then looked at predicting a persons attaining a threshold VO2 peak deemed sufficient for functional performance (≥20 ml*kg−1*min−1). In the group without dementia, age and gender were both sigificant factors associated with aerobic capacity below 20 ml*kg−1*min−1. PPT and MMSE were not contributing measures to the likelihood that a participant without dementia would be considered impaired (Table 2). The overall prediction accuracy of this equation was 80% (Nagelkerke r2 = 0.36). Using the mini-PPT did not improve the prediction accuracy as the final model using age and gender remained unchanged.
Table 2.
Final logistic regression model of functionally limiting aerobic capacity (VO2 peak<20ml* kg−1* min−1) on Physical Performance Test, Mini-Mental State Exam (MMSE) and demographics in the group without dementia.
| Logistic Coefficient b (SE) | p-value | Odds Ratio | 95% CI of Odds Ratio | -2 Log-Likelihood Ratio | Model chi square | |
|---|---|---|---|---|---|---|
| Constant | −13.83 (0.24) | 0.34 | 96.12 | |||
| Gender | −2.04 (0.62) | 0.001 | 0.13 | 0.04 – 0.44 | 85.09 | 11.04 |
| Age | 0.17 (0.05) | 0.002 | 1.18 | 1.06 – 1.31 | 74.08 | 22.05 |
| PPT | ns | 0.29 | ||||
| MMSE | ns | 0.49 |
Overall prediction accuracy 80.0%. Nagelkerke Rsqr = 0.36. The model does not change with mini-PPT sig. of MMSE p=0.56 and mini-PPT p=0.29.
Predictor variable are shown in the order of entry. -2 log-likelihood and chi square represent omnibus model statistics with entry of each predictor variable.
SE = Standard Error
ns – variable not selected for the final model.
Using the same methodology, age, gender and PPT score all contributed to the prediction of functionally-limiting aerobic capacity in individuals with AD. MMSE did not contribute to the model (Table 3). The overall accuracy of the model was 88.3% (Nagelkerke r2 = 0.55). Using the mini-PPT slightly reduced the prediction accuracy 78.3% (Nagelkerke r2 = 0.49). The probability that any participant, with or without dementia, would be considered to have functionally-limiting aerobic capacity can be calculated using Equation 1 from the logistic coefficients (b) in Tables 2 and 3.37
Table 3.
Final logistic regression model of functionally limiting aerobic capacity (VO2 peak<20ml* kg−1* min−1) on Physical Performance Test (A) and mini Physical Performance Test (B), Mini-Mental State Exam (MMSE) and demographics in the AD group.
| Logistic Coefficient b (SE) | p-value | Odds Ratio | 95% CI of Odds Ratio | -2 Log- Likelihood Ratio | Omnibus chi square | |
|---|---|---|---|---|---|---|
| Constant | −0.05 (0.27) | 0.99 | 77.70 | |||
| Gender | −3.28 (0.88) | <0.001 | 0.038 | 0.007 – 0.21 | 62.52 | 15.17 |
| PPT | −0.34 (0.17) | 0.04 | 0.71 | 0.51 – 0.98 | 51.85 | 25.85 |
| Age | 0.16 (0.08) | 0.051 | 1.17 | 1.00 – 1.38 | 47.40 | 30.30 |
| MMSE | ns | 0.36 | ||||
| Overall prediction accuracy 88.3%. Nagelkerke Rsqr = 0.55. | ||||||
| B) | ||||||
| Constant | 12.54 (3.84) | 0.001 | 96.12 | |||
| Gender | −2.61 (0.75) | <0.001 | 0.07 | 0.02 – 0.32 | 62.52 | 15.17 |
| mini-PPT | −0.84 (0.29) | 0.003 | 0.43 | 0.25 – 0.76 | 51.07 | 22.05 |
| Age | ns | 0.054 | ||||
| MMSE | ns | 0.37 | ||||
| Overall prediction accuracy 78.3%. Nagelkerke Rsqr = 0.49. | ||||||
Predictor variable are shown in the order of entry. -2 log-likelihood and chi square represent omnibus model statistics with entry of each predictor variable.
SE = Standard Error
ns – variable not selected for the final model.
-2 log-likelihood and Model chi square represent whole model statistics with entry of each predictor variable
| Eq. 1 |
where e is the natural logarithm, 2.718 and z = Constant + bAge * (Age) + b * Gender (Gender: 0 for female, 1 male) + bPPT * (PPT or mini-PPT score)
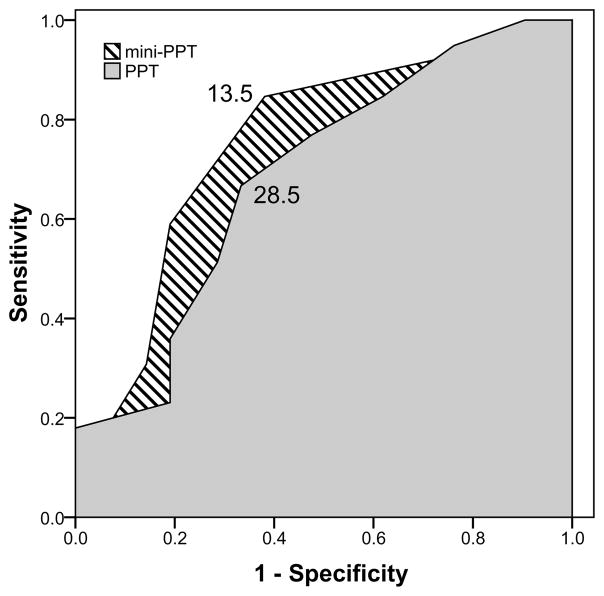
Calculation of the probability that an individual’s functional capacity is impaired may have limited clinical utility. We therefore identified PPT and mini-PPT cutoff values that would predict aerobic impairment with reasonable specificity and sensitivity using an ROC curve. Because PPT was not predictive of aerobic capacity in the group without dementia we did not perform this analysis on that group. Clinicians can use Eq. 1 to calculate the probability of functionally-limiting aerobic capacity using age and gender alone. Figure 1 shows the ROC curves for PPT (shaded) and mini-PPT (diagonals).
Figure 1.
ROC curves of sensitivity (y-axis) and 1-specificity (x-axis) for PPT (solid shade) and mini-PPT (diagonally hatched) to identify individuals with AD as having functionally-limiting aerobic capacity. At a cutoff score of 28.5, those who scored 28 or below on the PPT were correctly identified 67% of the time as having a VO2 peak below 20 ml*kg−1*min−1 (i.e. 67% sensitivity). The same cutoff point was 66.7% specific, meaning that two thirds of individuals scoring 29 or above were correctly classified as not having functionally-limiting aerobic capacity (area under curve = 0.69, p<0.02, 95%CI 0.55–0.83). The mini-PPT had slightly improved sensitivity. A cutoff score of 13.5 had 85% sensitivity and 62% specificity (area under curve = 0.75, p=0.001, 95%CI 0.62–0.89).
At a cutoff score of 28.5, those who scored 28 or below on the PPT were correctly identified 67% of the time as having a VO2 peak below 20 ml*kg−1*min−1 (i.e. 67% sensitivity). The same cutoff point was 67% specific, meaning that two thirds of individuals scoring 29 or above were correctly classified as not having functionally-limiting aerobic capacity. The area under the plot (0.69, p=0.02, 95%CI 0.55–0.83) can be taken as a measure of the diagnostic utility of the PPT and suggests that a cutoff score of 28.5 on the 9-item PPT provides better than chance ability to predict aerobic impairment in a group of individuals with dementia. The mini-PPT had slightly improved sensitivity. At a cutoff score of 13.5, the mini-PPT had 85% sensitivity and 62% specificity. The area under the plot was slightly higher than that of the PPT (0.75, p=0.001, 95%CI 0.62–0.89).
DISCUSSION
Physical function capacity sufficient to perform daily basic and instrumental activities is an essential component of maintaining independence as one ages. Traditional, self-reported measures of physical function and functional independence may lack sensitivity to early performance decline. 40 Subjective reports may also be confounded by poor self-awareness and recall for the patient 41 or by the perceived burden of the caregiver informant completing the report.42 An objective measure of physical performance and function such as the PPT or VO2 peak avoids the confounds of subjective reporting6 and provides an objective measures that is responsive to intervention.43, 44
It is important to identify objective measures of physical performance that have validity when compared to other, established measures. In the present study we compared the PPT with an establish objective threshold of aerobic capacity deemed necessary for functional performance.16 Our results suggest these measure are more strongly correlated in those with dementia than those who do not have dementia. We then sought to further test the clinical utility (sensitivity and specificity) of the PPT and mini-PPT to identify reduced aerobic capacity in the earliest stages of AD when compared to these established measures. The results suggest that a clinician can predict with reasonable accuracy (78%–88%) functionally-limiting aerobic capacity in an older adult with early-stage AD using just an age, gender and PPT or mini-PPT score.
Interestingly, the model including the PPT did not improve prediction of functionally-limiting aerobic capacity for those without dementia; age and gender alone are enough for 80% accuracy. It remains unclear why the PPT is more closely associated with aerobic function in those with dementia. One possible explanation is that even in the earliest stages of AD, subtle motor function changes occur that could alter both motor performance on a non-aerobic battery such as the PPT as well as treadmill-based maximal exercise testing. For example, gait speed can begin to decline up to 12 years before onset of mild cognitive impairment45 considered a prodromal stage of AD for many individuals. Gait variability and transfer are also altered in mild cognitive impairment and early-stage AD.46, 47 Thus, there appear to be subtle but meaningful motor changes early in the disease. It is possible that these changes, which may contribute to the physical function changes in AD detectable the PPT and mini-PPT,48 compromise aerobic capacity as well.
Assessment of aerobic function is within the purview of many rehabilitation professionals. Further, the rehabilitation professional can use the information derived from the exercise test for individualized exercise prescription. However, exercise testing is not always feasible in rehabilitative settings. The present results suggest that aerobic capacity as measured by a graded exercise test share common, measureable physical requirements with both versions of the PPT. This was seen in the correlation in both those with AD and without dementia. This supports previous work identifying what Binder et al. referred to as aerobic power (VO2 peak) as an important component of the PPT score, and therefore physical performance in older adults. As Binder et al. noted, this relationship is particularly striking because the PPT as employed in this study does not contain aerobically intense activities.39 Rather, the PPT along with age and gender, appears to be predictive of functionally-limiting aerobic capacity in those with AD. That is, individuals with AD who experience physical decline are also likely to experience a reduction in their aerobic capacity.
Cutoff scores for the PPT and mini-PPT provide a screening index for aerobic impairment of clients with dementia. We do not mean to suggest that the PPT is a replacement for clinical exercise testing. Rather, upon initial evaluation, these data suggest the PPT and mini-PPT provides information about the aerobic capacity and functional status of an individual. Based on this initial measurement, the rehabilitation professional may wish to specifically measure aerobic capacity using a maximal or sub-maximal aerobic exercise test to prescribe exercise and set goals within the plan of care. Clinically, those with AD who have a PPT score of 28 or below (or 12 and below on the mini-PPT), likely have diminish aerobic capacity that limits function and independence.
The 4-item abbreviated version of the PPT has been proposed as a useful alternative to the 9-item PPT.3 Purported benefit of this assessment are its brevity and ease of administration in the clinic; requiring only a penny, a chair and 50’ of floor length to administer. Certainly there is value in the 4-item PPT as a screening tool for physical function decline in early-stage AD. In fact, the present results would suggest it is slightly more sensitive to functionally-limiting aerobic capacity. That being said, we suggest that the full 9-item PPT has additional items of relevance to the rehabilitation professional. Specifically the 9-item PPT includes simulated basic daily activities such as feeding and dressing that are of particular interest to rehabilitation clinicians. Further, given that a full battery of physiological and psychological testing beyond that which is standard for clinical practice may be untenable, the PPT likely captures those components of aerobic function essential to performance of basic activities of daily living. Clinicians may chose to use either version (PPT or mini-PPT) depending on the needs of the clinical evaluation.
A significant limitation of the present analysis is the lack of clear measurement of other components of functional performance such as balance and flexibility which influence function.14 Though these are directly or indirectly captured by the PPT, they were not outcome measures in the Brain Aging Project. The model may have been improved were we to have captured additional physical function variables. In addition, the Brain Aging Project cohort are, as a group, relatively healthy compared to the greater population. This limits the generalizability of our findings. It is particularly important to note that the PPT and mini-PPT appear to hold little predictive value for functionally-limiting aerobic capacity in healthy older adults without dementia. While the PPT is weakly related to peak aerobic capacity in these individuals, and is a useful tool in this population for assessing function, frailty and fall risk, it appears to have no predictive value for clinicians in need of assessing functional aerobic capacity in those without dementia.
CONCLUSION
Both the 9-item PPT and 4-item mini-PPT are batteries of functional tasks useful for evaluation of individuals with early-stage AD. A score of 28 or less on the PPT, or 13 or less on the mini-PPT, indicates a reasonable likelihood of functionally-limiting aerobic capacity. For rehabilitation professionals, these results extend the utility of the PPT, which incorporates basic and instrumental activities of daily living. The PPT appears to be a multi-purpose clinical tool for assessing function, frailty, fall risk and potential aerobic compromise. While maximal exercise testing remains the gold standard method for indexing aerobic capacity level, the PPT could serve as an initial screening to for multiple problems in including impaired aerobic capacity for the individual with AD.
Acknowledgments
EDV is supported in part by a fellowship from the Foundation for Physical Therapy. Kansas Partners in Progress, Inc. supported SAB, CL and JH. The Brain Aging Project was supported by grants R03AG026374 and R21AG029615 from the National Institutes of Aging, grant K23NS058252 from the National Institute on Neurological Disorders and Stroke to JMB. The University of Kansas General Clinical Research Center (M01RR023940) provided essential space, expertise, and nursing support. There are no conflicts of interest to disclose.
Contributor Information
Eric D. Vidoni, Email: evidoni@kumc.edu.
Sandra A. Billinger, Email: sbillinger@kumc.edu.
Charesa Lee, Email: clee2@kumc.edu.
Jenna Hamilton, Email: jhamilton@kumc.edu.
Jeffrey M. Burns, Email: jburns2@kumc.edu.
References
- 1.Alzheimer’s Association. 2010 Alzheimer’s disease facts and figures. Alzheimers Dement. 2010 Mar;6(2):158–194. doi: 10.1016/j.jalz.2010.01.009. [DOI] [PubMed] [Google Scholar]
- 2.Krenz C, Larson EB, Buchner DM, Canfield CG. Characterizing patient dysfunction in Alzheimer’s-type dementia. Medical Care. 1988 May;26(5):453–461. doi: 10.1097/00005650-198805000-00002. [DOI] [PubMed] [Google Scholar]
- 3.Wilkins CH, Roe CM, Morris JC. A brief clinical tool to assess physical function: the mini-physical performance test. Arch Gerontol Geriatr. 2010 Jan–Feb;50(1):96–100. doi: 10.1016/j.archger.2009.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shah KR, Carr D, Roe CM, Miller JP, Coats M, Morris JC. Impaired Physical Performance and the Assessment of Dementia of the Alzheimer Type. Alzheimer Disease and Associated Disorders. 2004;18(3):112–118. doi: 10.1097/01.wad.0000127441.77570.f3. [DOI] [PubMed] [Google Scholar]
- 5.Sherman SE, Reuben D. Measures of functional status in community-dwelling elders. J Gen Intern Med. 1998 Dec;13(12):817–823. doi: 10.1046/j.1525-1497.1998.00245.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Reuben DB, Valle LA, Hays RD, Siu AL. Measuring physical function in community-dwelling older persons: a comparison of self-administered, interviewer-administered, and performance-based measures. J Am Geriatr Soc. 1995 Jan;43(1):17–23. doi: 10.1111/j.1532-5415.1995.tb06236.x. [DOI] [PubMed] [Google Scholar]
- 7.Beissner KL, Collins JE, Holmes H. Muscle force and range of motion as predictors of function in older adults. Phys Ther. 2000 Jun;80(6):556–563. [PubMed] [Google Scholar]
- 8.Reuben DB, Siu AL, Kimpau S. The predictive validity of self-report and performance-based measures of function and health. J Gerontol. 1992 Jul;47(4):M106–110. doi: 10.1093/geronj/47.4.m106. [DOI] [PubMed] [Google Scholar]
- 9.Delbaere K, Van den Noortgate N, Bourgois J, Vanderstraeten G, Tine W, Cambier D. The Physical Performance Test as a predictor of frequent fallers: a prospective community-based cohort study. Clin Rehabil. 2006 Jan;20(1):83–90. doi: 10.1191/0269215506cr885oa. [DOI] [PubMed] [Google Scholar]
- 10.VanSwearingen JM, Paschal KA, Bonino P, Chen TW. Assessing recurrent fall risk of community-dwelling, frail older veterans using specific tests of mobility and the physical performance test of function. J Gerontol A Biol Sci Med Sci. 1998 Nov;53(6):M457–464. doi: 10.1093/gerona/53a.6.m457. [DOI] [PubMed] [Google Scholar]
- 11.Farrell MK, Rutt RA, Lusardi MM, Williams AK. Reliability of the Physical Performance Test in People with Dementia. Phyiscal & Occupational Therapy in Geriatrics. 2010;28(2):144–153. [Google Scholar]
- 12.DeTurk WE, Cahalin LP. Cardiovascular and Pulmonary Physical Therapy: An Evidence-based Approach. 1. McGraw-Hill; 2004. [Google Scholar]
- 13.Huggett DL, Connelly DM, Overend TJ. Maximal aerobic capacity testing of older adults: a critical review. J Gerontol A Biol Sci Med Sci. 2005 Jan;60(1):57–66. doi: 10.1093/gerona/60.1.57. [DOI] [PubMed] [Google Scholar]
- 14.Morey MC, Pieper CF, Cornoni-Huntley J. Physical fitness and functional limitations in community-dwelling older adults. Med Sci Sports Exerc. 1998 May;30(5):715–723. doi: 10.1097/00005768-199805000-00012. [DOI] [PubMed] [Google Scholar]
- 15.Shephard R. Adapting physical activity to an aging population. J Sports Cardiol. 1987;4:1–13. [Google Scholar]
- 16.Cress ME, Meyer M. Maximal voluntary and functional performance levels needed for independence in adults aged 65 to 97 years. Phys Ther. 2003 Jan;83(1):37–48. [PubMed] [Google Scholar]
- 17.Arnett SW, Laity JH, Agrawal SK, Cress ME. Aerobic reserve and physical functional performance in older adults. Age Ageing. 2008 Jul;37(4):384–389. doi: 10.1093/ageing/afn022. [DOI] [PubMed] [Google Scholar]
- 18.Schrack JA, Simonsick EM, Ferrucci L. The energetic pathway to mobility loss: an emerging new framework for longitudinal studies on aging. J Am Geriatr Soc. 2010 Oct;58( Suppl 2):S329–336. doi: 10.1111/j.1532-5415.2010.02913.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Burns JM, Cronk BB, Anderson HS, et al. Cardiorespiratory fitness and brain atrophy in early Alzheimer disease. Neurology. 2008 Jul 15;71(3):210–216. doi: 10.1212/01.wnl.0000317094.86209.cb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vidoni ED, Honea RA, Burns JM. Neural correlates of impaired functional independence in early Alzheimer’s disease. J Alzheimers Dis. 2010 Jan;19(2):517–527. doi: 10.3233/JAD-2010-1245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Burns JM, Johnson DK, Watts A, Swerdlow RH, Brooks WM. Reduced lean mass in early Alzheimer disease and its association with brain atrophy. Arch Neurol. 2010 Apr;67(4):428–433. doi: 10.1001/archneurol.2010.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.White H, Pieper C, Schmader K, Fillenbaum G. Weight change in Alzheimer’s disease. J Am Geriatr Soc. 1996;44(3):265–272. doi: 10.1111/j.1532-5415.1996.tb00912.x. [DOI] [PubMed] [Google Scholar]
- 23.Johnson DK, Wilkins CH, Morris JC. Accelerated Weight Loss May Precede Diagnosis in Alzheimer Disease. Arch Neurol. 2006 September 1;63(9):1312–1317. doi: 10.1001/archneur.63.9.1312. [DOI] [PubMed] [Google Scholar]
- 24.Whitwell JL, Przybelski SA, Weigand SD, et al. 3D maps from multiple MRI illustrate changing atrophy patterns as subjects progress from mild cognitive impairment to Alzheimer’s disease. Brain. 2007 Jul;130(Pt 7):1777–1786. doi: 10.1093/brain/awm112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Barrett-Connor E, Edelstein SL, Corey-Bloom J, Wiederholt WC. Weight loss precedes dementia in community-dwelling older adults. J Am Geriatr Soc. 1996 Oct;44(10):1147–1152. doi: 10.1111/j.1532-5415.1996.tb01362.x. [DOI] [PubMed] [Google Scholar]
- 26.Buchman AS, Wilson RS, Bienias JL, Shah RC, Evans DA, Bennett DA. Change in body mass index and risk of incident Alzheimer disease. Neurology. 2005 Sep 27;65(6):892–897. doi: 10.1212/01.wnl.0000176061.33817.90. [DOI] [PubMed] [Google Scholar]
- 27.Swerdlow RH, Khan SM. The Alzheimer’s disease mitochondrial cascade hypothesis: an update. Exp Neurol. 2009 Aug;218(2):308–315. doi: 10.1016/j.expneurol.2009.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Swerdlow R, Marcus DL, Landman J, Kooby D, Frey W, 2nd, Freedman ML. Brain glucose metabolism in Alzheimer’s disease. Am J Med Sci. 1994 Sep;308(3):141–144. doi: 10.1097/00000441-199409000-00003. [DOI] [PubMed] [Google Scholar]
- 29.Morris JC, Storandt M, Miller JP, et al. Mild Cognitive Impairment Represents Early-Stage Alzheimer Disease. Archives of Neurology. 2001;58(3):397–405. doi: 10.1001/archneur.58.3.397. [DOI] [PubMed] [Google Scholar]
- 30.McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM. Clinical diagnosis of Alzheimer’s disease: Report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer’s disease. Neurology. 1984;34:939–944. doi: 10.1212/wnl.34.7.939. [DOI] [PubMed] [Google Scholar]
- 31.Morris JC. The Clinical Dementia Rating (CDR): current version and scoring rules. Neurology. 1993 November 1;43(11):2412b–2414. doi: 10.1212/wnl.43.11.2412-a. [DOI] [PubMed] [Google Scholar]
- 32.Folstein MF, Folstein SE, McHugh PR. Mini-mental State: A practical method for grading the cognitive state of patients for the clinicians. J Psychiatr Res. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 33.Reuben DB, Siu AL. An objective measure of physical function of elderly outpatients. The Physical Performance Test. J Am Geriatr Soc. 1990 Oct;38(10):1105–1112. doi: 10.1111/j.1532-5415.1990.tb01373.x. [DOI] [PubMed] [Google Scholar]
- 34.Brown M, Sinacore DR, Ehsani AA, Binder EF, Holloszy JO, Kohrt WM. Low-intensity exercise as a modifier of physical frailty in older adults. Arch Phys Med Rehabil. 2000 Jul;81(7):960–965. doi: 10.1053/apmr.2000.4425. [DOI] [PubMed] [Google Scholar]
- 35.Hollenberg M, Ngo LH, Turner D, Tager IB. Treadmill Exercise Testing in an Epidepiologic Study of Elderly Subjects. Journal of Gerontology: Biological Sciences. 1998;53A(4):259–267. doi: 10.1093/gerona/53a.4.b259. [DOI] [PubMed] [Google Scholar]
- 36.Burns JM, Mayo MS, Anderson HS, Smith HJ, Donnelly JE. Cardiorespiratory fitness in early-stage Alzheimer disease. Alzheimer Dis Assoc Disord. 2008 Jan–Mar;22(1):39–46. doi: 10.1097/WAD.0b013e31815a9ddc. [DOI] [PubMed] [Google Scholar]
- 37.Bewick V, Cheek L, Ball J. Statistics review 14: Logistic regression. Crit Care. 2005 Feb;9(1):112–118. doi: 10.1186/cc3045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Collinson P. Of bombers, radiologists, and cardiologists: time to ROC. Heart. 1998 Sep;80(3):215–217. doi: 10.1136/hrt.80.3.215. [DOI] [PubMed] [Google Scholar]
- 39.Binder EF, Birge SJ, Spina R, et al. Peak aerobic power is an important component of physical performance in older women. J Gerontol A Biol Sci Med Sci. 1999 Jul;54(7):M353–356. doi: 10.1093/gerona/54.7.m353. [DOI] [PubMed] [Google Scholar]
- 40.Jefferson AL, Byerly LK, Vanderhill S, et al. Characterization of activities of daily living in individuals with mild cognitive impairment. Am J Geriatr Psychiatry. 2008 May;16(5):375–383. doi: 10.1097/JGP.0b013e318162f197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sager MA, Dunham NC, Schwantes A, Mecum L, Halverson K, Harlowe D. Measurement of activities of daily living in hospitalized elderly: a comparison of self-report and performance-based methods. J Am Geriatr Soc. 1992 May;40(5):457–462. doi: 10.1111/j.1532-5415.1992.tb02011.x. [DOI] [PubMed] [Google Scholar]
- 42.Zanetti O, Geroldi C, Frisoni GB, Bianchetti A, Trabucchi M. Contrasting results between caregiver’s report and direct assessment of activities of daily living in patients affected by mild and very mild dementia: the contribution of the caregiver’s personal characteristics. J Am Geriatr Soc. 1999 Feb;47(2):196–202. doi: 10.1111/j.1532-5415.1999.tb04578.x. [DOI] [PubMed] [Google Scholar]
- 43.Zinzi P, Salmaso D, De Grandis R, et al. Effects of an intensive rehabilitation programme on patients with Huntington’s disease: a pilot study. Clin Rehabil. 2007 Jul;21(7):603–613. doi: 10.1177/0269215507075495. [DOI] [PubMed] [Google Scholar]
- 44.Binder EF, Brown M, Sinacore DR, Steger-May K, Yarasheski KE, Schechtman KB. Effects of extended outpatient rehabilitation after hip fracture: a randomized controlled trial. JAMA. 2004 Aug 18;292(7):837–846. doi: 10.1001/jama.292.7.837. [DOI] [PubMed] [Google Scholar]
- 45.Buracchio T, Dodge HH, Howieson D, Wasserman D, Kaye J. The trajectory of gait speed preceding mild cognitive impairment. Arch Neurol. 2010 Aug;67(8):980–986. doi: 10.1001/archneurol.2010.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Verghese J, Robbins M, Holtzer R, et al. Gait dysfunction in mild cognitive impairment syndromes. J Am Geriatr Soc. 2008 Jul;56(7):1244–1251. doi: 10.1111/j.1532-5415.2008.01758.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Manckoundia P, Mourey F, Pfitzenmeyer P, Papaxanthis C. Comparison of motor strategies in sit-to-stand and back-to-sit motions between healthy and Alzheimer’s disease elderly subjects. Neuroscience. 2006;137(2):385–392. doi: 10.1016/j.neuroscience.2005.08.079. [DOI] [PubMed] [Google Scholar]
- 48.Reger MA, Watson GS, Green PS, et al. Intranasal insulin improves cognition and modulates beta-amyloid in early AD. Neurology. 2008 Feb 5;70(6):440–448. doi: 10.1212/01.WNL.0000265401.62434.36. [DOI] [PubMed] [Google Scholar]