Abstract
Galectin-1 (Gal-1) is one of 15 evolutionarily conserved β-galactoside-binding proteins that display biologically-diverse activities in pathogenesis of inflammation and cancer. Gal-1 is variably expressed on immune cells and endothelial cells, though is commonly found and secreted at high levels in cancer cells. It induces apoptosis in effector T cells through homodimeric binding of N-acetyllactosamines on membrane glycoproteins (Gal-1 ligands). There is also compelling evidence in models of cancer and autoimmunity that recombinant Gal-1 (rGal-1) can potentiate immunoregulatory function of T cells. Here, we review Gal-1’s structural and functional features, while analyzing potential drawbacks and technical difficulties inherent to rGal-1’s nature. We also describe new Gal-1 preparations that exhibit dimeric stability and functional activity on T cells, providing renewed excitement for studying Gal-1 efficacy and/or use as anti-inflammatory therapeutics. We lastly summarize strategies targeting the Gal-1 – Gal-1 ligand axis to circumvent Gal-1-driven immune escape in cancer and boost anti-tumor immunity.
Keywords: Galectin-1, Galectin-1 ligands, Immunoregulation, Cancer Immune Evasion, Carbohydrate Therapeutics, Immunotherapy
2. Overview of Galectins
Galectins are a family of 15 carbohydrate-binding proteins (lectins) highly conserved throughout animal species [1]. Most galectins are widely distributed, though galectin (Gal)-5, -10 and -12 show tissue-specific distribution [2]. While galectins are variably expressed by all immune cells, they are upregulated in activated B and T cells, inflammatory macrophages, decidual natural killer (NK) cells, and FoxP3+ regulatory T cells [3; 4].
Galectins contain a variety of structural arrangements, but a relatively conserved carbohydrate recognition domain (CRD). The majority of galectins display a single CRD, and are biologically active as monomers (Gal-5, -7 and -10) [5], or require homodimerization for functional activity (Gal-1, -2, -11, -13, -14 and -15) [5]. Alternatively, tandem-repeat-type galectins (Gal-4, -8, -9, and -12) contain two CRDs separated by a short linker peptide, while Gal-3 (chimeric type) has a single CRD fused to a non-lectin domain that can be complexed with other Gal-3 monomers to form an oligomeric pentamer [5].
Galectins lack signal sequences required for conventional secretion. Nevertheless, many of them are soluble cytokine-like molecules secreted by an alternative pathway that resembles the Na+/K+ ATPase pump [6]. Galectins bind multiple glycoconjugates bearing the basic core disaccharide N-acetyllactosamine (LacNAc) found in O-linked and/or N-linked glycans [7; 8]. Importantly, carbohydrate-binding specificity within the galectin family is conferred by minor modifications in the lactosamine backbone, such as the extent of N-glycan branching, the multiplicity of LacNAc units, and terminal sialylation or fucosylation [9; 10]. Of note, some galectins, such as Gal-10, bind to mannose-containing glycans, suggesting other relatively unexplored functions like pathogen-recognition [11].
Cumulative evidence suggests a major role for galectins as mediators of inflammatory resolution phases by virtue of their pro-apoptotic activities on activated leukocytes [8; 12; 13]. However, under certain circumstances, galectins, notably Gal-3, have been shown to amplify pro-inflammatory responses [14; 15], suggesting that galectin-mediated effects depend on multiple factors that include mono/multivalency and concentrations in experimental conditions, the extracellular environment, and the target cell assayed.
Gal-1 (Galaptin or LGALS1) is a ~14kDa protein that was the first described galectin family member and, due to the abundance of cysteine residues (sulfhydryl groups), is referred to as an S-type lectin [16]. Gal-1 is widely expressed in tissues of many vertebrate and invertebrate organisms, where upon secretion, requires rapid binding to extracellular ligands in order to maintain activity and stability [17]. In general, Gal-1-mediated activities/effects can be divided into two cellular contexts: (a) T-cell-related (apoptosis and cell turn-over [18; 19], immunoregulation [20; 21] and cancer immune evasion [22]), and (b) Non-T cell related (cell adhesion [23], B cell development [24], mRNA splicing [25], angiogenesis [26], and nerve and muscle differentiation/homeostasis [27; 28], among others). Gal-1 activities influencing T cell fate, immunoregulation, and formation of immune-privileged sites in cancer are the focus of this report and will be discussed in more detail in the following sections.
3. Structural Biochemistry of Galectin-1 Binding
Gal-1’s structure is influenced by 2 anti-parallel β-sheets with a conserved topology of the CRD among other members of the galectin family [29]. Gal-1’s CRD avidly recognizes and binds LacNAc-bearing structures via van der Waals interactions and hydrogen bond formation [29]. These activities are mediated by key amino acids that include His45, Asn47, Arg49, Val60, Asn62, Trp69, Glu72, and Arg74 [29].
Gal-1 is a pleotropic molecule, wherein its function is dictated by its cellular location and concentration thereof. Intracellular Gal-1 is predominantly monomeric, and mediates cell growth regulation via protein-protein interactions with cytoplasmic RAS [30]. Gal-1’s lectin activity, to the contrary, is based on β-galactoside interactions predominantly found on the extracellular compartment [31].
Secreted Gal-1 spontaneously dimerizes, inducing autocrine and/or paracrine activities through engagement of LacNAc-bearing structures on O-linked and/or N-linked glycans [31]. Glycan structures decorate membranes of most cell types, as a result of synchronized activities of glycan-modifying enzymes; glycosyltransferases and glycosidases. However, for creation of Gal-1-binding determinants, cell activation triggers 3 important post-translational events: (1) enhancement of core 2 N-acetylglucosaminyltransferase 1 (C2GNT1) activity and synthesis of core 2 O-glycans, commonly found as the backbone of Gal-1 ligands [32], (2) suppression of glycosyltransferase α2,6-sialyltransferase 1 (ST6Gal1) activity, which catalyzes the transfer of α 2,6 neuraminic acid to terminal LacNAc, abrogating Gal-1 binding [33], and (3) branching of Asn-linked complex N-glycans by N-acetylglucosaminyltransferases (Mgat genes) [34; 35]. Notably, dimeric Gal-1 binds more avidly to multiple LacNAc units (poly-LacNAc) than to a single Type 1 (Galβ1-3GlcNAc) or Type 2 (Galβ1-4GlcNAc) LacNAc unit.
Dimeric Gal-1 binds to numerous glycoproteins on activated leukocytes, including CD4, CD7, CD43 and CD45 [8; 36; 37; 38], and to extracellular matrix glycoproteins, such as laminin, fibronectin and vitronectin [17; 39]. Among its well characterized functions, Gal-1 mediates homotypic and heterotypic cell aggregation, cell adhesion to the extracellular matrix, and induces pro-apoptotic activity on activated leukocytes [17]. All of these activities are important for tumor nucleation, invasion into surrounding tissues, and immune escape; therefore, Gal-1 has become an important biomarker for disease progression in cancer, and a speculative target for the development of novel chemotherapeutic agents.
4. Galectin-1 and the Regulation of T cell Survival
Dimeric Gal-1 is universally recognized as a negative regulator of immune responses. Initially, Gal-1 had been described as a pro-adhesive molecule, mediating cancer cell interactions with components of the extracellular matrix [23]. Nevertheless, previous pioneering studies on electrolectin, a Gal-1 homologue purified from the fish Electrophorus electricus, suggested pro-apoptotic effects mediated by β-galactoside interactions [40]. Years later, Perillo et al. demonstrated that Gal-1 presence in the thymic stroma induces deletion of autoreactive immature thymocytes, and experimentally validated their findings using rGal-1 to induce apoptosis on human activated T cells and leukemic cell lines [8]. In addition, they show that Gal-1 engagement to extracellular glycoconjugates on the surface of leukemic and primary human T cells triggered clustering and polarization of CD7, CD43, and CD45 microdomains, followed by signaling and transcriptional activation that, despite many efforts, still remain controversial [38; 41]. Initial reports of Gal-1-driven apoptosis suggested a direct involvement of caspase-8 and caspase-3 activation with subsequent mitochondrial amplification mediated by cytochrome c release [42; 43]. Conversely, Hahn et al. demonstrated that Gal-1-mediated T cell death occurs independent of caspase activation through nuclear translocation of mitochondrial endonuclease G that is not accompanied by cytochrome c release [44]. Furthermore, a third group has recently challenged the Gal-1 apoptosis paradigm [45]. Using T cell leukemic lines treated with rGal-1 in the absence of DTT, exposure of phosphatidylserine residues (early sign of apoptosis) became evident 4h post-rGal-1 incubation, but significantly reversed within the next 24h, with no changes in mitochondrial potential or nuclear morphology [45]. These data offered 2 important insights into Gal-1/T cell biology: (1) DTT added to preserve functionality in Gal-1 preparations is itself able to induce pro-apoptotic changes [45] and (2) Gal-1-driven exposure of phosphatidylserine residues in vivo, leads to opsonization and further phagocytic removal, unveiling a new mechanism of cell turnover [45].
A thorough description of the glycan repertoire in mouse and human T helper (Th) subsets revealed that Gal-1 selectively induces apoptosis on pro-inflammatory Th1 and Th17 cell subsets, but not on naïve, Th2 or regulatory FoxP3+ T cells[10]. In a comprehensive analysis, Toscano et al. suggested that the susceptibility to undergo Gal-1-mediated cell death is dictated by 3 elements: (1) expression of core-2 O- glycan epitopes; (2) presence of large amounts of peanut agglutinin (PNA)-reactive asialocore-1 O-glycans; and (3) expression of Sambucus nigra agglutinin (SNA)-reactive N-acetylneuraminic acid α2,6-galactose residues on their cell surfaces [10]. In addition, their data show that Th2 binding and susceptibility to Gal-1-mediated apoptosis can be greatly enhanced after treatment with C. perfringens α2,6 sialidase, but not with S. thyphimurium α 2,3 neuraminidase [10].
Although the use of rGal-1 has offered valuable mechanistic data in regards to Gal-1 biology, Gal-1-mediated mechanisms of T cell death still remain inconclusive due to variations among targeted cell types, concentrations used, and inclusion of reducing agents, which themselves, induce pro-apoptotic activities. Nevertheless, recently described Gal-1 preparations with constitutive dimerization, either by leucine-zippered domains [46], Gal-9 peptide linkers [31], or through fusion via hFc immunoglobulin domains (Gal-1hFc) [47; 48], have shown enhanced stability in the absence of reducing agents and have overcome monomer-dimer equilibrium problems, representing novel valuable tools to study Gal-1-mediated biological effects.
5. Gal-1 and its T cell Immunomodulatory Signature
Over the past 10 years, T cell immunologists have centered their attention on particular Th subsets that prevent autoimmunity and efficiently suppress inflammatory responses. Regulatory CD4+ T cells (Treg) co-expressing high levels of CD25 and the transcription factor FoxP3, represent approximately 5% of murine circulating T cells in homeostatic conditions [49]. A similar phenotype and percentage of Tregs have also been found in human peripheral blood and promote immunosuppression and facilitate cancer progression as well [50; 51; 52; 53]. The relevance of mouse and human naturally-occurring Tregs mediating peripheral tolerance is well established, but their mechanisms of action still remain inconclusive. Although in vivo data suggest that IL-10 and transforming growth factor-β (TGF-β) are important mediators of Treg suppressive activity; regulatory effects in vitro are cytokine-independent and thought to be mediated by contact-dependent mechanisms [54]. As a result of transcriptomic and proteomic analyses to identify Treg-specific immunosuppressive molecules, Gal-1 was found to be significantly expressed and secreted by mouse and human activated Tregs [20]. In fact, Garin et al. showed that Treg immunosuppressive activity is blocked by the use of anti-Gal-1 neutralizing antibodies in vitro [20]. Although Treg cell numbers in Gal-1−/− mice are similar to wt mice, their suppressive potential ex vivo is significantly compromised [20]. Interestingly, recent evident shows that Treg cell immunosuppressive potential might also be facilitated by the expression of another member of the galectin family, Gal-10 [55].
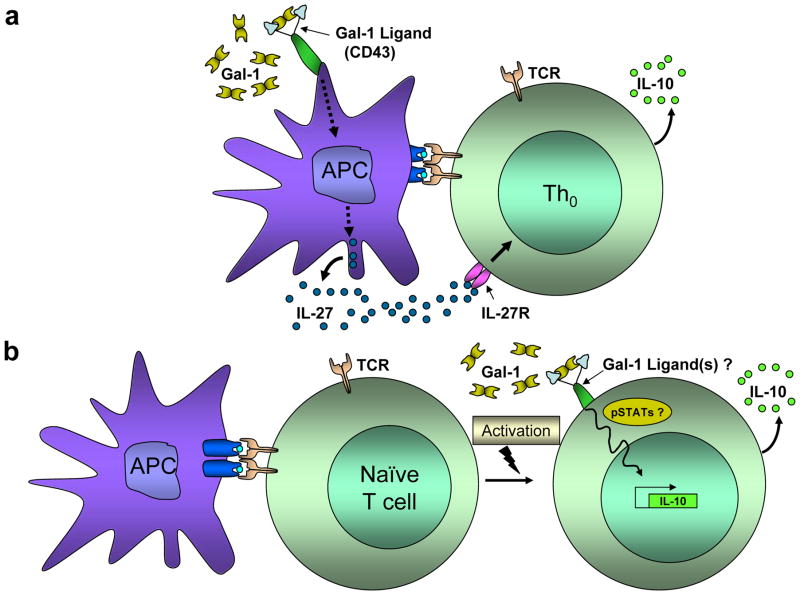
In contrast to naturally-occurring Treg cells that emerge directly from the thymus, there are peripheral mechanisms that support the development of other types of regulatory Th subsets. Regulatory type 1 (Tr1) cells, and TGF-β–producing, Th3 cells, are 2 relatively new cell subsets induced by antigen stimulation in the presence of tolerogenic signals [56]. Tr1 cells phenotypically express high levels of IL-10, show limited proliferative capacity, express low levels of IL-2, variable amounts of TGF-β, and no IL-4 [57]. In vivo, IL-10, and IL-27 significantly induce the production of Tr1 cells [58]. Notably, fully-differentiated Th1 and Th2 cell subsets can become Tr1 cells upon chronic stimulation, impairing T cell function in patients with asthma [59]. Recent reports also demonstrate that stimulation of human CD4+ T cells with anti-CD46 mAbs can efficiently induce synthesis of Tr1 cells [60]. In addition, it has been shown that CD45 ligation through specific anti-carbohydrate CD45RO/RB mAbs can regulate the formation of Tr1 cells in vitro, representing a promising and potent form of immunomodulation [61]. Alternatively, Tr1 cells can be generated in vitro, using exogenous IL-10 and IFN-γ [62], IL-27 [63], IL-21 [64], vitamin D3 and dexamethasone [65], or by repetitive stimulation with immature APCs [66]. Recently, Ilarregui et al. suggested that Gal-1 can significantly enhance the synthesis of Tr1-like IL-10+ T cells via the generation of tolerogenic APCs [21]. Upon Gal-1 engagement of CD43, APCs generate IL-27, which in turn, binds to the IL-27 receptor on Th cells, favoring the synthesis of IL-10 (Figure 1a) [21]. These Gal-1-generated Tr1 cells efficiently suppress inflammation in murine models of autoimmunity, and are thought to favor tumor growth and immune evasion in syngeneic mouse cancer models [21]. Similarly, two independent groups have shown direct Gal-1 influence on de novo synthesis of IL-10-expressing T cells, using genetically-engineered forms of Gal-1 that constitutively dimerize [46; 47; 48]. These data suggest that dimeric Gal-1 binding to activated Th cells might be able to directly promote Tr1 differentiation, although the precise mechanism and Gal-1 ligands involved in this process still remain unknown and are subject of ongoing investigation (Figure 1b).
Figure 1. Gal-1 induces the synthesis of IL-10+ T cells through direct and indirect mechanisms.
(a) Gal-1 binding to poly-LacNAc glycans (blue antennae) on the surface of antigen presenting cells (APCs) triggers the release of IL-27, which in turn, binds to the IL-27 receptor (IL-27r) on T cells, favoring the synthesis of IL-10. (b) Alternatively, stably dimeric Gal-1 preparations directly induce T cells to synthesize IL-10, though the Gal-1 inducing mechanism is still unclear and under intense investigation.
Th3 cells are another regulatory Th subset by means of their high expression of TGF-β [67]. These cells efficiently suppress antigen-specific immune responses, and have an important role of the establishment of tolerogenic environments by favoring induction of other regulatory Th cells [68]. At present, Gal-1-mediated induction of TGF-β, or expression levels of Gal-1 on Th3 cells have not been addressed.
6. Galectin-1 in T cell-Mediated Inflammation and Establishment of Cancer Immune Evasion
Gal-1 modulates T cell-mediated immune responses by virtue of its pro-apoptotic effect on pro-inflammatory Th subsets, induction of IL-10 synthesis, and Th2 cytokine skewing. In fact, expression of Gal-1 at the site of inflammation is most abundant from the peak through the resolution phases of autoimmune conditions, mirroring the expression of other regulatory molecules such as programmed death 1 (PD-1) and its ligand, PD-1L [21]. Therefore, inflammatory phenotypes of Gal-1−/− mice in models of autoimmunity tend to be more severe than in wt mice [21]. Importantly, rGal-1 has been used to ameliorate acute and chronic inflammation in different models of disease. In early 1990s, Offner et al. showed prevention and milder forms of autoimmune encephalomyelitis (EAE) in Lewis rats treated with rGal-1 [69]. Years later, Rabinovich et al. found that daily rGal-1 treatments in a mouse model of collagen-induced arthritis, shifted the cytokine milieu from a Th1 towards a Th2 response [70]. Similarly, rGal-1 treatments have been shown to prevent liver injury in a model of concanavalin A-induced hepatitis [71], and efficiently decrease T cell infiltration in models of hapten-mediated inflammatory bowel syndrome [72], and experimental autoimmune uveitis [73]. Moreover, rGal-1 treatment has been shown to delay the onset of murine Type-1 diabetes [74] via induction of high levels of IL-10, and prevent graft-versus-host disease, through suppression of IL-2 synthesis [75]. More recently, the use of a chimeric mouse Gal-1-human immunoglobulin-Fc fusion protein revealed amelioration of afferent and efferent phases of hapten-driven contact dermatitis [47], cementing the potential use of stable Gal-1 dimers as therapeutic options in inflammatory conditions.
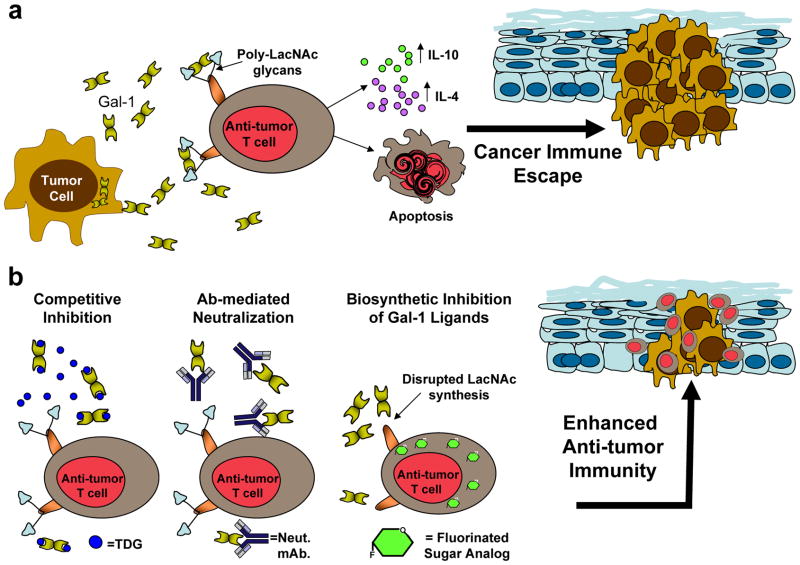
In a similar fashion, Gal-1 has been shown to play a pivotal role in cancer, promoting the formation of immune-privileged sites. Cumulative evidence shows that Gal-1 expression is correlated with aggressive phenotypes in many types of tumors [22; 76; 77]. Experimental abrogation of tumor-derived Gal-1 favors tumor rejection, derived from enhanced anti-tumor T cell proliferation and greater levels of IFN-γ [22]. Similarly, Gal-1, highly expressed in Reed Stemberg’s cells of Hodgkin’s lymphoma, has shown to promote Th2-prone environments, featured by elevated levels of IL-4, IL-13 and IL-10, and accompanied by increased numbers of Tregs that prevent effective anti-tumor immune responses (Figure 2a) [78]. As a result, novel cancer therapeutics that interfere with Gal-1 immunological activities (e.g. Gal-1 neutralizing antibodies [79], competent inhibitors of Gal-1-binding [80; 81], and metabolic modifiers of LacNAc synthesis [23; 48; 82]) (Figure 2b), are currently under investigation, as they may enhance anti-tumor immune responses and longer periods of sustained remission. In fact, we have found that treatment with a fluorinated analog of N-acetylglucosamine, 2-acetamido-1,3,6-tri-O-acetyl-4-deoxy-4-fluoro-D-glucopyranose (4-F-GlcNAc), in mice bearing syngeneic tumors, accentuates anti-tumor immunity by disrupting the synthesis of Gal-1-binding determinants on anti-tumor immunocytes [48]. 4-F-GlcNAc-treated mice exhibit significantly smaller tumors than control-treated mice through elevated levels of IFN-γ-producing T cells and tumor-specific cytotoxic T cells, and decreased synthesis of IL-10 [48].
Figure 2. Targeting the Gal-1 – Gal-1 ligand axis in tumors abrogates Gal-1-mediated immune privilege.
(a) Tumor-derived Gal-1 induces apoptosis on anti-tumor immunocytes, and skews the cytokine milieu towards a Th2 profile, promoting tumor growth. (b) Novel approaches to interfere with the Gal-1–Gal-1 ligand axis include competitive inhibition with thiodigalactoside (TDG), a lactose-based polysaccharide; neutralization with anti-Gal-1 monoclonal antibodies and metabolic inhibition of Gal-1 ligands through fluorinated sugar analogs (e.g. fully acetylated 4-fluoro-glucosamine).
7. Use of Recombinant Gal-1 in Research and Its Associated Technical Difficulties
Gal-1 is a cytokine-like molecule with promising therapeutic potential for inflammatory disorders, and a candidate target for cancer therapy. Due to its lack of post-translational modifications, Gal-1 can be easily synthesized in recombinant bacteria and purified by lactosyl sepharose columns [83]. Nonetheless, research using recombinant Gal-1 (rGal-1) has numerous drawbacks and limitations inherent to the nature of the molecule itself, and to confounding effects mediated by chemicals used to stabilize Gal-1 preparations.
Gal-1, as many other galectins, contains numerous sulfhydryl groups that mediate intermolecular and intra-molecular disulfide bonding [84]. Consequently, its rapid oxidation conditions oligomerization and abnormal folding of the CRD, restricting rGal-1’s functionality in culturing conditions to less than 8h [45].
To avoid oxidative inactivation, rGal-1 preparations are commonly supplemented with reducing agents, such as dithiothreitol (DTT) [85]. Although inclusion of DTT stabilizes Gal-1 preparations, Gal-1 concentrations ≥7μM are necessary in order prevent dissociation into monomeric forms that have weaker binding capacity [17; 86; 87]. Moreover, DTT itself has been shown to induce apoptosis, independent of the β-galactoside activity mediated by Gal-1 [45]. In fact, recent data using rGal-1 treatments in HL-60 promyeloid leukemic cells and MOLT-4 leukemic T cell cultures have failed to demonstrate full apoptotic activity in the absence of DTT [85]. Initial phosphatidylserine exposure observed 4h after rGal-1 treatment is reversible after 24h, and not accompanied by further changes in the mitochondria or nucleus suggestive of apoptosis [45]. Other approaches to overcome oxidative inactivation include the mutation of a key sulfhydryl in the cysteine-2 position for serine-2 (C2S Gal-1 variant) [17], alkylation of Gal-1 preparations with iodoacetamide (iGal-1) [88], and a genetically engineered variant with pan-deletion of all cysteine residues (CS Gal-1) [84].
Additionally, many investigators have questioned the use of ≥7μM Gal-1 as a physiological concentration. Indeed, most rGal-1 assays use concentrations ranging from 10–30μM in order to preserve a functional dimeric structure; however, this methodology has precluded the study of other immunomodulatory activities independent of apoptosis that have been attributed to Gal-1 in vivo. To this end, investigating Gal-1-mediated cytokine induction on Th cells in vitro using currently available tools has been technically challenging; nonetheless, the use of constitutively dimeric Gal-1 preparations, via leucine-zipper [46], Gal-9 linker peptides [31], or hFc immunoglobulin domains [47; 48], have already shown its potential to study these understudied Gal-1 properties experimentally.
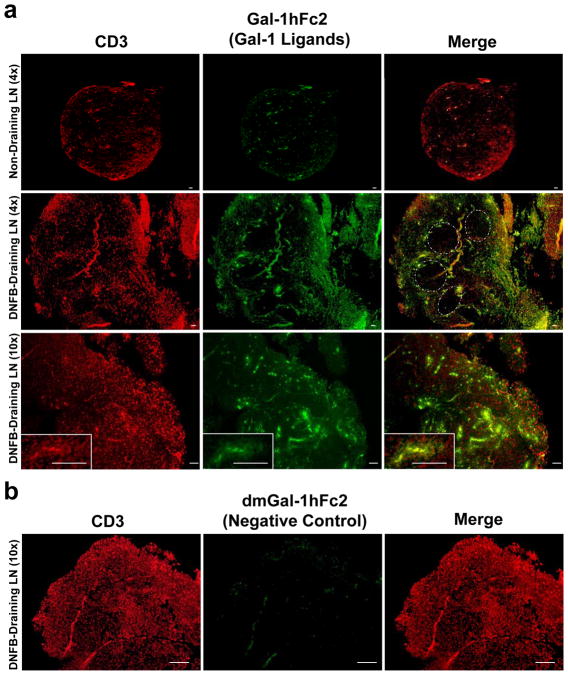
Elucidating the identity of Gal-1 ligands has also posed a major challenge in the Gal-1 field. Currently, rGal-1 is biotinylated and used as a probe in flow cytometry. In addition, biotinylated rGal-1 can be coupled to sepharose beads in order to precipitate ligands from cell lysates; however, this is a complicated procedure that requires high amounts of Gal-1 and cell lysate. In addition, biotinylated Gal-1 is not suitable for use in Western blot, due to the presence of avidin and streptavidin non-specific reactive proteins. However, a recently described Gal-1hFc fusion protein [47; 48; 82], has demonstrated Gal-1 ligand probing by multiple approaches including Western blot and immunofluorescence (Figure 3), potentially solving this long-standing problem in the field of immunoglycobiology.
Figure 3. Gal-1-binding N-acetyllactosamines are abundantly expressed in para-cortical T cell zones in a DNFB-induced model of T cell activation.
(a) Lymph nodes (LNs) draining dinitrofluorobenzene (DNFB)-sensitized skin were harvested and embedded in paraffin, and 4μm sections were stained with rabbit IgG anti-mouse CD3 (1:2000) (Abcam, San Francisco, CA) and Gal-1hFc2 (green) (20μg/ml) for 1h and counterstained with Cy-3 (red) anti-rabbit IgG (1:200) (Invitrogen, Carlsbad, CA) and rabbit anti-human IgG (1:2000) (Abcam, San Francisco, CA), followed by a 30 min incubation with Cy-5 anti-rabbit IgG (1:200) (Invitrogen, Carlsbad, CA). Note the absence of Gal-1hFc2 staining on B cell zones (dotted lines), and the clustering pattern of T cells decorated with Gal-1 ligands, suggestive of clonal expansion (inset). Representative microphotographs taken at 4X (upper and middle panels) and 10x (lower panel) are shown (Inset = 40x) (bars =100μm). (b) β-galactoside-specific Gal-1 ligand recognition on activated T cells is validated by using anti-CD3 (red) and a non-binding mutant form of Gal-1hFc2, dmGal-1hFc2 (green). Representative microphotographs from 3 experiments are shown (bars =200μm).
8. Future directions of Gal-1 research in T cell immunity
Multiple drawbacks inherent to the nature and biology of native and rGal-1 have precluded their therapeutic use, and have obscured conclusions about the precise mechanisms of Gal-1-mediated effects on T cells. Nevertheless, there is a growing number of reports describing novel approaches to enhance Gal-1’s dimeric structure, with concomitant increased stability and long-lasting biological effects [31; 46; 47]. The use of these constitutively dimeric Gal-1 preparations will help overcome mechanistic caveats, and due to their enhanced functionality, might perhaps become promising therapeutic options for treating autoimmune and inflammatory T cell-mediated conditions.
In addition, to counteract Gal-1-driven immunoregulatory effects in cancer, where Gal-1 over-expression often marks poor prognosis and advanced disease, neutralization of Gal-1 binding via blocking antibodies [79], competitive binding inhibition [80; 81], or modification of T cell Gal-1 binding determinants using fluorinated sugar analogs [23; 48; 82], are remarkable approaches with enormous potential to boost anti-tumor T cell immunity.
In summary, while Gal-1 research in the past encountered many limitations, new methods to detect Gal-1 ligands and to enhance Gal-1 stability without perturbing its biological properties have recently been reported. They represent valuable advancements in Gal-1 research, which will likely help clarify cell death paradigms, offer mechanistic insights into Gal-1-mediated cytokine regulation, and hopefully translate into effective therapies for inflammatory disorders.
Highlights.
Galectin-1 (Gal-1) has profound effects on inflammation and cancer.
Gal-1 research is limited due to the structural instability of Gal-1.
New Gal-1 formulations offer new glycobiological tools for exploring the Gal-1 – Gal-1 ligand axis in inflammation and cancer.
The Gal-1 – Gal-1 axis is a compelling anti-inflammatory and -cancer therapeutic target.
Acknowledgments
This work was funded by an NIH/NCI RO1 grant CA118124 to C. Dimitroff and an NIH/NCCAM RO1 grant AT004268 to C. Dimitroff. None of the authors has any potential financial conflict of interest related to this manuscript.
References
- 1.Gray CA, Adelson DL, Bazer FW, Burghardt RC, Meeusen EN, Spencer TE. Discovery and characterization of an epithelial-specific galectin in the endometrium that forms crystals in the trophectoderm. Proc Natl Acad Sci U S A. 2004;101:7982–7. doi: 10.1073/pnas.0402669101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yang RY, Rabinovich GA, Liu FT. Galectins: structure, function and therapeutic potential. Expert Rev Mol Med. 2008;10:e17. doi: 10.1017/S1462399408000719. [DOI] [PubMed] [Google Scholar]
- 3.Kopcow HD, Rosetti F, Leung Y, Allan DS, Kutok JL, Strominger JL. T cell apoptosis at the maternal-fetal interface in early human pregnancy, involvement of galectin-1. Proc Natl Acad Sci U S A. 2008;105:18472–7. doi: 10.1073/pnas.0809233105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rabinovich GA, Toscano MA. Turning 'sweet' on immunity: galectin-glycan interactions in immune tolerance and inflammation. Nat Rev Immunol. 2009;9:338–52. doi: 10.1038/nri2536. [DOI] [PubMed] [Google Scholar]
- 5.Camby I, Le Mercier M, Lefranc F, Kiss R. Galectin-1: a small protein with major functions. Glycobiology. 2006;16:137R–157R. doi: 10.1093/glycob/cwl025. [DOI] [PubMed] [Google Scholar]
- 6.Nickel W. Unconventional secretory routes: direct protein export across the plasma membrane of mammalian cells. Traffic. 2005;6:607–14. doi: 10.1111/j.1600-0854.2005.00302.x. [DOI] [PubMed] [Google Scholar]
- 7.Baum LG, Pang M, Perillo NL, Wu T, Delegeane A, Uittenbogaart CH, Fukuda M, Seilhamer JJ. Human thymic epithelial cells express an endogenous lectin, galectin-1, which binds to core. 2 O-glycans on thymocytes and T lymphoblastoid cells. J Exp Med. 1995;181:877–87. doi: 10.1084/jem.181.3.877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Perillo NL, Pace KE, Seilhamer JJ, Baum LG. Apoptosis of T cells mediated by galectin-1. Nature. 1995;378:736–9. doi: 10.1038/378736a0. [DOI] [PubMed] [Google Scholar]
- 9.Stowell SR, Arthur CM, Mehta P, Slanina KA, Blixt O, Leffler H, Smith DF, Cummings RD. Galectin-1, -2, and -3 exhibit differential recognition of sialylated glycans and blood group antigens. J Biol Chem. 2008;283:10109–23. doi: 10.1074/jbc.M709545200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Toscano MA, Bianco GA, Ilarregui JM, Croci DO, Correale J, Hernandez JD, Zwirner NW, Poirier F, Riley EM, Baum LG, Rabinovich GA. Differential glycosylation of TH1, TH2 and TH-17 effector cells selectively regulates susceptibility to cell death. Nat Immunol. 2007;8:825–34. doi: 10.1038/ni1482. [DOI] [PubMed] [Google Scholar]
- 11.Swaminathan GJ, Leonidas DD, Savage MP, Ackerman SJ, Acharya KR. Selective Recognition of Mannose by the Human Eosinophil Charcot-Leyden Crystal Protein (Galectin-10): A Crystallographic Study at 1.8 A Resolution. Biochemistry. 1999;38:15406. doi: 10.1021/bi995093f. [DOI] [PubMed] [Google Scholar]
- 12.Hadari YR, Arbel-Goren R, Levy Y, Amsterdam A, Alon R, Zakut R, Zick Y. Galectin-8 binding to integrins inhibits cell adhesion and induces apoptosis. J Cell Sci. 2000;113(Pt 13):2385–97. doi: 10.1242/jcs.113.13.2385. [DOI] [PubMed] [Google Scholar]
- 13.Tsuchiyama Y, Wada J, Zhang H, Morita Y, Hiragushi K, Hida K, Shikata K, Yamamura M, Kanwar YS, Makino H. Efficacy of galectins in the amelioration of nephrotoxic serum nephritis in Wistar Kyoto rats. Kidney Int. 2000;58:1941–52. doi: 10.1111/j.1523-1755.2000.00366.x. [DOI] [PubMed] [Google Scholar]
- 14.Hsu DK, Yang RY, Pan Z, Yu L, Salomon DR, Fung-Leung WP, Liu FT. Targeted disruption of the galectin-3 gene results in attenuated peritoneal inflammatory responses. Am J Pathol. 2000;156:1073–83. doi: 10.1016/S0002-9440(10)64975-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yamaoka A, Kuwabara I, Frigeri LG, Liu FT. A human lectin, galectin-3 (epsilon bp/Mac-2), stimulates superoxide production by neutrophils. J Immunol. 1995;154:3479–87. [PubMed] [Google Scholar]
- 16.Lee RT, Ichikawa Y, Allen HJ, Lee YC. Binding characteristics of galactoside-binding lectin (galaptin) from human spleen. J Biol Chem. 1990;265:7864–71. [PubMed] [Google Scholar]
- 17.Cho M, Cummings RD. Galectin-1, a beta-galactoside-binding lectin in Chinese hamster ovary cells. I. Physical and chemical characterization. J Biol Chem. 1995;270:5198–206. doi: 10.1074/jbc.270.10.5198. [DOI] [PubMed] [Google Scholar]
- 18.Perillo NL, Marcus ME, Baum LG. Galectins: versatile modulators of cell adhesion, cell proliferation, and cell death. J Mol Med. 1998;76:402–12. doi: 10.1007/s001090050232. [DOI] [PubMed] [Google Scholar]
- 19.Dias-Baruffi M, Zhu H, Cho M, Karmakar S, McEver RP, Cummings RD. Dimeric galectin-1 induces surface exposure of phosphatidylserine and phagocytic recognition of leukocytes without inducing apoptosis. J Biol Chem. 2003;278:41282–93. doi: 10.1074/jbc.M306624200. [DOI] [PubMed] [Google Scholar]
- 20.Garin MI, Chu CC, Golshayan D, Cernuda-Morollon E, Wait R, Lechler RI. Galectin-1: a key effector of regulation mediated by CD4+CD25+ T cells. Blood. 2007;109:2058–65. doi: 10.1182/blood-2006-04-016451. [DOI] [PubMed] [Google Scholar]
- 21.Ilarregui JM, Croci DO, Bianco GA, Toscano MA, Salatino M, Vermeulen ME, Geffner JR, Rabinovich GA. Tolerogenic signals delivered by dendritic cells to T cells through a galectin-1-driven immunoregulatory circuit involving interleukin 27 and interleukin 10. Nat Immunol. 2009;10:981–91. doi: 10.1038/ni.1772. [DOI] [PubMed] [Google Scholar]
- 22.Rubinstein N, Alvarez M, Zwirner NW, Toscano MA, Ilarregui JM, Bravo A, Mordoh J, Fainboim L, Podhajcer OL, Rabinovich GA. Targeted inhibition of galectin-1 gene expression in tumor cells results in heightened T cell-mediated rejection; A potential mechanism of tumor-immune privilege. Cancer Cell. 2004;5:241–51. doi: 10.1016/s1535-6108(04)00024-8. [DOI] [PubMed] [Google Scholar]
- 23.Woynarowska B, Skrincosky DM, Haag A, Sharma M, Matta K, Bernacki RJ. Inhibition of lectin-mediated ovarian tumor cell adhesion by sugar analogs. J Biol Chem. 1994;269:22797–803. [PubMed] [Google Scholar]
- 24.Gauthier L, Rossi B, Roux F, Termine E, Schiff C. Galectin-1 is a stromal cell ligand of the pre-B cell receptor (BCR) implicated in synapse formation between pre-B and stromal cells and in pre-BCR triggering. Proc Natl Acad Sci U S A. 2002;99:13014–9. doi: 10.1073/pnas.202323999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Patterson RJ, Wang W, Wang JL. Understanding the biochemical activities of galectin-1 and galectin-3 in the nucleus. Glycoconj J. 2004;19:499–506. doi: 10.1023/B:GLYC.0000014079.87862.c7. [DOI] [PubMed] [Google Scholar]
- 26.Thijssen VL, Postel R, Brandwijk RJ, Dings RP, Nesmelova I, Satijn S, Verhofstad N, Nakabeppu Y, Baum LG, Bakkers J, Mayo KH, Poirier F, Griffioen AW. Galectin-1 is essential in tumor angiogenesis and is a target for antiangiogenesis therapy. Proc Natl Acad Sci U S A. 2006;103:15975–80. doi: 10.1073/pnas.0603883103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gaudet AD, Steeves JD, Tetzlaff W, Ramer MS. Expression and functions of galectin-1 in sensory and motoneurons. Curr Drug Targets. 2005;6:419–25. doi: 10.2174/1389450054021864. [DOI] [PubMed] [Google Scholar]
- 28.Goldring K, Jones GE, Thiagarajah R, Watt DJ. The effect of galectin-1 on the differentiation of fibroblasts and myoblasts in vitro. J Cell Sci. 2002;115:355–66. doi: 10.1242/jcs.115.2.355. [DOI] [PubMed] [Google Scholar]
- 29.Lopez-Lucendo MF, Solis D, Andre S, Hirabayashi J, Kasai K, Kaltner H, Gabius HJ, Romero A. Growth-regulatory human galectin-1: crystallographic characterisation of the structural changes induced by single-site mutations and their impact on the thermodynamics of ligand binding. J Mol Biol. 2004;343:957–70. doi: 10.1016/j.jmb.2004.08.078. [DOI] [PubMed] [Google Scholar]
- 30.Paz A, Haklai R, Elad-Sfadia G, Ballan E, Kloog Y. Galectin-1 binds oncogenic H-Ras to mediate Ras membrane anchorage and cell transformation. Oncogene. 2001;20:7486–93. doi: 10.1038/sj.onc.1204950. [DOI] [PubMed] [Google Scholar]
- 31.Earl LA, Bi S, Baum LG. Galectin multimerization and lattice formation are regulated by linker region structure. Glycobiology. 21:6–12. doi: 10.1093/glycob/cwq144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hernandez JD, Nguyen JT, He J, Wang W, Ardman B, Green JM, Fukuda M, Baum LG. Galectin-1 binds different CD43 glycoforms to cluster CD43 and regulate T cell death. J Immunol. 2006;177:5328–36. doi: 10.4049/jimmunol.177.8.5328. [DOI] [PubMed] [Google Scholar]
- 33.Amano M, Galvan M, He J, Baum LG. The ST6Gal I sialyltransferase selectively modifies N-glycans on CD45 to negatively regulate galectin-1-induced CD45 clustering, phosphatase modulation, and T cell death. J Biol Chem. 2003;278:7469–75. doi: 10.1074/jbc.M209595200. [DOI] [PubMed] [Google Scholar]
- 34.Grigorian A, Torossian S, Demetriou M. T-cell growth, cell surface organization, and the galectin-glycoprotein lattice. Immunol Rev. 2009;230:232–46. doi: 10.1111/j.1600-065X.2009.00796.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Boscher C, Dennis JW, Nabi IR. Glycosylation, galectins and cellular signaling. Curr Opin Cell Biol. 2011;23:383–92. doi: 10.1016/j.ceb.2011.05.001. [DOI] [PubMed] [Google Scholar]
- 36.Fulcher JA, Chang MH, Wang S, Almazan T, Hashimi ST, Eriksson AU, Wen X, Pang M, Baum LG, Singh RR, Lee B. Galectin-1 co-clusters CD43/CD45 on dendritic cells and induces cell activation and migration through Syk and PKC signaling. J Biol Chem. 2009 doi: 10.1074/jbc.M109.037507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pang M, He J, Johnson P, Baum LG. CD45-mediated fodrin cleavage during galectin-1 T cell death promotes phagocytic clearance of dying cells. J Immunol. 2009;182:7001–8. doi: 10.4049/jimmunol.0804329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nguyen JT, Evans DP, Galvan M, Pace KE, Leitenberg D, Bui TN, Baum LG. CD45 modulates galectin-1-induced T cell death: regulation by expression of core 2 O-glycans. J Immunol. 2001;167:5697–707. doi: 10.4049/jimmunol.167.10.5697. [DOI] [PubMed] [Google Scholar]
- 39.Ozeki Y, Matsui T, Yamamoto Y, Funahashi M, Hamako J, Titani K. Tissue fibronectin is an endogenous ligand for galectin-1. Glycobiology. 1995;5:255–61. doi: 10.1093/glycob/5.2.255. [DOI] [PubMed] [Google Scholar]
- 40.Levi G, Teichberg VI. Isolation and physicochemical characterization of electrolectin, a beta-D-galactoside binding lectin from the electric organ of Electrophorus electricus. J Biol Chem. 1981;256:5735–40. [PubMed] [Google Scholar]
- 41.Pace KE, Hahn HP, Pang M, Nguyen JT, Baum LG. CD7 delivers a pro-apoptotic signal during galectin-1-induced T cell death. J Immunol. 2000;165:2331–4. doi: 10.4049/jimmunol.165.5.2331. [DOI] [PubMed] [Google Scholar]
- 42.Brandt B, Buchse T, Abou-Eladab EF, Tiedge M, Krause E, Jeschke U, Walzel H. Galectin-1 induced activation of the apoptotic death-receptor pathway in human Jurkat T lymphocytes. Histochem Cell Biol. 2008;129:599–609. doi: 10.1007/s00418-008-0395-x. [DOI] [PubMed] [Google Scholar]
- 43.Matarrese P, Tinari A, Mormone E, Bianco GA, Toscano MA, Ascione B, Rabinovich GA, Malorni W. Galectin-1 sensitizes resting human T lymphocytes to Fas (CD95)-mediated cell death via mitochondrial hyperpolarization, budding, and fission. J Biol Chem. 2005;280:6969–85. doi: 10.1074/jbc.M409752200. [DOI] [PubMed] [Google Scholar]
- 44.Hahn HP, Pang M, He J, Hernandez JD, Yang RY, Li LY, Wang X, Liu FT, Baum LG. Galectin-1 induces nuclear translocation of endonuclease G in caspase- and cytochrome c-independent T cell death. Cell Death Differ. 2004;11:1277–86. doi: 10.1038/sj.cdd.4401485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Stowell SR, Karmakar S, Arthur CM, Ju T, Rodrigues LC, Riul TB, Dias-Baruffi M, Miner J, McEver RP, Cummings RD. Galectin-1 induces reversible phosphatidylserine exposure at the plasma membrane. Mol Biol Cell. 2009;20:1408–18. doi: 10.1091/mbc.E08-07-0786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.van der Leij J, van den Berg A, Harms G, Eschbach H, Vos H, Zwiers P, van Weeghel R, Groen H, Poppema S, Visser L. Strongly enhanced IL-10 production using stable galectin-1 homodimers. Mol Immunol. 2007;44:506–13. doi: 10.1016/j.molimm.2006.02.011. [DOI] [PubMed] [Google Scholar]
- 47.Cedeno-Laurent F, Barthel SR, Opperman MJ, Lee DM, Clark RA, Dimitroff CJ. Development of a nascent galectin-1 chimeric molecule for studying the role of leukocyte galectin-1 ligands and immune disease modulation. J Immunol. 2010;185:4659–72. doi: 10.4049/jimmunol.1000715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Cedeno-Laurent F, Opperman MJ, Barthel SR, Hays D, Schatton T, Zhan Q, He X, Matta KL, Frank MH, Supko JG, Murphy GF, Dimitroff CJ. Metabolic inhibition of galectin-1-binding carbohydrates accentuates anti-tumor immunity. J Invest Dermatol. 2011 doi: 10.1038/jid.2011.335. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sakaguchi S, Sakaguchi N, Asano M, Itoh M, Toda M. Immunologic self-tolerance maintained by activated T cells expressing IL-2 receptor alpha-chains (CD25). Breakdown of a single mechanism of self-tolerance causes various autoimmune diseases. J Immunol. 1995;155:1151–64. [PubMed] [Google Scholar]
- 50.Baecher-Allan C, Brown JA, Freeman GJ, Hafler DA. CD4+CD25high regulatory cells in human peripheral blood. J Immunol. 2001;167:1245–53. doi: 10.4049/jimmunol.167.3.1245. [DOI] [PubMed] [Google Scholar]
- 51.Jonuleit H, Schmitt E, Stassen M, Tuettenberg A, Knop J, Enk AH. Identification and functional characterization of human CD4(+)CD25(+) T cells with regulatory properties isolated from peripheral blood. J Exp Med. 2001;193:1285–94. doi: 10.1084/jem.193.11.1285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Tan W, Zhang W, Strasner A, Grivennikov S, Cheng JQ, Hoffman RM, Karin M. Tumour-infiltrating regulatory T cells stimulate mammary cancer metastasis through RANKL-RANK signalling. Nature. doi: 10.1038/nature09707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Klages K, Mayer CT, Lahl K, Loddenkemper C, Teng MW, Ngiow SF, Smyth MJ, Hamann A, Huehn J, Sparwasser T. Selective depletion of Foxp3+ regulatory T cells improves effective therapeutic vaccination against established melanoma. Cancer Res. 70:7788–99. doi: 10.1158/0008-5472.CAN-10-1736. [DOI] [PubMed] [Google Scholar]
- 54.Belkaid Y, Piccirillo CA, Mendez S, Shevach EM, Sacks DL. CD4+CD25+ regulatory T cells control Leishmania major persistence and immunity. Nature. 2002;420:502–7. doi: 10.1038/nature01152. [DOI] [PubMed] [Google Scholar]
- 55.Kubach J, Lutter P, Bopp T, Stoll S, Becker C, Huter E, Richter C, Weingarten P, Warger T, Knop J, Mullner S, Wijdenes J, Schild H, Schmitt E, Jonuleit H. Human CD4+CD25+ regulatory T cells: proteome analysis identifies galectin-10 as a novel marker essential for their anergy and suppressive function. Blood. 2007;110:1550–8. doi: 10.1182/blood-2007-01-069229. [DOI] [PubMed] [Google Scholar]
- 56.Groux H, O'Garra A, Bigler M, Rouleau M, Antonenko S, de Vries JE, Roncarolo MG. A CD4+ T-cell subset inhibits antigen-specific T-cell responses and prevents colitis. Nature. 1997;389:737–42. doi: 10.1038/39614. [DOI] [PubMed] [Google Scholar]
- 57.Roncarolo MG, Levings MK. The role of different subsets of T regulatory cells in controlling autoimmunity. Curr Opin Immunol. 2000;12:676–83. doi: 10.1016/s0952-7915(00)00162-x. [DOI] [PubMed] [Google Scholar]
- 58.Pot C, Apetoh L, Awasthi A, Kuchroo VK. Molecular pathways in the induction of interleukin-27-driven regulatory type 1 cells. J Interferon Cytokine Res. 30:381–8. doi: 10.1089/jir.2010.0047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hawrylowicz CM, O'Garra A. Potential role of interleukin-10-secreting regulatory T cells in allergy and asthma. Nat Rev Immunol. 2005;5:271–83. doi: 10.1038/nri1589. [DOI] [PubMed] [Google Scholar]
- 60.Kemper C, Chan AC, Green JM, Brett KA, Murphy KM, Atkinson JP. Activation of human CD4+ cells with CD3 and CD46 induces a T-regulatory cell 1 phenotype. Nature. 2003;421:388–92. doi: 10.1038/nature01315. [DOI] [PubMed] [Google Scholar]
- 61.Gregori S, Mangia P, Bacchetta R, Tresoldi E, Kolbinger F, Traversari C, Carballido JM, de Vries JE, Korthauer U, Roncarolo MG. An anti-CD45RO/RB monoclonal antibody modulates T cell responses via induction of apoptosis and generation of regulatory T cells. J Exp Med. 2005;201:1293–305. doi: 10.1084/jem.20040912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Levings MK, Sangregorio R, Galbiati F, Squadrone S, de Waal Malefyt R, Roncarolo MG. IFN-alpha and IL-10 induce the differentiation of human type 1 T regulatory cells. J Immunol. 2001;166:5530–9. doi: 10.4049/jimmunol.166.9.5530. [DOI] [PubMed] [Google Scholar]
- 63.Pot C, Jin H, Awasthi A, Liu SM, Lai CY, Madan R, Sharpe AH, Karp CL, Miaw SC, Ho IC, Kuchroo VK. Cutting edge: IL-27 induces the transcription factor c-Maf, cytokine IL-21, and the costimulatory receptor ICOS that coordinately act together to promote differentiation of IL-10-producing Tr1 cells. J Immunol. 2009;183:797–801. doi: 10.4049/jimmunol.0901233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Spolski R, Kim HP, Zhu W, Levy DE, Leonard WJ. IL-21 mediates suppressive effects via its induction of IL-10. J Immunol. 2009;182:2859–67. doi: 10.4049/jimmunol.0802978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Barrat FJ, Cua DJ, Boonstra A, Richards DF, Crain C, Savelkoul HF, de Waal-Malefyt R, Coffman RL, Hawrylowicz CM, O'Garra A. In vitro generation of interleukin 10-producing regulatory CD4(+) T cells is induced by immunosuppressive drugs and inhibited by T helper type 1 (Th1)- and Th2-inducing cytokines. J Exp Med. 2002;195:603–16. doi: 10.1084/jem.20011629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Jonuleit H, Schmitt E, Schuler G, Knop J, Enk AH. Induction of interleukin 10-producing, nonproliferating CD4(+) T cells with regulatory properties by repetitive stimulation with allogeneic immature human dendritic cells. J Exp Med. 2000;192:1213–22. doi: 10.1084/jem.192.9.1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Carrier Y, Yuan J, Kuchroo VK, Weiner HL. Th3 cells in peripheral tolerance. I. Induction of Foxp3-positive regulatory T cells by Th3 cells derived from TGF-beta T cell-transgenic mice. J Immunol. 2007;178:179–85. doi: 10.4049/jimmunol.178.1.179. [DOI] [PubMed] [Google Scholar]
- 68.Bali MS, Lang J, Jaggy A, Spreng D, Doherr MG, Forterre F. Comparative study of vertebral fractures and luxations in dogs and cats. Vet Comp Orthop Traumatol. 2009;22:47–53. [PubMed] [Google Scholar]
- 69.Offner H, Celnik B, Bringman TS, Casentini-Borocz D, Nedwin GE, Vandenbark AA. Recombinant human beta-galactoside binding lectin suppresses clinical and histological signs of experimental autoimmune encephalomyelitis. J Neuroimmunol. 1990;28:177–84. doi: 10.1016/0165-5728(90)90032-i. [DOI] [PubMed] [Google Scholar]
- 70.Rabinovich GA, Daly G, Dreja H, Tailor H, Riera CM, Hirabayashi J, Chernajovsky Y. Recombinant galectin-1 and its genetic delivery suppress collagen-induced arthritis via T cell apoptosis. J Exp Med. 1999;190:385–98. doi: 10.1084/jem.190.3.385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Santucci L, Fiorucci S, Cammilleri F, Servillo G, Federici B, Morelli A. Galectin-1 exerts immunomodulatory and protective effects on concanavalin A-induced hepatitis in mice. Hepatology. 2000;31:399–406. doi: 10.1002/hep.510310220. [DOI] [PubMed] [Google Scholar]
- 72.Santucci L, Fiorucci S, Rubinstein N, Mencarelli A, Palazzetti B, Federici B, Rabinovich GA, Morelli A. Galectin-1 suppresses experimental colitis in mice. Gastroenterology. 2003;124:1381–94. doi: 10.1016/s0016-5085(03)00267-1. [DOI] [PubMed] [Google Scholar]
- 73.Toscano MA, Commodaro AG, Ilarregui JM, Bianco GA, Liberman A, Serra HM, Hirabayashi J, Rizzo LV, Rabinovich GA. Galectin-1 suppresses autoimmune retinal disease by promoting concomitant Th2- and T regulatory-mediated anti-inflammatory responses. J Immunol. 2006;176:6323–32. doi: 10.4049/jimmunol.176.10.6323. [DOI] [PubMed] [Google Scholar]
- 74.Perone MJ, Bertera S, Shufesky WJ, Divito SJ, Montecalvo A, Mathers AR, Larregina AT, Pang M, Seth N, Wucherpfennig KW, Trucco M, Baum LG, Morelli AE. Suppression of autoimmune diabetes by soluble galectin-1. J Immunol. 2009;182:2641–53. doi: 10.4049/jimmunol.0800839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Baum LG, Blackall DP, Arias-Magallano S, Nanigian D, Uh SY, Browne JM, Hoffmann D, Emmanouilides CE, Territo MC, Baldwin GC. Amelioration of graft versus host disease by galectin-1. Clin Immunol. 2003;109:295–307. doi: 10.1016/j.clim.2003.08.003. [DOI] [PubMed] [Google Scholar]
- 76.Szoke T, Kayser K, Baumhakel JD, Trojan I, Furak J, Tiszlavicz L, Horvath A, Szluha K, Gabius HJ, Andre S. Prognostic significance of endogenous adhesion/growth-regulatory lectins in lung cancer. Oncology. 2005;69:167–74. doi: 10.1159/000087841. [DOI] [PubMed] [Google Scholar]
- 77.Jung EJ, Moon HG, Cho BI, Jeong CY, Joo YT, Lee YJ, Hong SC, Choi SK, Ha WS, Kim JW, Lee CW, Lee JS, Park ST. Galectin-1 expression in cancer-associated stromal cells correlates tumor invasiveness and tumor progression in breast cancer. Int J Cancer. 2007;120:2331–8. doi: 10.1002/ijc.22434. [DOI] [PubMed] [Google Scholar]
- 78.Juszczynski P, Ouyang J, Monti S, Rodig SJ, Takeyama K, Abramson J, Chen W, Kutok JL, Rabinovich GA, Shipp MA. The AP1-dependent secretion of galectin-1 by Reed Sternberg cells fosters immune privilege in classical Hodgkin lymphoma. Proc Natl Acad Sci U S A. 2007;104:13134–9. doi: 10.1073/pnas.0706017104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Ouyang J, Juszczynski P, Rodig SJ, Green MR, O'Donnell E, Currie T, Armant M, Takeyama K, Monti S, Rabinovich GA, Ritz J, Kutok JL, Shipp MA. Viral induction and targeted inhibition of galectin-1 in EBV+ posttransplant lymphoproliferative disorders. Blood. 2011;117:4315–22. doi: 10.1182/blood-2010-11-320481. [DOI] [PubMed] [Google Scholar]
- 80.Stannard KA, Collins PM, Ito K, Sullivan EM, Scott SA, Gabutero E, Darren Grice I, Low P, Nilsson UJ, Leffler H, Blanchard H, Ralph SJ. Galectin inhibitory disaccharides promote tumour immunity in a breast cancer model. Cancer Lett. 2010;299:95–110. doi: 10.1016/j.canlet.2010.08.005. [DOI] [PubMed] [Google Scholar]
- 81.Ito K, Scott SA, Cutler S, Dong LF, Neuzil J, Blanchard H, Ralph SJ. Thiodigalactoside inhibits murine cancers by concurrently blocking effects of galectin-1 on immune dysregulation, angiogenesis and protection against oxidative stress. Angiogenesis. 2011 doi: 10.1007/s10456-011-9213-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Barthel SR, Antonopoulos A, Cedeno-Laurent F, Schaffer L, Hernandez G, Patil SA, North SJ, Dell A, Matta KL, Neelamegham S, Haslam SM, Dimitroff CJ. Peracetylated 4-fluoro-glucosamine reduces the content and repertoire of N- and O-glycans without direct incorporation. J Biol Chem. 2011;286:21717–31. doi: 10.1074/jbc.M110.194597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Baum LG, Seilhamer JJ, Pang M, Levine WB, Beynon D, Berliner JA. Synthesis of an endogeneous lectin, galectin-1, by human endothelial cells is up-regulated by endothelial cell activation. Glycoconj J. 1995;12:63–8. doi: 10.1007/BF00731870. [DOI] [PubMed] [Google Scholar]
- 84.Nishi N, Abe A, Iwaki J, Yoshida H, Itoh A, Shoji H, Kamitori S, Hirabayashi J, Nakamura T. Functional and structural bases of a cysteine-less mutant as a long-lasting substitute for galectin-1. Glycobiology. 2008;18:1065–73. doi: 10.1093/glycob/cwn089. [DOI] [PubMed] [Google Scholar]
- 85.Stowell SR, Karmakar S, Stowell CJ, Dias-Baruffi M, McEver RP, Cummings RD. Human galectin-1, -2, and -4 induce surface exposure of phosphatidylserine in activated human neutrophils but not in activated T cells. Blood. 2007;109:219–27. doi: 10.1182/blood-2006-03-007153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Cho M, Cummings RD. Characterization of monomeric forms of galectin-1 generated by site-directed mutagenesis. Biochemistry. 1996;35:13081–8. doi: 10.1021/bi961181d. [DOI] [PubMed] [Google Scholar]
- 87.Cho M, Cummings RD. Galectin-1, a beta-galactoside-binding lectin in Chinese hamster ovary cells. II. Localization and biosynthesis. J Biol Chem. 1995;270:5207–12. doi: 10.1074/jbc.270.10.5207. [DOI] [PubMed] [Google Scholar]
- 88.Stowell SR, Cho M, Feasley CL, Arthur CM, Song X, Colucci JK, Karmakar S, Mehta P, Dias-Baruffi M, McEver RP, Cummings RD. Ligand reduces galectin-1 sensitivity to oxidative inactivation by enhancing dimer formation. J Biol Chem. 2009;284:4989–99. doi: 10.1074/jbc.M808925200. [DOI] [PMC free article] [PubMed] [Google Scholar]