Abstract
Rationale
Subtypes of 50-kHz ultrasonic vocalizations (USVs) in rats are thought to reflect positive affect and occur with cocaine or amphetamine delivery. In contexts predicting forthcoming cocaine, pre-drug anticipatory USVs are initially minimal during daily sessions but gradually escalate over several weeks, presumably as the animal learns to expect and look forward to impending drug access. To gain more insight into motivational aspects of cocaine intake in animal models of drug dependence studies, it is important to compare experience-dependent changes in lever response rate, USVs and locomotion during cocaine conditioning and extinction trials.
Objective
To address whether cocaine-induced increases in lever responding and locomotor activity correspond with USV production. The study also determined whether short-term cocaine and context deprivation effects could be detected during conditioning or extinction.
Methods
Rats underwent 20 days of 60-min sessions of self- or yoked administration of cocaine (0.75 mg/kg/infusion, i.v.), followed by 19 days of extinction training (8 weeks total, weekends off).
Results
Lever responding for cocaine and cocaine-induced locomotor activity increased across conditioning sessions. In contrast, the number of frequency modulated (FM) 50-kHz USVs evoked in response to cocaine infusion decreased with cocaine experience, suggesting perhaps tolerance to the rewarding properties of the drug. In addition, USVs but not lever pressing or locomotion are affected after brief periods of drug and/or drug context abstinence.
Conclusions
Except for initial drug exposure, increased cocaine seeking during cocaine delivery could reflect either enhanced drug motivation or the development of drug tolerance, but not enhanced positive affect.
Keywords: Addiction, craving, cocaine seeking, deprivation effect, self-administration, yoked rats, reward, relapse, extinction
INTRODUCTION
Cocaine is a drug with psychoactive and stimulating properties. The psychological effects of low doses of cocaine are described as enhanced euphoria, sense of well-being and self-esteem, accompanied by stimulating effects such as increased energy and mental alertness (Spotts et al. 1984). However, chronic cocaine use in humans has been reported to lead to a decrease of its pleasurable euphoric effects, accompanied by an increase in frequency of cocaine use and the administration of escalating cocaine doses (i.e., development of apparent drug tolerance) (Small et al. 2009). Increased doses eventually lead to the desired “high”, but are usually accompanied by feelings of irritability, anxiety and paranoia (Resnick et al. 1977; Spotts and Shontz 1984; Trinkoff et al. 1989; Trinkoff et al. 1990; Breiter et al. 1997).
In animal models of drug dependence, the rewarding properties of cocaine have been inferred from observable animal behavior such as increased effort to obtain self-administered cocaine infusions and preference for cocaine-paired environments (Spyraki et al. 1987; Kalivas et al. 1988; Roberts et al. 1989; Hooks et al. 1994; Caine et al. 1995; McBride et al. 1999; Campbell et al. 2000; Zakharova et al. 2009). In addition, negative effects of cocaine have been observed in several animal studies, in which cocaine induces or enhances defensive behaviors, such as freezing, crouching and flight (Blanchard et al. 1998; Blanchard et al. 1999; Blanchard et al. 2000), and cocaine delivery in a runway setup has been shown to trigger conflict behavior (e.g., approach/avoidance) in rats (Ettenberg et al. 1993).
During episodes of major significance humans and animals have emotional reactions that involve responses such as physiological activation, motivational, perceptual, evaluative and learning processes (Peper 2006). Ultrasonic vocalizations (USVs) emitted by rats in response to significant events in their environment can be used as real-time reflection of emotional status. For instance, food presentation and social encounters have been shown to cause an increase in the emission of high frequency (“50-kHz”) calls (Knutson et al. 1998; Burgdorf et al. 2000), whereas the presence of a predator, aversive footshock or the touch by an unfamiliar human has been reported to elicit low frequency (“22-kHz”) calls (Blanchard et al. 1991; Brudzynski et al. 1992).
USVs have lately received increased attention in drug dependence studies because short-term administration of amphetamine or cocaine has been shown to evoke the emission of positive, in particular frequency modulated (FM), 50-kHz USVs (Wintink et al. 2001; Ahrens et al. 2009; Mu et al. 2009; Simola et al. 2009; Barker et al. 2010; Williams et al. 2010; Browning et al. 2011). Dopamine (DA) activation in the mesolimbic DA system by central amphetamine and glutamate administration as well as by electrical stimulation of the brain (e.g., nucleus accumbens, ventral tegmental area) increases the emission of 50-kHz calls (Fu et al. 1994; Burgdorf et al. 2001; Burgdorf et al. 2007). Furthermore, increased emissions of 50-kHz USVs have been reported in environments associated with cocaine, morphine and amphetamine administration and in response to cues that indicate impending drug availability (Knutson et al. 1999; Ma et al. 2010; Maier et al. 2010). These effects are of particular relevance since cocaine-induced USV data of the same animals emitting cocaine-anticipatory USVs (Ma et al. 2010; Maier et al. 2010) are shown here.
The two main goals of this study were to examine 1) hypothesized changes in cocaine-induced USVs during relatively long-term administration and extinction training sessions, and 2) how they correlate with common measures, such as locomotor activity and lever responding for intravenous self-administration of the drug. Our results may help to further understand the development of cocaine dependence and to lead to improvements in modeling drug dependence.
MATERIALS AND METHODS
Animals
Five-week old male Sprague Dawley rats (Charles River Laboratories, Inc., Wilmington, MA) were obtained for this study. During handling and lever response training (four weeks total) the rats were group-housed in polypropylene cages. After surgery, the animals were single-housed. All animals were kept under a reversed 12:12 light/dark cycle (lights on at 8 p.m.). Only during daily handling, after lever response acquisition and recovery from surgery, was food provided ad libitum. At all other times, the animals were food restricted to maintain body weight. Animals were tested daily between 9 a.m. – 12 p.m., with weekends off.
Apparatus
Lever response training, conditioning and extinction sessions were conducted in single-lever operant chambers (28 cm × 22 cm × 21 cm). The operant chambers were located within sound-attenuating boxes (Med-Associates, St. Albans, VT) to minimize external noise and light. During conditioning, catheterized rats were intravenously connected via tubing to a sterile cocaine (0.75 mg/kg/injection) or saline syringe (0.1ml/injection) mounted on a motorized pump (Razel Scientific Instruments, Model A, St. Albans, VT). Lever responses elicited during signaled intervals resulted in a 6-second infusion of either cocaine or saline solution, accompanied by the blinking of a stimulus light above the lever (i.e.,15 times during the 6-sec infusion). Three sets of photobeams, two positioned 5 cm from each sidewall and one in the center, were used to assess locomotor activity. Ultrasonic microphones (PCB Piezotronics, Buffalo, NY; working frequency range: 5–126,000 Hz) were positioned in the center of the operant chamber with a maximal distance of 22 cm to ensure digital recording of USVs. Photobeam interruptions and USVs were assessed for the entire session in 10-minute intervals.
Self-Administration and Yoked Groups
The rats were randomly assigned to one of the following four groups: cocaine self-administration (SA), cocaine yoked (Y), saline SA and saline Y. Only SA groups had access to the lever during operant training and experimental sessions. Yoked animals did not have access to the lever at any time during the experiment. Each SA rat was paired with a Y rat that passively received identical amounts of food pellets and cocaine or saline infusions.
Lever Response Training
SA rats underwent lever response training for sucrose pellets (45 mg, P.J. Noyes, Lancester, NH) on a fixed ratio-1 (FR-1) reinforcement schedule. Animals were trained for lever response for 12 days in 10-minute sessions. Lever presses resulted in the delivery of a sucrose pellet cued by a short illumination of a stimulus light above the lever. Each Y animal received the identical amount of sucrose pellets as its counterpart, with no lever being available.
Jugular Catheterization Surgery
The catheterization surgical procedure was performed as previously described (Depoortere et al. 1993). Briefly, all animals were surgically implanted with a catheter made out of Silastic tubing (8.5 cm, 0.64 mm o.d.), of which one end was connected to a cannula endpiece (Plastics One, Roanoke, VA). Anesthesia was maintained and delivered through a gas delivery system (VetEquip, Inc, Pleasanton, CA) and consisted of an isoflurane (2.5 – 4 %; AErrane, Baxter Healthcare, Deerfield, IL) and oxygen (0.8 l/min; Airgas Southwest, Corpus Christi, TX) mixture. After the catheter was inserted into the right jugular vein and secured with surgical suture, its connected endpiece was subcutaneously guided towards an incision on top of the head. Before closing the jugular incision, three drops of the antibiotic gentamicin sulfate (50 mg/ml) were applied to inhibit infection. The catheter was fixed on the skull with four stainless steel anchor screws (Plastics One, Roanoke, VA) embedded in acrylic cement. The anti-inflammatory and analgesic drug Carprofen (5 mg/kg) was also administered subcutaneously. 0.1 ml of the antibiotic Timentin (100 mg/1.5 ml), diluted in heparinized saline (1 U/ml heparin), was intravenously administered to the animals during the recovery period. Conditioning sessions commenced after a one-week recovery from the surgery. Heparinized saline (0.1 ml, i.v.) was daily administered to the animals to maintain catheter patency and to test catheter function.
Conditioning and Extinction Sessions
Conditioning and extinction sessions were conducted over 4 weeks each (i.e., 8 weeks total), 5 days per week, resulting in 20 days of conditioning and 19 days of extinction sessions. Stimulus conditions were the same between conditioning and extinction sessions except that all animals received non-reinforcing saline infusions during extinction training. Sessions commenced as follows: Animals were placed into dark operant chambers for 10 minutes prior to drug availability for assessment of drug/saline anticipatory USVs as previously reported (Ma et al. 2010; Maier et al. 2010). (Please note that more animals were added to the experiment after the two previously published studies were submitted for publication.) After the pre-drug interval, the house light was illuminated and olfactory as well as visual cues (rose or cinnamon scents and black or white wall covering) were introduced to the animals. For the next 60 minutes, the lever was available for fourteen 30-second intervals for the SA groups at variable intervals (i.e., VI schedule; at time points 610s, 700s, 1060s, 1310s, 1760s, 2070s, 2160s, 2520s, 2770s, 3220s, 3530s, 3710, 3900s, 4060s), the stimulus light located above the lever slot was illuminated for SA and Y animals. Lever responses by SA animals during the availability intervals (FR-1) resulted in the administration of either sterile cocaine or saline solution to themselves and their yoked counterparts (see more details under ‘Apparatus’). Animals were kept in their home cages over the 2-day weekend to determine whether drug and/or cue/ test context deprivation behavioral effects (enhanced lever responding, USVs and/or locomotion) might be observed upon relief from a brief abstinence.
Data Collection
Vocalization (USVs), locomotor and lever response behaviors during the 60-minute period of cocaine or saline administration of each experimental session were recorded and analyzed. MED-PC software (Med Associates, St. Albans, VT) was used to detect locomotion and lever responses, and ultrasonic microphones to detect rat USVs. USVs were recorded and assessed with RECORDER multi-channel and SASLab Pro software (Avisoft Bioacoustics, Berlin, Germany). Recording settings were as follows: Sampling rate: 22050 Hz; Format: 16 bit; Buffer: 0.2 s; Range: 40 % 250 kHz; FFT size: 256; Resolution: 86 Hz.
Assessment of Rat USVs
Detected USVs were assessed using SASLab Pro software as follows: Each recorded 10-minute interval was converted to a spectrogram and imported to a data file. Trained data analyzers used visual and auditory confirmation to assess the number of verified USVs. Experimenters performing USV differentiation were blind to the experimental groups and were individually trained by Esther Maier to assure consistent analyses. The proportion of flat/FM 50-kHz USVs were determined from a data subset consisting of one session per week (8 total) in 3 animals per group. Flat calls were defined as those varying < 5 kHz (as displayed on software-generated spectrogram) and lacking detectable auditory frequency variation in playbacks set at 11.025 kHz. Flat calls were determined to equal less than 1% of total calls across all reviewed sessions, therefore total USVs reported here include both flat and FM USVs. Additional assessment methods can also be observed in video format (Maier et al. 2010). It is important to mention that the co-occurrence of USVs and light cue could not be determined due to technical limitations.
Drug
Cocaine HCl (0.75 mg/kg/injection; RTI International, Triangle Park, NC) was dissolved in sterile 0.9 % sodium chloride. Animals were weighed daily to assure a cocaine dose of 0.75 mg/kg body weight per infusion delivered immediately after each lever response.
Data Analyses
USVs and locomotor activity recorded during the 60-minute conditioning and extinction sessions were analyzed separately using three-way repeated ANOVAs [Drug Condition (cocaine, saline) × Mode (self-administered vs. yoked) × Session Days (1–20 or 21–39)] (data for one SA cocaine and one Y cocaine animal were assessed during conditioning but not during extinction). Two-way ANOVAs were conducted on lever response data, and one-way ANOVAs were performed to examine within-group changes associated with cocaine and saline experience and to monitor weekly changes in USV-eliciting effects of cocaine injections (USVs/lever responses). To evaluate the effects of cocaine and cue deprivation during conditioning and extinction sessions, paired samples t-tests were used to compare USVs, lever responses and locomotor activity between Fridays (i.e., days 5, 10, 15 and 25, 30, 35) and the following Mondays (i.e., days 6, 11, 16 and 26, 31, 36) within cocaine and control groups. Independent samples t-tests were used to compare these values between the cocaine and control conditions. Posthoc analyses (Least Significant Difference tests) to determine precise between- and within-group differences were performed in the event of significant interaction effects. Levene’s Test for Equality of Variances was performed for all data sets. When equality of variance was violated, log transformations were applied and analysis of variance was conducted on transformed data, including locomotor activity and USV/lever response ratio during conditioning sessions and 50-kHz USVs during conditioning and extinction sessions. Data lost due to equipment recording errors (i.e., 1.5 % of total USVs and less than 1 % of locomotor activity and lever response measures) were replaced as mean group values.
RESULTS
Conditioning Sessions
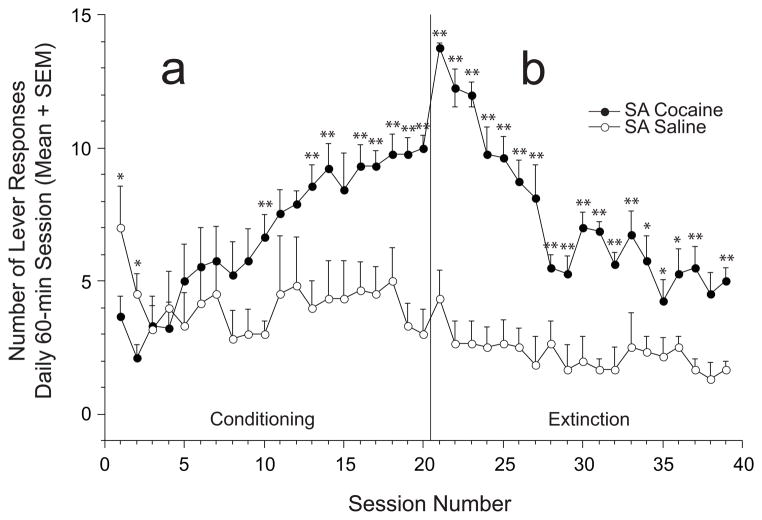
Lever Responses during Conditioning (Figure 1a)
Fig. 1.
Lever responses (mean + SEM) during conditioning and extinction sessions: Self-administration (SA) groups. Response rates of the SA cocaine group across conditioning and extinction sessions were significantly greater than the SA saline (control) group. a. During the first 2 conditioning sessions (sessions 1–20), control animals (n = 5) showed significantly greater lever responses than SA cocaine rats (n = 9), but cocaine-reinforced responding gradually increased to significantly greater levels by the 10th session. b. Across all extinction sessions (sessions 21–39), cocaine-experienced animals (n = 8) maintained significantly greater lever response rates compared to saline controls (n = 5). *, ** = significantly greater compared to matched session between groups; p < 0.05 or p < 0.01, respectively.
Two-way repeated measures ANOVA between SA cocaine and SA saline groups showed significant Drug (F(1,12) = 5.415; p = 0.038), Day (F(19,228) = 4.703; p < 0.001) and Drug × Day interaction effects (F(19,228) = 4.326; p < 0.001). One-way ANOVAs revealed significant within-group Day effects in SA cocaine (F(19,152) = 11.71; p < 0.001), but not the SA saline group (F(19,95) < 1.0; n.s.). Posthoc tests showed that saline control animals performed significantly more lever responses than cocaine rats during the first 2 sessions. However, significantly higher lever responses for the cocaine animals were observed during the last two weeks of sessions.
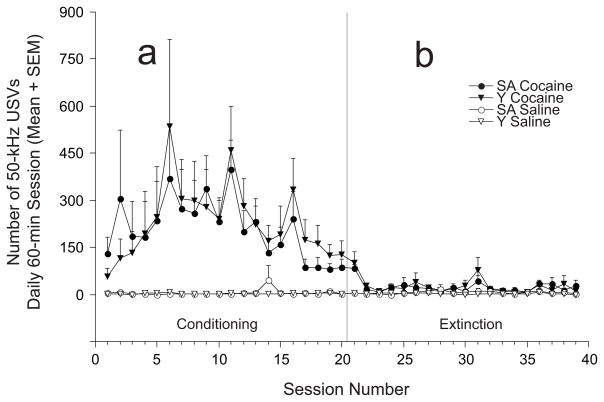
50-kHz USVs during Conditioning (Figure 2a)
Fig. 2.
50-kHz USVs (mean + SEM) during conditioning and extinction sessions: Self-administering (SA) and Yoked (Y) groups. a. Daily 50-kHz USVs were significantly greater in the cocaine groups (SA cocaine, n = 9; Y cocaine, n = 7) compared to saline groups (SA saline, n = 6; Y saline, n = 5). No significant differences were detected between SA and Y groups. b. Cocaine-experienced animals (SA cocaine, n = 8 and Y cocaine, n = 6) emitted significantly more 50-kHz USVs than saline controls (SA saline, n = 6 and Y saline, n = 5) over extinction training, but remained at control levels for the majority of the sessions.
Three-way repeated measures ANOVA showed significant Drug (F(1,23) = 142.22 p < 0.001) and Day (F(19,437) = 2.73; p < 0.01), but no significant Mode or Drug × Day interaction effects. One-way ANOVAs performed on data from combined cocaine and control groups (e.g., SA and Y groups for each drug condition) revealed significant within-group Day effects for the cocaine (F(19,285) = 3.57; p<0.001), but not the saline control groups (F(19,190) < 1.0; n.s.)
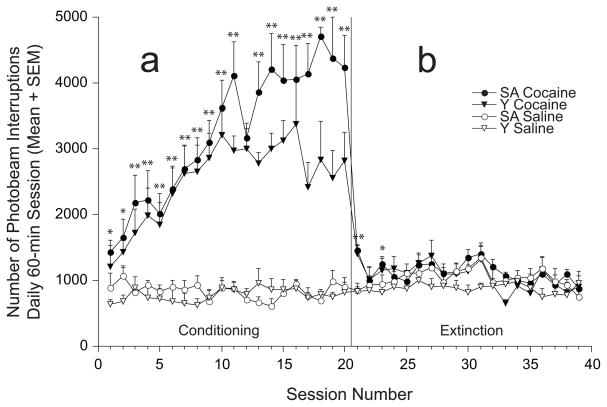
Locomotor Activity during Conditioning (Figure 3a)
Fig. 3.
Locomotor activity (mean + SEM) during conditioning and extinction sessions: SA and Y groups. a. Across all conditioning sessions, mean activity scores were significantly greater in cocaine groups (SA cocaine, n = 9; Y cocaine, n = 7) compared to controls (SA saline, n = 6; Y saline, n = 5), with no significant differences within SA and Y subgroups of each condition. b. During extinction training, a significant decrease from the first to last session was detected in the cocaine, but not saline groups. *, ** = significantly greater activity levels for both cocaine groups compared to both saline control conditions during the same session; p < 0.05 or p < 0.01, respectively.
Three-way repeated measures ANOVA showed significant effects of Drug (F(1,23) = 201.7; p < 0.001), Day (F(19,437) = 5.36; p < 0.001) and Drug × Day interaction (F(19,437) = 4.73; p < 0.001) effects, but not Mode or any other interaction effects. One-way ANOVAs revealed significant Day effects for the cocaine-receiving groups (F(19,285) = 11.55; p < 0.001), but not the saline control conditions (F(19,190) < 1.0; n.s.). Significantly greater levels of locomotor activity in both cocaine groups compared to the saline control groups at every tested session were shown.
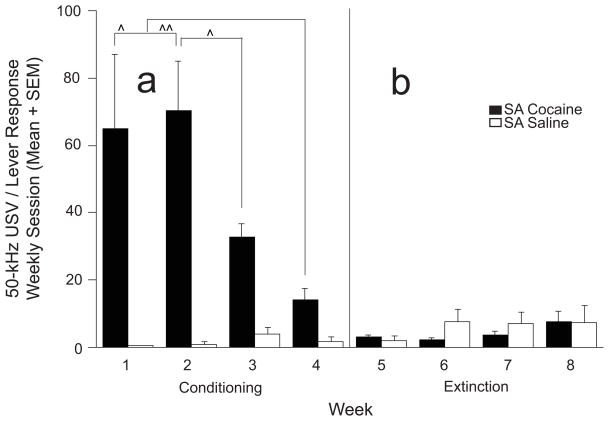
USV/Lever Response Ratio during Conditioning (Figure 4a)
Fig. 4.
USV/Lever response ratio (Weekly means + SEM) during conditioning and extinction: SA groups. a. Including rats eliciting at least 3 lever responses/week, the number of 50-kHz USVs induced by each unit dose of cocaine (0.75 mg/kg; n = 7) decreased significantly during the last 2 weeks of conditioning compared to the first two weeks. The USV/lever response ratio across all four weeks of conditioning was comparable in control animals receiving the same volume of saline injections (e.g., 0.1 ml; n = 6) during each lever b. No significant differences in weekly USV/lever response ratios were detected between SA cocaine (n = 7) and SA saline (n = 6) groups during extinction sessions. ^, ^^ = significantly smaller within-treatment effect; p < 0.05 or p < 0.01, respectively.
To compare weekly changes in the number of USVs elicited per cocaine or saline injection, one-way repeated measures ANOVA were performed on USVs/lever response ratio data (number of USVs per 0.75 mg/kg cocaine or 0.1 ml saline in SA cocaine and saline groups) from rats with at least 3 lever responses/week. Significant week effects (F(3,18) = 11.038; p < 0.001) were revealed in SA cocaine (n = 7) rats but not SA saline (n = 6) animals (F(3,15) < 1.0; n.s.), reflecting the significant decrease in USVs/lever response ratio from the first two weeks compared to the last two weeks in the SA cocaine, but not SA saline groups.
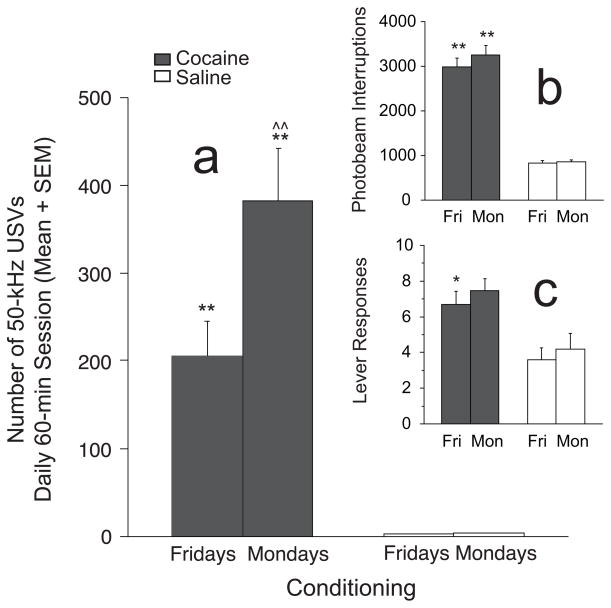
USVs, Lever Responses and Locomotor Activity before and after 2-Day Cocaine Abstinence Intervals during Conditioning (Figure 5)
Fig. 5.
50-kHz USVs, locomotor activity and lever responses (means + SEM) before and after “weekend” cocaine and cue abstinence during conditioning sessions: SA and Y combined. a. Significantly greater cocaine-induced 50-kHz USVs (n = 16) were detected on the Mondays following 2-day cocaine and cue abstinence (e.g., remaining in home cage over weekend) compared to the 5th day of consecutive cocaine administration sessions (e.g., Fridays). No significant differences were detected in cocaine-naïve animals (n = 11) after identical home cage confinement. b and c. Locomotor activity and lever responses in the cocaine animals were significantly greater than saline groups on Friday and Monday sessions, but no group showed significant differences between sessions in these measures. ^^= significantly greater within-treatment effects on Mondays vs. Fridays; p < 0.01; *, ** = significantly greater than saline control condition on same day; p < 0.05 or p < 0.01, respectively.
T-tests of USV, locomotor activity and lever response data between Fridays and the following Mondays were performed to determine the effects of 2-day cocaine and cue abstinence. Since no significant effects of Mode on USVs and locomotor activity were detected during conditioning sessions, SA and Y groups were combined for these comparisons. USVs for cocaine, but not saline groups, were significantly higher on Mondays compared to Fridays (t(15) = 4.03, p < 0.001; Paired samples t-tests) and significantly greater than USVs in the saline groups (Monday: t(25) = 3.81; p < 0.001; Friday: t(25) = 3.65; p < 0.001 for both; Independent samples t-test) (see Fig. 5a). Lever responses in cocaine-reinforced animals (i.e., SA animals) were significantly greater than SA saline controls on Fridays (t(13) = 2.409; p < 0.05) but not on Mondays (t(13) = 2.033; n.s.), but neither group showed significant 2-day abstinence effects (t(8) = 0.142 and t(5) = 1.32 for cocaine and saline, respectively; n.s. for both) (see Fig. 5b). Locomotor activity was significantly higher in the cocaine compared to saline groups on Friday (t(25) = 9.24; p < 0.001) and Monday (t(25) = 7.97; p < 0.05). However, comparisons between Fridays and Mondays in the cocaine (t(15) = 1.99; n.s.) and saline (t(10) = 0.37; n.s.) groups showed no significant abstinence effects on locomotion (see Fig. 5c).
Extinction Sessions
Lever Responses during Extinction (Figure 1b)
Two-way repeated measures ANOVA showed significant Drug (F(1,12) = 75.231; p < 0.001), Day (F(18,216) = 12.326; p < 0.001) and Drug × Day interaction effects (F(18,216) = 6.222; p < 0.001). One-way ANOVAs revealed significant within-group Day effects in SA cocaine (F(18,126) = 15.61; p < 0.001), but not the SA saline group (F(18,90) < 1.0; n.s.). Animals with previous cocaine self-administration experience maintained significantly greater lever response rates compared to controls across virtually all extinction sessions (i.e., 18/19 sessions).
50-kHz USVs during Extinction (Figure 2b)
Three-way repeated measures ANOVA showed significant Drug (F(1,21) = 25.04; p < 0.001) and Day (F(18,378) = 2.76; p < 0.001), but no other significant interactions. One-way ANOVAs revealed significant Day effects in rats that had previously received cocaine during conditioning (e.g., SA and Y cocaine groups; F(18,234) = 2.95; p < 0.001), but not in animals with no previous cocaine experience (e.g., saline control conditions; F(18,180) = 1.31; n.s.).
Locomotor Activity during Extinction (Figure 3b)
Three-way repeated measures ANOVA showed significant Day (F(18,378) = 3.027; p < 0.001) and Drug × Day interaction effects (F(18,378) = 1.797; p = 0.024), but no Drug, Mode (F(1,21) = 3.522 and 2.066, respectively, both n.s.) or other interaction effects. One-way ANOVAs revealed significant Day effects for animals with previous cocaine experience (F(18,234) = 4.15; p < 0.001), but not saline control conditions (F(18,180) < 1.0; n.s.). Locomotor activity significantly decreased in cocaine, but not saline groups, from the first to last extinction session.
USV/Lever Response Ratio during Extinction (Figure 4b)
One-way ANOVAs performed on USV/Lever responses (number of USVs per 0.1 ml saline in SA cocaine and saline groups) from rats with at least 3 lever responses/week showed no significant differences across Weeks for SA cocaine (n = 7) or SA saline (n = 6) groups (F(3,18) = 2.05 and F(3,15) < 1.0, respectively; both n.s.)
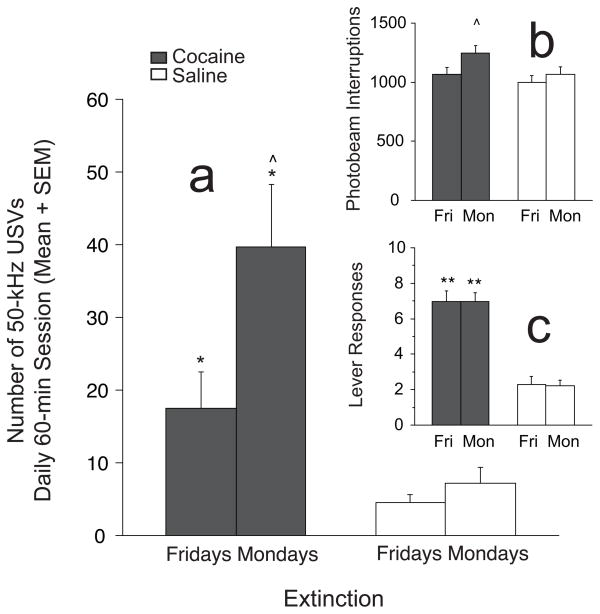
USVs, Lever Responses and Locomotor Activity before and after 2-Day Cue Abstinence Intervals during Extinction (Figure 6)
Fig. 6.
50-kHz USVs, locomotor activity and lever responses (means + SEM) before and after “weekend” cue abstinence during extinction sessions: SA and Y combined. a. Significantly greater cocaine-induced 50-kHz USVs (n =14) were detected on the Mondays following 2 days of cue abstinence (e.g., weekend in home cage only) compared to the 5th day of consecutive extinction sessions (e.g., Fridays). No significant differences were detected in cocaine-naïve animals (n = 11) after identical home cage confinement. b. Cocaine-experienced rats showed significantly greater locomotor activity on Mondays compared to Fridays and compared to saline controls. c. Lever responses in the cocaine animals were significantly greater than saline groups on Friday and Monday sessions, but lever responses were comparable between sessions. ^^ = significantly greater within-treatment effects on Mondays vs. Fridays; p < 0.01; *, ** = significantly greater than saline control condition on same day; p < 0.05 or p < 0.01, respectively.
T-tests were performed on USVs, lever responses and locomotor activity occurring on Fridays and the following Mondays to determine effects of 2-day cue abstinence in cocaine-experienced and cocaine-naïve groups. Cocaine-experienced rats showed significantly greater USVs (t(13) = 2.32; p < 0.05) and locomotor activity (t(13) = 2.53; p < 0.05) when placed back into the operant chamber after 2 days of home cage only. USVs were significantly greater in cocaine-experienced versus cocaine-naïve rats on Fridays (t(23) = 2.05; p < 0.05) and Mondays (USVs: t(23) = 2.52; p < 0.05) compared to saline (see Fig. 6a and c). Lever responses by both cocaine-experienced (t(7) = 0.00; n.s.) and cocaine-naïve (t(5) = 0.09; n.s.) rats did not differ on these days (see Fig. 6b).
DISCUSSION
During the course of cocaine conditioning, both the number of lever responses and locomotor activity gradually escalated in the cocaine groups over several weeks, as expected. However, although cocaine-induced 50-kHz USVs also escalated during the first week of cocaine administration, it reached a plateau in the second week and gradually dropped off over the last two weeks despite the continuing escalation of lever responses for cocaine. Indeed, analyses of the number of USVs elicited per cocaine injection were significantly higher in the first two weeks, compared to the last two weeks during conditioning. Moreover, for the cocaine groups the number of 50-kHz USVs was significantly elevated on the session following 2-day (weekend) abstinence, but locomotor activity and lever response rates were not.
Our study confirms previous findings that cocaine administration induces 50-kHz USVs in rats (Mu et al. 2009; Barker et al. 2010; Williams and Undieh 2010; Browning et al. 2011), as has been found with amphetamine (Burgdorf et al. 2001; Ahrens et al. 2009; Simola et al. 2009; Barker et al. 2010; Wright et al. 2010) but not caffeine (Simola et al. 2009). As previously reported (Maier et al. 2008), animals in the cocaine self-administration group progressively increased lever responding across conditioning sessions. These findings are in contrast to other limited-access studies (i.e., 1-hour sessions) that show little or no enhancement of drug intake behavior (Ahmed et al. 1998; Ben-Shahar et al. 2004; Mantsch et al. 2004). However, in the present experiment, cocaine was available on a variable interval (VI) schedule. Therefore, the observed escalation in lever responses over sessions may reflect increasing expertise in learning the task rather than a “transition to drug dependence” primarily associated with extended-access sessions (Depoortere et al. 1993; Ahmed and Koob 1998; Ben-Shahar et al. 2004; Mantsch et al. 2004; Liu et al. 2005). In addition, an increase of cocaine-induced locomotor activity was evident throughout conditioning, which progressively increased with cocaine experience. While this finding is consistent with other reports of cocaine-induced behavioral sensitization after repeated administration of equal doses (Post et al. 1976; Kalivas et al. 1988; Hooks et al. 1994; Crombag et al. 2002; Williams and Undieh 2010), our study does not specifically address the development of sensitization effects, since animals increased cocaine intake as well as locomotor activity across sessions. A similar increase in cocaine-induced locomotor activity during self-administration was previously reported in our laboratory (Maier et al. 2008).
However, it is quite notable that over the 20 days (4 weeks, weekends off) of cocaine conditioning, the ratio of FM 50-kHz USVs per lever response (i.e., drug intake) was significantly lower during the last 2 weeks of conditioning, compared to the first 2 weeks. Since FM 50-kHz and not flat 50-kHz USVs are thought to be strongly associated with a reward state (Burgdorf et al. 2007; Burgdorf et al. 2008; Wohr et al. 2008; Ahrens et al. 2009; Simola et al. 2009; Wright et al. 2010), the observed decline in FM 50-kHz USVs, despite increased drug intake suggests a tolerance-like response developing to the rewarding properties of cocaine parallel to a sensitization to the reinforcing effects of the drug. However, since positive affect USVs can also be decreased by the presence of aversive stimuli (Knutson et al. 1998), alternate explanations are also conceivable. For instance, it has been reported that cocaine in high doses can cause aversive states in human users (Spotts and Shontz 1984). As observed in the present study, there was a close correspondence between increasing levels of cocaine intake and the significant decrease in the number of 50-kHz USVs emitted per each cocaine injection. Although seemingly counter-intuitive (i.e., decrease in rewarding state despite higher drug intake), the higher cocaine intake seen in the last several cocaine sessions could have caused an increase in the non-rewarding effects of cocaine, resulting in fewer cocaine-induced USVs.
Nevertheless, with or without increased drug intake, a crucial factor likely contributing to our present findings concerns long-term effects of drug exposure. For example, even though previous work has reported that experimenter-delivered i.p. cocaine-, (Mu et al. 2009; Williams and Undieh 2010) or i.v. amphetamine-induced (Ahrens et al. 2009) USVs progressively increase with repeated administration of a constant drug dose, these studies were conducted over short intervals (5 days). However, Browning and colleagues recently published a long-term SA cocaine study that shows an increase of 50-kHz USVs during long-term cocaine self-administration (Browning et al. 2011). USVs were measured at four different time points during the experiment, in which the recordings were started immediately after the session commenced without a preceding anticipation period. This is of particular interest since recent publications from our lab (Ma et al. 2010; Maier et al. 2010) showed that during a drug-free 10-minute interval, the same rats used in the current study chronically emitted anticipatory 50-kHz USVs prior to cocaine self- or yoked administration that gradually and markedly increased over the entire 20 days of conditioning (Ma et al. 2010). Taken together, the eventual decrease in cocaine-induced USVs during cocaine availability may imply that a gradual temporal shift of cocaine reward occurred, from the reward itself to the conditioned environment, as observed in people and non-human primates [see review, (Schultz 2010)].
Following four weeks of cocaine conditioning all rats underwent an additional four weeks of extinction training. A rapid decline in 50-kHz USVs occurred and the number of calls dropped down to control levels only two days into extinction. Note that even prior to the start of extinction training, the number of USVs elicited by each cocaine injection reduced markedly. Thus, the process that decreased the rewarding properties of cocaine already appeared to be underway. This process may have combined with extinction learning to inhibit reward and/or expectancy. Interestingly, a comparably rapid or extensive drop was not evident for lever response behavior. Throughout the entire extinction training, cocaine-experienced SA animals made substantially more non-reinforced lever responses than SA saline controls. These data suggest that lever responses in these animals were perhaps the result of an over-learned motor behavior rather than via motivational processes (Horvitz 2001). Alternatively, the high number of lever responses might reflect “checking”; a behavior that exists despite diminished expectations of changes in outcome (Djodari-Irani et al. 2011; Schwabe et al. 2011). In this case, the complete decline in USVs suggests that cocaine delivery was not anticipated though lever responding was persistent. Indeed, our SA saline rats showed a similar pattern during the first two days of conditioning; 50-kHz USVs at low levels during high rates of lever responding. Taken together, USVs may be a more sensitive measure of changes in reward conditions, and may prove to be a vital assessment tool in animal studies of cocaine dependence.
During cocaine conditioning, there was no effect of mode of drug administration (i.e., self- or experimenter administration) on the emission of FM 50-kHz USVs. Mode of drug administration has been shown to elicit different cocaine effects, such as cocaine and cocaine-associated taste cue avoidance, release of glutamate in the ventral tegmental area and dopamine receptor levels (Stefanski et al. 2007; You et al. 2007; Twining et al. 2009). However, our current findings may imply that the mechanisms that trigger cocaine-induced FM 50-kHz USVs under the experimental conditions utilized here are independent of the motivational effects associated with cocaine delivery.
Note that in the present study, negative-affect 22-kHz calls were not observed in SA or Y animals during conditioning or extinction conditions. Others have reported emission of long flat 22-kHz calls during withdrawal from chronic cocaine exposure, but only when triggered by startle conditions (Mutschler et al. 1998; Mutschler et al. 1998). Long flat 22-kHz USVs are generally known to reflect a negative emotional state (Blanchard et al. 1991; Brudzynski and Ociepa 1992; Knutson et al. 2002). In a recent study utilizing 6-hr cocaine access sessions, short 22-kHz USVs were observed in rats self-administering two different cocaine doses (0.355 and 0.71 mg/kg/infusion), though more prominently in the low dose (Barker et al. 2010). Since the conditions under which short, as opposed to long, 22-kHz calls remain unclear at this time, further research should explore which conditions typically evoke various 22-kHz calls. Methodological differences among cocaine administration studies exploring USVs are likely to affect USV emissions and result in inconsistencies between findings. For example, in some experimental designs, USV data are only collected from a subset of the total number of cocaine administration sessions, so daily changes in USVs are not tracked (Barker et al. 2010). In addition, differences in the duration of cocaine access (e.g., short- or long-access) or whether animals are ever removed from the cocaine-paired context would likely influence cocaine-induced or cocaine-conditioned USVs. In the current study, 1-hr cocaine administration sessions occurred 5 days/week, animals occupied a separate home cage when not participating in training sessions and USVs were recorded during each and every 1-hr conditioning and extinction session. Therefore, the current methodology is unique for the ability to determine the effects of test context, cue abstinence and daily changes in USVs.
Cocaine-experienced yoked and SA rats similarly emitted more 50-kHz USVs after 2 days of abstinence (i.e., more 50-kHz USVs on Mondays vs. the previous Fridays) during conditioning and during extinction training. Drug deprivation-type effects have been reported not only for cocaine (Tran-Nguyen et al. 1998; Grimm et al. 2001; Lu et al. 2004; Kerstetter et al. 2008; Maier et al. 2010) but also for alcohol and nicotine (Sinclair et al. 1968; Rodd et al. 2004; Bell et al. 2006; O'Dell et al. 2007). However, in contrast to the cocaine deprivation effect described for anticipatory 50-kHz USVs (Maier et al. 2010), which is evident even at the Monday following the first weekend deprivation, a marked increase in alcohol consumption per se has so far been shown to occur only with many weeks of extended experience or long periods of abstinence (Sinclair and Senter 1968; Rodd et al. 2004; Bell et al. 2006; O'Dell and Koob 2007). Likewise, in the present report only the number of 50-kHz USVs, but not the amount of horizontal locomotion and reinforced or non-reinforced lever responses, was significantly enhanced after weekends. Since the 2-days of abstinence occurred only on weekends, it is possible that non-specific events occurring only on these particular days may alter behaviors observed on Mondays. Only USVs and not locomotor activity or lever responding were significantly altered, thus, USVs could be more sensitive to these non-specific events. Previous “incubation of cocaine seeking” studies report that a minimum of 7 or more abstinence days is required to produce increased lever response behavior during extinction training (Grimm et al. 2001; Kerstetter et al. 2008; Lu et al. 2004; Tran-Nguyen et al. 1998). Therefore, our data might indicate that a 2-day drug deprivation period was not long enough to affect lever response rates, but was long enough to enhance events that mediate USVs. These data suggest that USVs may be a more sensitive measurement of learned associations between the environment and cocaine delivery (i.e., “cocaine expectation”) than lever response behavior. However, lever responses and USVs are fundamentally different indices of cocaine reinforcement and may not be comparable using absolute values (i.e., number of occurrences). Additional analyses using percent change in lever responses from Monday to Friday were performed, yet no significant differences were detected using these data conversions of lever response rates.
Sensitization of drug-induced 50-kHz USVs after repeated same-dose drug exposure has been previously shown for short-term cocaine and amphetamine treatment (Ahrens et al. 2009; Mu et al. 2009; Williams & Undieh, 2010). Thus, the weekly enhancement of cocaine-induced 50-kHz USVs on Mondays (e.g., after 2 days of total cocaine and context abstinence) during conditioning might be the result of USV-specific incubation effects since lever responding for drug intake does not yet differ between those days. Alternatively, the Monday boost may reflect a transiently slight degradation of conditioned tolerance to the drug due to forced abstinence away from cues, context and/or drug. During extinction, the elevation of 50-kHz calls on Mondays (e.g., after 2 days of context abstinence) might be triggered by the presentation of direct cocaine-paired cues, such as olfactory and visual cues within the operant environment, lever presentation and/or the illumination of stimulus lights. This finding is consistent with previous work reporting animals that previously received amphetamine, morphine or cocaine for several days in a distinct environment, vocalize more in a drug-free state in the drug-paired environment (Knutson et al. 1999; Ma et al. 2010; Maier et al. 2010).
In summary, increasing levels of cocaine experience resulted in higher response rates for SA cocaine rats. Interestingly, the number of FM 50-kHz USVs per cocaine injection declined after approximately two weeks of cocaine sessions. During extinction, USVs dropped off rapidly compared to lever responding. In addition, 2-day periods of abstinence from cocaine and/or cocaine cues exaggerated USVs, but not drug consumption or non-reinforced lever responding. The data support the possibility that USVs can provide meaningful information in cocaine-experienced rats that cannot be accessed using only lever pressing or locomotor activity behaviors.
Acknowledgments
Funding: This work was supported by NIH Grants DA014640 and DA014640-05S1, The University of Texas VP Research Office (C.L.D.), NIDA Drug Supply Program, The Davis Phinney Foundation (T.S.) and The University of Texas Waggoner Center for Alcohol and Addiction Research Bruce-Jones Graduate Fellowship (E.Y.M., A.M.A.).
We thank Sean Ma for his technical support in USV recording and analysis, Todd Maddox for his assistance in USV data analyses, Leah McAleer and Neha Thakore for their assistance with technical and personnel management, Tiffany Nguyen, John Jiles, Sam Ryo, Hunter Owen, Donna Chan, Ernest Tong, Suk Hyun Lee,Tian Tian, Linda Ju, Rachel Chavana, Rosie Maddox, Amarachi Amuneke, Ellen Kusey and Michael Rotko for their help with USV data organization and assessment.
References
- Ahmed SH, Koob GF. Transition from moderate to excessive drug intake: Change in hedonic set point. Science. 1998;282(5387):298–300. doi: 10.1126/science.282.5387.298. [DOI] [PubMed] [Google Scholar]
- Ahrens AM, Ma ST, Maier EY, Duvauchelle CL, Schallert T. Repeated intravenous amphetamine exposure: Rapid and persistent sensitization of 50-khz ultrasonic trill calls in rats. Behav Brain Res. 2009;197(1):205–209. doi: 10.1016/j.bbr.2008.08.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barker DJ, Root DH, Ma S, Jha S, Megehee L, Pawlak AP, West MO. Dose-dependent differences in short ultrasonic vocalizations emitted by rats during cocaine self-administration. Psychopharmacology (Berl) 2010;211(4):435–442. doi: 10.1007/s00213-010-1913-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell RL, Rodd ZA, Lumeng L, Murphy JM, McBride WJ. The alcohol-preferring p rat and animal models of excessive alcohol drinking. Addict Biol. 2006;11(3–4):270–288. doi: 10.1111/j.1369-1600.2005.00029.x. [DOI] [PubMed] [Google Scholar]
- Ben-Shahar O, Ahmed SH, Koob GF, Ettenberg A. The transition from controlled to compulsive drug use is associated with a loss of sensitization. Brain Res. 2004;995(1):46–54. doi: 10.1016/j.brainres.2003.09.053. [DOI] [PubMed] [Google Scholar]
- Blanchard DC, Blanchard RJ. Cocaine potentiates defensive behaviors related to fear and anxiety. Neurosci Biobehav Rev. 1999;23(7):981–991. doi: 10.1016/s0149-7634(99)00031-7. [DOI] [PubMed] [Google Scholar]
- Blanchard RJ, Blanchard DC, Agullana R, Weiss SM. Twenty-two khz alarm cries to presentation of a predator, by laboratory rats living in visible burrow systems. Physiol Behav. 1991;50(5):967–972. doi: 10.1016/0031-9384(91)90423-l. [DOI] [PubMed] [Google Scholar]
- Blanchard RJ, Hebert M, Dulloog L, Markham C, Figueira R, Nishimura O, Newsham K, Kaawaloa JN, Blanchard DC. Cocaine-induced sniffing stereotypy changes in response to threat. Pharmacol Biochem Behav. 2000;66(2):249–256. doi: 10.1016/s0091-3057(00)00183-0. [DOI] [PubMed] [Google Scholar]
- Blanchard RJ, Hebert MA, Dulloog L, Kaawaloa N, Nishimura O, Blanchard DC. Acute cocaine effects on stereotype and defense: An ethoexperimental approach. Neurosci Biobehav Rev. 1998;23(2):179–188. doi: 10.1016/s0149-7634(98)00019-0. [DOI] [PubMed] [Google Scholar]
- Breiter HC, Gollub RL, Weisskoff RM, Kennedy DN, Makris N, Berke JD, Goodman JM, Kantor HL, Gastfriend DR, Riorden JP, Mathew RT, Rosen BR, Hyman SE. Acute effects of cocaine on human brain activity and emotion. Neuron. 1997;19(3):591–611. doi: 10.1016/s0896-6273(00)80374-8. [DOI] [PubMed] [Google Scholar]
- Browning JR, Browning DA, Maxwell AO, Dong Y, Jansen HT, Panksepp J, Sorg BA. Positive affective vocalizations during cocaine and sucrose self-administration: A model for spontaneous drug desire in rats. Neuropharmacology. 2011;61(1–2):268–275. doi: 10.1016/j.neuropharm.2011.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brudzynski SM, Ociepa D. Ultrasonic vocalization of laboratory rats in response to handling and touch. Physiol Behav. 1992;52(4):655–660. doi: 10.1016/0031-9384(92)90393-g. [DOI] [PubMed] [Google Scholar]
- Burgdorf J, Knutson B, Panksepp J. Anticipation of rewarding electrical brain stimulation evokes ultrasonic vocalization in rats. Behav Neurosci. 2000;114(2):320–327. [PubMed] [Google Scholar]
- Burgdorf J, Knutson B, Panksepp J, Ikemoto S. Nucleus accumbens amphetamine microinjections unconditionally elicit 50-khz ultrasonic vocalizations in rats. Behav Neurosci. 2001;115(4):940–944. doi: 10.1037//0735-7044.115.4.940. [DOI] [PubMed] [Google Scholar]
- Burgdorf J, Kroes RA, Moskal JR, Pfaus JG, Brudzynski SM, Panksepp J. Ultrasonic vocalizations of rats (rattus norvegicus) during mating, play, and aggression: Behavioral concomitants, relationship to reward, and self-administration of playback. J Comp Psychol. 2008;122(4):357–367. doi: 10.1037/a0012889. [DOI] [PubMed] [Google Scholar]
- Burgdorf J, Wood PL, Kroes RA, Moskal JR, Panksepp J. Neurobiology of 50-khz ultrasonic vocalizations in rats: Electrode mapping, lesion, and pharmacology studies. Behav Brain Res. 2007;182(2):274–283. doi: 10.1016/j.bbr.2007.03.010. [DOI] [PubMed] [Google Scholar]
- Caine SB, Heinrichs SC, Coffin VL, Koob GF. Effects of the dopamine d-1 antagonist sch 23390 microinjected into the accumbens, amygdala or striatum on cocaine self-administration in the rat. Brain Res. 1995;692(1–2):47–56. doi: 10.1016/0006-8993(95)00598-k. [DOI] [PubMed] [Google Scholar]
- Campbell JO, Wood RD, Spear LP. Cocaine and morphine-induced place conditioning in adolescent and adult rats. Physiol Behav. 2000;68 (4):487–493. doi: 10.1016/s0031-9384(99)00225-5. [DOI] [PubMed] [Google Scholar]
- Crombag HS, Jedynak JP, Redmond K, Robinson TE, Hope BT. Locomotor sensitization to cocaine is associated with increased fos expression in the accumbens, but not in the caudate. Behav Brain Res. 2002;136(2):455–462. doi: 10.1016/s0166-4328(02)00196-1. [DOI] [PubMed] [Google Scholar]
- Depoortere RY, Li DH, Lane JD, Emmett-Oglesby MW. Parameters of self-administration of cocaine in rats under a progressive-ratio schedule. Pharmacol Biochem Behav. 1993;45(3):539–548. doi: 10.1016/0091-3057(93)90503-l. [DOI] [PubMed] [Google Scholar]
- Djodari-Irani A, Klein J, Banzhaf J, Joel D, Heinz A, Harnack D, Lagemann T, Juckel G, Kupsch A, Morgenstern R, Winter C. Activity modulation of the globus pallidus and the nucleus entopeduncularis affects compulsive checking in rats. Behav Brain Res. 2011;219(1):149–158. doi: 10.1016/j.bbr.2010.12.036. [DOI] [PubMed] [Google Scholar]
- Ettenberg A, Geist TD. Qualitative and quantitative differences in the operant runway behavior of rats working for cocaine and heroin reinforcement. Pharmacol Biochem Behav. 1993;44(1):191–198. doi: 10.1016/0091-3057(93)90298-8. [DOI] [PubMed] [Google Scholar]
- Fu XW, Brudzynski SM. High-frequency ultrasonic vocalization induced by intracerebral glutamate in rats. Pharmacol Biochem Behav. 1994;49 (4):835–841. doi: 10.1016/0091-3057(94)90231-3. [DOI] [PubMed] [Google Scholar]
- Grimm JW, Hope BT, Wise RA, Shaham Y. Neuroadaptation. Incubation of cocaine craving after withdrawal. Nature. 2001;412(6843):141–142. doi: 10.1038/35084134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hooks MS, Duffy P, Striplin C, Kalivas PW. Behavioral and neurochemical sensitization following cocaine self-administration. Psychopharmacology (Berl) 1994;115(1–2):265–272. doi: 10.1007/BF02244782. [DOI] [PubMed] [Google Scholar]
- Horvitz JC. The effects of d1 and d2 receptor blockade on the acquisition and expression of a conditioned appetitive response. Appetite. 2001;37 (2):119–120. doi: 10.1006/appe.2001.0419. [DOI] [PubMed] [Google Scholar]
- Kalivas PW, Duffy P, DuMars LA, Skinner C. Behavioral and neurochemical effects of acute and daily cocaine administration in rats. J Pharmacol Exp Ther. 1988;245(2):485–492. [PubMed] [Google Scholar]
- Kerstetter KA, Aguilar VR, Parrish AB, Kippin TE. Protracted time-dependent increases in cocaine-seeking behavior during cocaine withdrawal in female relative to male rats. Psychopharmacology (Berl) 2008;198(1):63–75. doi: 10.1007/s00213-008-1089-8. [DOI] [PubMed] [Google Scholar]
- Knutson B, Burgdorf J, Panksepp J. Anticipation of play elicits high-frequency ultrasonic vocalizations in young rats. J Comp Psychol. 1998;112(1):65–73. doi: 10.1037/0735-7036.112.1.65. [DOI] [PubMed] [Google Scholar]
- Knutson B, Burgdorf J, Panksepp J. High-frequency ultrasonic vocalizations index conditioned pharmacological reward in rats. Physiol Behav. 1999;66(4):639–643. doi: 10.1016/s0031-9384(98)00337-0. [DOI] [PubMed] [Google Scholar]
- Knutson B, Burgdorf J, Panksepp J. Ultrasonic vocalizations as indices of affective states in rats. Psychol Bull. 2002;128(6):961–977. doi: 10.1037/0033-2909.128.6.961. [DOI] [PubMed] [Google Scholar]
- Liu Y, Roberts DC, Morgan D. Effects of extended-access self-administration and deprivation on breakpoints maintained by cocaine in rats. Psychopharmacology (Berl) 2005;179(3):644–651. doi: 10.1007/s00213-004-2089-y. [DOI] [PubMed] [Google Scholar]
- Lu L, Grimm JW, Dempsey J, Shaham Y. Cocaine seeking over extended withdrawal periods in rats: Different time courses of responding induced by cocaine cues versus cocaine priming over the first 6 months. Psychopharmacology (Berl) 2004;176(1):101–108. doi: 10.1007/s00213-004-1860-4. [DOI] [PubMed] [Google Scholar]
- Ma ST, Maier EY, Ahrens AM, Schallert T, Duvauchelle CL. Repeated intravenous cocaine experience: Development and escalation of pre-drug anticipatory 50-khz ultrasonic vocalizations in rats. Behav Brain Res. 2010;212(1):109–114. doi: 10.1016/j.bbr.2010.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maier EY, Ahrens AM, Ma ST, Schallert T, Duvauchelle CL. Cocaine deprivation effect: Cue abstinence over weekends boosts anticipatory 50-khz ultrasonic vocalizations in rats. Behav Brain Res. 2010;214:75–79. doi: 10.1016/j.bbr.2010.04.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maier EY, Ledesma RT, Seiwell AP, Duvauchelle CL. Diazepam alters cocaine self-administration, but not cocaine-stimulated locomotion or nucleus accumbens dopamine. Pharmacol Biochem Behav. 2008;91(1):202–207. doi: 10.1016/j.pbb.2008.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maier EY, Ma ST, Ahrens A, Schallert TJ, Duvauchelle CL. Assessment of ultrasonic vocalizations during drug self-administration in rats. J Vis Exp. 2010:41. doi: 10.3791/2041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mantsch JR, Yuferov V, Mathieu-Kia AM, Ho A, Kreek MJ. Effects of extended access to high versus low cocaine doses on self-administration, cocaine-induced reinstatement and brain mrna levels in rats. Psychopharmacology (Berl) 2004;175(1):26–36. doi: 10.1007/s00213-004-1778-x. [DOI] [PubMed] [Google Scholar]
- McBride WJ, Murphy JM, Ikemoto S. Localization of brain reinforcement mechanisms: Intracranial self-administration and intracranial place-conditioning studies. Behav Brain Res. 1999;101(2):129–152. doi: 10.1016/s0166-4328(99)00022-4. [DOI] [PubMed] [Google Scholar]
- Mu P, Fuchs T, Saal DB, Sorg BA, Dong Y, Panksepp J. Repeated cocaine exposure induces sensitization of ultrasonic vocalization in rats. Neurosci Lett. 2009;453(1):31–35. doi: 10.1016/j.neulet.2009.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mutschler NH, Miczek KA. Withdrawal from a self-administered or non-contingent cocaine binge: Differences in ultrasonic distress vocalizations in rats. Psychopharmacology (Berl) 1998;136(4):402–408. doi: 10.1007/s002130050584. [DOI] [PubMed] [Google Scholar]
- Mutschler NH, Miczek KA. Withdrawal from i.V. Cocaine “binges” in rats: Ultrasonic distress calls and startle. Psychopharmacology (Berl) 1998;135(2):161–168. doi: 10.1007/s002130050497. [DOI] [PubMed] [Google Scholar]
- O'Dell LE, Koob GF. 'Nicotine deprivation effect' in rats with intermittent 23-hour access to intravenous nicotine self-administration. Pharmacol Biochem Behav. 2007;86(2):346–353. doi: 10.1016/j.pbb.2007.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peper M. Imaging emotional brain functions: Conceptual and methodological issues. J Physiol Paris. 2006;99(4–6):293–307. doi: 10.1016/j.jphysparis.2006.03.009. [DOI] [PubMed] [Google Scholar]
- Post RM, Rose H. Increasing effects of repetitive cocaine administration in the rat. Nature. 1976;260(5553):731–732. doi: 10.1038/260731a0. [DOI] [PubMed] [Google Scholar]
- Resnick RB, Kestenbaum RS, Schwartz LK. Acute systemic effects of cocaine in man: A controlled study by intranasal and intravenous routes. Science. 1977;195(4279):696–698. doi: 10.1126/science.841307. [DOI] [PubMed] [Google Scholar]
- Roberts DC, Loh EA, Vickers G. Self-administration of cocaine on a progressive ratio schedule in rats: Dose-response relationship and effect of haloperidol pretreatment. Psychopharmacology (Berl) 1989;97(4):535–538. doi: 10.1007/BF00439560. [DOI] [PubMed] [Google Scholar]
- Rodd ZA, Bell RL, Sable HJ, Murphy JM, McBride WJ. Recent advances in animal models of alcohol craving and relapse. Pharmacol Biochem Behav. 2004;79(3):439–450. doi: 10.1016/j.pbb.2004.08.018. [DOI] [PubMed] [Google Scholar]
- Schultz W. Dopamine signals for reward value and risk: Basic and recent data. Behav Brain Funct. 2010;6:24. doi: 10.1186/1744-9081-6-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwabe L, Wolf OT. Stress-induced modulation of instrumental behavior: From goal-directed to habitual control of action. Behav Brain Res. 2011 doi: 10.1016/j.bbr.2010.12.038. [DOI] [PubMed] [Google Scholar]
- Simola N, Ma ST, Schallert T. Influence of acute caffeine on 50-khz ultrasonic vocalizations in male adult rats and relevance to caffeine-mediated psychopharmacological effects. Int J Neuropsychopharmacol. 2009:1–10. doi: 10.1017/S1461145709990113. [DOI] [PubMed] [Google Scholar]
- Sinclair JD, Senter RJ. Development of an alcohol-deprivation effect in rats. Q J Stud Alcohol. 1968;29(4):863–867. [PubMed] [Google Scholar]
- Small AC, Kampman KM, Plebani J, De Jesus Quinn M, Peoples L, Lynch KG. Tolerance and sensitization to the effects of cocaine use in humans: A retrospective study of long-term cocaine users in philadelphia. Subst Use Misuse. 2009;44(13):1888–1898. doi: 10.3109/10826080902961179. [DOI] [PubMed] [Google Scholar]
- Spotts JV, Shontz FC. Drug-induced ego states. I. Cocaine: Phenomenology and implications. Int J Addict. 1984;19(2):119–151. doi: 10.3109/10826088409057173. [DOI] [PubMed] [Google Scholar]
- Spyraki C, Nomikos GG, Varonos DD. Intravenous cocaine-induced place preference: Attenuation by haloperidol. Behav Brain Res. 1987;26(1):57–62. doi: 10.1016/0166-4328(87)90016-7. [DOI] [PubMed] [Google Scholar]
- Stefanski R, Ziolkowska B, Kusmider M, Mierzejewski P, Wyszogrodzka E, Kolomanska P, Dziedzicka-Wasylewska M, Przewlocki R, Kostowski W. Active versus passive cocaine administration: Differences in the neuroadaptive changes in the brain dopaminergic system. Brain Res. 2007;1157:1–10. doi: 10.1016/j.brainres.2007.04.074. [DOI] [PubMed] [Google Scholar]
- Tran-Nguyen LT, Fuchs RA, Coffey GP, Baker DA, O'Dell LE, Neisewander JL. Time-dependent changes in cocaine-seeking behavior and extracellular dopamine levels in the amygdala during cocaine withdrawal. Neuropsychopharmacology. 1998;19(1):48–59. doi: 10.1016/S0893-133X(97)00205-4. [DOI] [PubMed] [Google Scholar]
- Trinkoff AM, Ritter C, Anthony JC. The prevalence and self-reported consequences of cocaine use: An exploratory and descriptive analysis. Drug Alcohol Depend. 1990;26(3):217–225. doi: 10.1016/0376-8716(90)90163-9. [DOI] [PubMed] [Google Scholar]
- Trinkoff AM, Ritter CJ, Anthony JC. The prevalence and self-reported consequences of cocaine use. NIDA Res Monogr. 1989;95:329. [PubMed] [Google Scholar]
- Twining RC, Bolan M, Grigson PS. Yoked delivery of cocaine is aversive and protects against the motivation for drug in rats. Behav Neurosci. 2009;123(4):913–925. doi: 10.1037/a0016498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams SN, Undieh AS. Brain-derived neurotrophic factor signaling modulates cocaine induction of reward-associated ultrasonic vocalization in rats. J Pharmacy Exp Ther. 2010;332(2):463–468. doi: 10.1124/jpet.109.158535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wintink AJ, Brudzynski SM. The related roles of dopamine and glutamate in the initiation of 50-khz ultrasonic calls in adult rats. Pharmacol Biochem Behav. 2001;70(2–3):317–323. doi: 10.1016/s0091-3057(01)00615-3. [DOI] [PubMed] [Google Scholar]
- Wohr M, Houx B, Schwarting RK, Spruijt B. Effects of experience and context on 50-khz vocalizations in rats. Physiology & Behavior. 2008;93(4–5):766–776. doi: 10.1016/j.physbeh.2007.11.031. [DOI] [PubMed] [Google Scholar]
- Wright JM, Gourdon JC, Clarke PB. Identification of multiple call categories within the rich repertoire of adult rat 50-khz ultrasonic vocalizations: Effects of amphetamine and social context. Psychopharmacology (Berl) 2010;211(1):1–13. doi: 10.1007/s00213-010-1859-y. [DOI] [PubMed] [Google Scholar]
- You ZB, Wang B, Zitzman D, Azari S, Wise RA. A role for conditioned ventral tegmental glutamate release in cocaine seeking. J Neurosci. 2007;27(39):10546–10555. doi: 10.1523/JNEUROSCI.2967-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zakharova E, Leoni G, Kichko I, Izenwasser S. Differential effects of methamphetamine and cocaine on conditioned place preference and locomotor activity in adult and adolescent male rats. Behav Brain Res. 2009;198(1):45–50. doi: 10.1016/j.bbr.2008.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]