Abstract
Background
In specific vole and primate species the neuropeptide Oxytocin (OT) plays a central role in the regulation of pair-bonding behavior. Here we investigate to what extent genetic variants in the oxytocin receptor gene (OXTR) are associated with pair-bonding and related social behaviors in humans.
Methods
We first genotyped twelve Single Nucleotide Polymorphisms (SNPs) in the Twin and Offspring Study in Sweden (TOSS, N=2309) and the Swedish Twin Study of CHild and Adolescent Development (TCHAD, N=1240) comprising measures of self-reported pair-bonding behavior. In the TOSS-sample we further investigated one the SNPs for measures of marital status and quality. Moreover, in the TCHAD sample we explored the longitudinal relationship between precursors of pair-bonding during childhood and subsequent behavior in romantic relationships. Finally, in TCHAD and in the Child and Adolescent Twin Study of Sweden (CATSS, N=1771) the association between the same SNP and childhood behaviors was investigated.
Results
One SNP (rs7632287) in OXTR was associated with traits reflecting pair-bonding in women in the TOSS and TCHAD samples. In girls the rs7632287 SNP was further associated with childhood social problems, which longitudinally predicted pair-bonding behavior in the TCHAD-sample. This association was replicated in the CATSS-sample in which an association between the same SNP and social interaction deficit symptoms from the autism spectrum was detected.
Conclusion
These results suggest an association between variation in OXTR and human pair-bonding and other social behaviors, possibly indicating that the well described influence of OT on affiliative behavior in voles could also be of importance for humans.
Keywords: Monogamy, Neuropeptide, Polymorphism, Autism, Affiliative behavior, Social Problems
Introduction
Pair-bonded relationships are dyadic coalitions between sexual partners based on selective social attachments, often characterized by partner preference, biparental care and intrasexual aggression. Although it is debatable whether humans are truly monogamous lasting bonds between sexual partners are widespread throughout nearly all modern human societies. In humans pair-bonding probably evolved to increase paternal provisioning (1, 2) or as a consequence of male mating competition (3, 4). Furthermore, it has recently been suggested that pair-bonding behavior shaped the evolution of human society (5). Although pair-bonds are common in our species and an important part of human nature, knowledge about the biological foundations of the human propensity to engage in enduring pair-bonded relationships remain sparse.
Oxytocin (OT) is a neuropeptide well known for its role in the periphery, particularly in contraction of the uterus during labour and in ejection of milk during lactation. More recently OT has also been acknowledged for its influences on a wide range of social behaviors including social motivation and approach behavior (6, 7). In many species OT is also important for the formation and expression of social memory as well as for the regulation of aggression, sexual behavior and maternal care (8). In addition, similar to the role of the closely related peptide vasopressin for pair-bonding behavior in male voles, OT has a central role in the formation and regulation of pair-bonding behavior in female voles (9). In the socially monogamous and highly social prairie vole (Microtus ochrogaster) central infusions of OT facilitate (10), whereas a selective oxytocin receptor (OTR) antagonist inhibits, pair-bond formation in female individuals of this species (11). Moreover, there are notable differences in OTR distribution patterns among prairie voles and the non-monogamous montane voles (Microtus montanus), mainly in the brain region nucleus accumbens (12), and an OTR antagonist applied directly to this region blocks mating-induced partner preference formation (13). Furthermore, overexpression of the OTR in nucleus accumbens accelerates partner preference formation in female prairie voles (14). The molecular mechanism behind the differences in OTR expression has not yet been described, although differences in potential regulatory elements in the oxytocin receptor gene (OXTR), which could reflect variation in gene expression, have been found between prairie and montane voles (15). Similar to what has been shown in voles, a recent study has found that manipulations of OT activity alter partner-directed social behavior during pair interactions in the pair-bonding primate Black-tufted Marmoset (Callithrix penicillata) (16).
In humans several experimental pharmacological studies have found positive effects of intra-nasally administered OT on a wide spectrum of social behaviors, including trust (17), generosity (18), judgments of facial trustworthiness and attractiveness (19), face recognition (20), parochial altruism (21), emotion perception (22), social behaviors in autism spectrum disorder (23, 24) and more pair-bonding related phenotypes such as communication and behavior in a conflict discussion between couples (25). Studies have also shown associations between plasma levels of OT and romantic bonding related outcomes (26). Moreover, there is also evidence from neuroimaging studies suggesting effects of OT treatment on amygdala activity (27–29), a brain region known to be of importance for regulation of social behaviors. Further, genetic studies have shown that variation in OXTR is associated with social behaviors in humans such as empathy (30) attachment style in patients with depression (31), social cognition in ADHD (32), emotional support seeking (33), prosocial temperament (34), maternal sensitivity (35), prosocial decision making (36) and autism (37–46). Finally, variation in OXTR has been associated with the functioning (34) as well as the size of the amygdala (47, 48). Although these previous studies are valuable for the general understanding of the influences of OT and OTR on social behavior in humans, studies specifically investigating the extent to which OT and its receptor are implicated in pair-bonding behavior are warranted. We have previously reported an association between variation in the vasopressin receptor 1a gene and pair-bonding behavior in men (49), possibly analogous to how variation in this gene correlates with affiliative behavior in male voles. The aim of this study was to investigate whether variation in OXTR is associated with social functioning in general and pair-bonding in particular amongst humans. Considering the sexually dimorphic effects of OT shown in voles, our prime focus was on women.
Materials and Methods
An overview of the different study samples, including information about the phenotypes and the order of analyses is presented in Figure 1. More extensive descriptions of the samples and the measures used are presented below as well as in Supplemental Information.
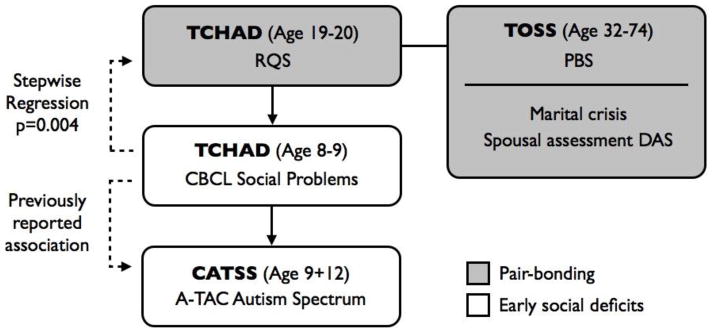
Figure 1. Overview of the order of analysis, samples and measures used to investigate associations between oxytocin receptor gene (OXTR) polymorphisms and pair-bonding behaviour as well as childhood deficits in social behaviours.
Firstly, associations between 12 SNPs in the oxytocin receptor gene (OXTR) and measures of pair-bonding were investigated in the TCHAD and TOSS samples, comprising men and women in romantic relationships (see Tables 2 and 3 for the results from these analyses ). Secondly, the SNP positively associated with pair-bonding behavior in women in the first step (rs7632287) was further studied in relation to marital crisis and measures of marital quality as perceived by spouses in the TOSS sample (Table 4a,b). Thirdly, the relation between behaviors in young girls and adult pair-bonding behavior was explored using the longitudinal design in TCHAD (Table 4c). The CBCL subscale social problems at age 8–9 was revealed to predict pair-bonding behavior in adulthood. Fourthly, the rs7632287 SNP was studied in relation to the social problems scale in TCHAD. The CBCL social problems scale has previously been shown to be associated with measures of the autism spectrum (65) and in the final step the rs7632287 SNP was studied in relation to symptoms of autism assessed in an additional sample, CATSS (Table 5). See text for further details and references regarding the samples and the different measures. Sample names are in bold text. RQS=Relationship Quality Survey, PBS=Partner Bonding Scale, CBCL=Child Behavior Checklist, A-TAC= the Autism—Tics, AD/HD, and other Comorbidities inventory.
Subjects
TOSS
The study consisted of 909 twin pairs and their spouses from both the first and second cohort of the Twin and Offspring Study in Sweden (TOSS), a two-cohort study of twin parents, one adolescent child and the spouse/partner. A more detailed description of the sample is available in a previous paper by Neiderhiser and colleagues (50). The same-sex twins included in the study were required to have a relationship of at least 5 years with their partner; while 82% were married, 18% were cohabiting but unmarried. This study used three different measures of pair-bonding behavior in the TOSS sample; the Partner Bonding Scale (PBS) (49), a martial crisis item from a life event questionnaire based on the Social Readjustment Rating Scale (51) and spouse reports of marital quality using the Dyadic Adjustment Scale (DAS) (52). DNA samples were collected for all twins participating in TOSS and all family members who participated as part of cohort 2. Since the first TOSS cohort only includes twin mothers, DNA is available for a larger amount of adult women than men. DNA was extracted from saliva samples that were collected using OraGeneR DNA self-collection kit. Zygosity was determined primarily by genotyping. A total of 2309 individuals (1463 women and 947 men) provided usable DNA samples.
TCHAD
The Twin study of CHild and Adolescent Development (TCHAD) is a longitudinal study on the health and behavior of 1480 Swedish twin pairs born between May 1985 and December 1986 (53). This study used self-report questionnaire data regarding behavior in romantic relationships from the Relationship Quality Survey (RQS) and parent-report questionnaire data regarding child behavior from the Child Behavior Checklist (CBCL) from two different times. In 1994, 1106 (75%) parents of twins 8–9 years old replied to the questionnaire and in the 2005 follow-up assessment the 19–20 year old twins’ self-reports had a response rate of 59% (n=1705). Zygosity was determined using a discriminant analysis that was previously developed using information from 385 twin pairs with known zygosity (54). DNA was extracted from mouthwash samples that were collected using DNA self-collection kit. In total, 1240 (528 boys and 712 girls) provided usable DNA samples. Of these 142 boys and 323 girls were in a romantic relationship at the age of 19–20.
CATSS
The Child and Adolescent Twin Study in Sweden (CATSS) is a nation-wide cohort that focuses on all Swedish twins turning 9 or 12 years since July 2004. CATSS has an 80% response rate, making it a highly representative population sample. Data were available on 12 446 children: N= 5944 for 9-year olds and 6496 for 12-year olds (the two samples were independent). The Autism—Tics, AD/HD, and other Comorbidities inventory (A-TAC) (55) was used for phenotypic assessment in the CATSS sample. This study used genetic material from the first DNA collection from CATSS, including information from 887 girls. DNA was extracted from saliva samples that were collected using OraGeneR DNA self-collection kit. Zygosity was determined primarily by genotyping.
Measures
Assessment Strategy
This study included a variety of measures that were constructed to assess social functioning during different developmental periods. During adulthood and late adolescence, these measures focused on pair-bonding through the assessment of current romantic relationships. For child participants, measures focused on broader indices of social functioning conceived as precursors of pair bonding.
PBS
In the TOSS sample the Partner Bonding Scale was used to assess pair-bonding behavior amongst the adult twins and their spouses. It includes items that are analogous to measures of behavioral patterns observed amongst nonhuman primates. The scale comprises items from the Dyadic Adjustment Scale (DAS) (52), a frequently used assessment of the quality of marital relationships and similar dyads, the Support Seeking and Giving (SSG) (56) assessment measuring subjects’ engagement with other people, and the Marital Instability Scale (MIS) (57). A more detailed description of the scale is presented elsewhere (49).
RQS
Within the TCHAD sample the affection subscale of the Relationship Quality Survey (58, 59) was used to assess pair-bonding behavior. This subscale was selected due to its similar to the partner-bonding behavior scale used in TOSS. The affection subscale asks participants to describe the quality of interactions with their romantic partners. Specifically, this subscale includes items such as “How affectionate are you with this person?” and “How important is it that this person likes you?” reflecting individual characteristics related to pair-bonding behavior.
CBCL
Emotional and behavioral functioning within the TCHAD study were also assessed via parent report, using the Child Behavior Checklist when the twins were 8–9 years old. The CBCL includes 118 items that describe the child’s behavioral, emotional and social problems, and 8 subscales: withdrawn, somatic complaints anxious/depressed, social problems, thought problems, attention problems, delinquent behavior and aggressive behavior. All 8 subscales were included in a stepwise regression analyses in the TCHAD sample.
A-TAC
Parents in the CATSS sample were interviewed with the Autism—Tics, AD/HD, and other Comorbidities inventory, a telephone interview designed for large-scale epidemiological research in neuropsychiatry (55). This measure was used in the current study because it assesses social functioning in great detail. Autism spectrum disorders (ASDs) are defined by current diagnostic criteria as being characterized by a triad of features: social impairments, communication impairments, and restricted repetitive behaviors and interests (DSM-IV), and thus there are different modules in A-TAC for these domains (60). A recent study from the CATSS-project showed that these domains are correlated, but have different genetic etiologies (61). Here we used A-TAC scores comprising all autism related A-TAC items (rather than dichotomous scales or scales based only on DSM-IV items) for the three domains separately as well as the A-TAC total autism spectrum score (60), because these measures had most variability and therefore provide most power to detect genetic effects in association analyses.
SNP Genotyping
At present, there are no consistent evidence for association between any specific OXTR SNP and social behaviors in the literature. There are however several reports of nominal associations to various social behaviors. In this study we have chosen polymorphisms located in as well as up- and downstream of the OXTR with a relatively even distribution that have been associated to risk of autism or other aspects of social behaviors in at least in one previous paper (with the exception of the non-synonomus SNP rs4686302). Proposed association, references and further information about the studied SNPs are found in Table 1. Genotyping of SNPs rs75775, rs1488467, rs4564970, rs53576, rs237897, rs237887, rs11720238 was performed by KBioscience (http://www.kbioscience.co.uk) using the KASPar chemistry, which is a competitive allele specific PCR SNP genotyping system using FRET quencher cassette oligos (http://www.kbioscience.co.uk/genotyping/genotyping-chemistry.htm). The remaining five SNPs rs4686302, rs2254298, rs2268493, rs1042778 and rs7632287 were genotyped using commercially available 5′ nuclease (TaqMan) assays on an ABI Prism 7900HT instrument (Applied Biosystems, Foster City, CA, USA).
Table 1.
Information about analyzed SNPs
| SNP | Minor Allele | MAF | SNP Position | Position | Proposed Associations (References) |
|---|---|---|---|---|---|
| rs75775 | T | 0.15 | 8820732 | 5′ | Autism (42) |
| rs1488467 | C | 0.05 | 8813231 | 5′ | Autism (41) |
| rs4564970 | C | 0.07 | 8810408 | Intron 1 | Autsism (41) |
| rs4686302 | T | 0.12 | 8809222 | Exon 3 | Non-synonomus SNP (A218T) |
| rs237897 | A | 0.40 | 8808285 | Intron 3 | Prosociality (36), Allelic imbalance (41) |
| rs53576 | A | 0.35 | 8804371 | Intron 3 | Social cognition/Prosociality (30–35) Amygdala activation (34) Autism (37) |
| rs2254298 | A | 0.09 | 8802228 | Intron 3 | Prosociality (31, 36), Autism (37, 38, 44, 46) Amygdala size (47, 48) |
| rs2268493 | C | 0.32 | 8800840 | Intron 3 | Autism (40, 43) |
| rs237887 | G | 0.41 | 8797042 | Intron 3 | Prosociality (36), Autism (44) |
| rs1042778 | T | 0.39 | 8794545 | 3′UTR | Prosociality (36), Autism (39, 43) |
| rs7632287 | A | 0.25 | 8791446 | 3′ | Autism (41, 43) |
| rs11720238 | T | 0.14 | 8791339 | 3′ | Autsim (41) |
MAF=Minor allele frequency.
Statistical Analysis
Statistical associations between the continuous and categorical predictors and continuous and binary outcome variables were estimated using Generalized Linear Mixed Effects Models (GLMM) in the PROC GLIMMIX procedure of SAS (SAS Institute, Inc., Cary, NC). This procedure allowed us to account for the dependent nature of the twin observations (all twins were included in the analyses) similarly to what has been shown previously (49).
Results
Twelve OXTR SNPs, evenly distributed over the gene and previously proposed to be associated with autism and other aspects of social behaviors (Table 1), were genotyped in the adult men and women from the Twin and Offspring Study in Sweden (TOSS, N=2309) (50) and in the participants of the Swedish Twin Study of CHild and Adolescent Development (TCHAD, N=1240) sample (53). The allele and genotype distributions of the studied SNPs were similar to what has been reported in previous studies and did not deviate from Hardy-Weinberg equilibrium.
Our first set of analyses revealed a significant association between the rs7632287 SNP and self reported partner-bonding behavior within the TOSS sample, as assessed by the Partner Bonding Scale (PBS) (49). For the younger women in the TCHAD sample, the same SNP was significantly associated with the affection subscale of the Relationship Quality Survey (RQS) (62) (Table 2). The P-values from both studies were combined using Fisher’s method (63), taking effect direction into account by regarding the TCHAD sample as a replication sample and performing a one-tailed test for the association between rs7632287 and the RQS scale. This resulted in a combined P-value of 0.004, significant (P<0.05) after Bonferroni correction for the twelve tests performed. We found no significant associations between any other OXTR SNPs and PBS or RQS scores in women and in line with our hypothesis based on animal data no consistent association was found in men. Consequently, all subsequent analyses focused upon women.
Table 2.
Associations between the analyzed SNPs in OXTR and two different measures of pair-bonding behavior in women (TOSS, N=1413; TCHAD, N=323)
| TOSS (Partner Bonding Scale)
|
TCHAD (Relationship Quality Survey)
|
||||||
|---|---|---|---|---|---|---|---|
| Snp | df | F | P | Snp | df | F | P |
|
|
|
||||||
| rs75775 | 2, 1054 | 1.35 | 0.26 | rs75775 | 2, 200 | 1.59 | 0.21 |
| rs1488467 | 2,512 | 1.51 | 0.22 | rs1488467 | 2, 171 | 1.03 | 0.36 |
| rs4564970 | 2, 649 | 2.41 | 0.09 | rs4564970 | 2, 166 | 1.26 | 0.29 |
| rs4686302 | 2, 1024 | 0.89 | 0.41 | rs4686302 | 2, 212 | 2.88 | 0.06 |
| rs237897 | 2, 1123 | 2.70 | 0.07 | rs237897 | 2, 271 | 0.25 | 0.78 |
| rs53576 | 2, 1119 | 2.69 | 0.07 | rs53576 | 2, 263 | 0.64 | 0.53 |
| rs2254298 | 2, 682 | 1.02 | 0.36 | rs2254298 | 2, 118 | 0.50 | 0.61 |
| rs2268493 | 2, 1102 | 1.51 | 0.22 | rs2268493 | 2, 274 | 1.30 | 0.27 |
| rs237887 | 2, 1114 | 0.72 | 0.49 | rs237887 | 2, 282 | 1.62 | 0.20 |
| rs1042778 | 2, 1139 | 0.92 | 0.40 | rs1042778 | 2, 279 | 0.61 | 0.54 |
| rs7632287 | 2, 1093 | 3.88 | 0.02 | rs7632287 | 2, 238 | 3.16 | 0.04 |
| rs11720238 | 2, 1002 | 1.09 | 0.34 | rs11720238 | 2, 204 | 0.22 | 0.80 |
When examining the mean scores of females carrying the different rs7632287 genotypes, the same pattern was seen in both the TOSS and TCHAD samples: women carrying one or two copies of the A-allele scored lower on both the PBS (d=0.15) and RQS (d=0.28) scales than did women carrying two copies of the G-allele. No difference between carriers of one or two copies of A-alleles was detected (Table 3). Therefore, in further analyses of these two cohorts A-allele carriers were combined into one category and compared to women carrying two copies of the G-allele.
Table 3.
Association between the rs7632287 SNP and pair-bonding behavior in the TOSS and TCHAD samples (N=TOSS/TCHAD)
| A/A (N=88/30) | A/G (N=515/122) | G/G (N=810/171) | df | F | P1 | |
|---|---|---|---|---|---|---|
|
|
||||||
| TOSS: Partner Bonding Scale | 46.78 (6.09) | 46.46 (6.96) | 47.52 (6.48) | 1, 1162 | 7.59 | 0.006 |
| TCHAD: Relationship Quality Survey | 36.56 (6.40) | 36.81 (3.82) | 37.81 (3.12) | 1, 296 | 6.27 | 0.01 |
Analyses were performed by comparing individuals carrying one or two copies of the A-allele with G-allele homozygotes. Values for the PBS and the RQS scale are means with standard deviation within brackets.
To investigate potential associations between rs7632287 and measures of marital quality other than the PBS and RQS scales, we used an item from a life event questionnaire based on the Social Readjustment Rating Scale (51) in the TOSS sample. This item asked whether the female subjects had experienced marital crisis or threat of divorce during the last year. We found that women carrying the A-allele more often answered affirmatively to this question than women not carrying this allele (Table 4a). Eleven percent of women carrying no copy of the A-allele reported marital crisis, whereas 16% of the women carrying this allele reported marital crisis, suggesting approximately a 50% increase in risk of marital crisis for carriers of the A-allele compared to noncarriers of this allele.
Table 4.
Associations between the rs7632287 SNP and additional behavioral phenotypes in the TOSS (A/B) and THCAD samples (C).
| A/A + A/G | G/G | df | F | P | |
|---|---|---|---|---|---|
|
|
|||||
| Have you experienced marital crisis or threat of divorce during the last year? | |||||
| No | 520(84%) | 729(89%) | 1, 1084 | 9.03 | 0.003 |
| Yes | 96(16%) | 86(11%) | |||
| Spouse report | |||||
| N=616 | N=815 | ||||
| Dyadic consensus | 63.53 (7.98) | 64.48 (7.10) | 1, 1147 | 5.64 | 0.02 |
| Affectional expression | 17.79 (2.98) | 17.96 (2.78) | 1, 1188 | 1.20 | 0.27 |
| Dyadic satisfaction | 43.71 (3.02) | 43.85 (2.95) | 1, 1173 | 0.73 | 0.39 |
| Dyadic cohesion | 18.89 (4.07) | 19.05 (3.93) | 1, 1162 | 0.55 | 0.46 |
| Parent report | |||||
| N=318 | N=382 | ||||
| Social problems | 0.96 (1.79) | 0.64 (1.15) | 1, 460 | 7.42 | 0.007 |
Next we investigated whether the rs7632287 genotype in women was related to their partners’ perceptions of marital quality within the TOSS sample. This analysis was intended to further identify potential effects of the women’s genotype upon their current relationships. For this purpose, we used the four subscales (Dyadic Consensus, Dyadic Satisfaction, Dyadic Cohesion and Affectional Expression) from the Dyadic Adjustment Scale (52), commonly used to measure quality of marital relationships. The correlations between the DAS subscales in men and PBS scores in women ranged from r=0.37 to r=0.42. In line with our hypothesis marital quality, as assessed by the husbands, was associated with the rs7632287 genotype of their wives. Men married to women carrying the A-allele reported significantly lower scores on the Dyadic Consensus (measuring agreement on matters important to dyadic functioning) subscale than did men married to women not carrying this allele (d=0.13) (Table 4b).
In summary, rs7632287 and measures indicative of pair-bonding were associated within two independent samples. To gain better understanding of pair-bonding in a broader phenotypic sense and to be able to investigate rs7632287 in relation to other relevant phenotypes related to pair-bonding behavior, this investigation was extended to include potential developmental precursors of pair-bonding behavior in adulthood. The longitudinal design of the TCHAD sample allowed us to investigate whether early childhood behaviors predict future behavior in romantic relationship. To this end we used the Child Behavioral Checklist inventory (CBCL) (64) reported by parents in the first wave of the TCHAD study (at age 8–9). Using stepwise regression, we found that the parent-reported CBCL social problems subscale (a scale assessing a variety of difficulties that children may display in their relationships with peers and adults), but no other CBCL subscale, was significantly (r=−0.14, p=0.004) associated with girls’ behavior in romantic relationships at ages nineteen to twenty years. Further, we demonstrated that for girls the CBCL social problems scale was associated with the rs7632287 genotype (Table 4c). Consistent with the association between the rs7632287 SNP and pair-bonding behavior described previously, girls carrying the A-allele experienced more social problems than girls not carrying this allele (d=0.21).
Since the CBCL subscale social problems measures a behavioral domain related to autism (65), we investigated if the rs7632287 SNP is associated with autism spectrum syndromes. To do so we turned to the Child and Adolescent Twin Study of Sweden (CATSS, N=1771) sample (66) using parent interviews with the Autism-Tics, AD/HD, and other Co-morbidities inventory (A-TAC) (55). In a recent paper (65), comparing A-TAC and CBCL outcomes, the CBCL social problems scale showed the strongest correlation with the A-TAC autism spectrum score and the autism spectrum symptoms of deficient capacity for social interaction. We therefore hypothesized that there would be a relationship between the rs7632287 SNP and aspects of social behaviors in girls as assessed by the A-TAC. In line with our hypothesis, in girls – but not in boys - the rs7632287 SNP was significantly associated with the A-TAC total autism spectrum score as well as with the social interaction and communication domains in the autism spectrum (Table 5).
Table 5.
Associations between the rs7632287 SNP and autism related traits using total scores from the A-TAC in the CATSS sample
| A/A | A/G | G/G | df | F | P | |
|---|---|---|---|---|---|---|
|
|
||||||
| Total score | N=45 | N=327 | N=447 | |||
| Autism spectrum score | 2.56 (5.85) | 0.89 (2.38) | 1.10 (3.11) | 2, 539 | 5.70 | 0.004 |
| Sub-domains of autism spectrum | ||||||
| Social Interaction score | 1.48(3.50) | 0.40 (1.38) | 0.54 (1.90) | 2, 494 | 6.94 | 0.001 |
| Communication score | 0.76 (1.97) | 0.31 (0.97) | 0.38 (1.02) | 2, 557 | 3.36 | 0.04 |
| Flexibility score | 0.26 (0.88) | 0.15 (0.44) | 0.16(0.58) | 2, 493 | 0.84 | 0.43 |
Discussion
Our current results propose an association between a SNP in OXTR (rs7632287) and pair-bonding behavior in adult and adolescent women as well as with measures of social problems in childhood, both reflected through normal variation in behavior as well as with measures of autism spectrum traits. The rs7632287 SNP was significantly associated with two different measures of pair-bonding related behaviors including the Partner Bonding Scale as well as the affection subscale from the Relationship Quality Survey. Furthermore, when investigating the influence of the rs7632287 SNP on marital problems assessed by the women and the quality of the marital relationship described by their spouses significant associations were detected. We also showed that in girls, the rs7632287 SNP was associated with childhood social problems (which longitudinally predict behavior in romantic relationships in early adulthood). Finally, the rs7632287 SNP was shown to be associated with social problems and autism related traits. Taken together, these results are congruent with previous quantitative genetics research showing genetic influences on marital outcomes (67), social problems (68) and autism spectrum disorders (69).
Although variation in OXTR in previous studies has been shown to associate with different measures of social behavior (30–46) no one has previously linked it to a phenotype in humans directly corresponding to the species-unspecific and evolutionarily important concept of pair-bonding. Pair-bonding has been studied extensively in voles and shown to be facilitated through the actions of OT in females of this species. The fact that we are able to show an association between OXTR and behaviors reflecting pair-bonding in women but not in men is therefore consistent with previous research in animals. This study adds to previous findings and gives support to the hypothesis that OT in humans, as in other animals, is important for affiliative bonds between individuals of our species (70, 71).
By taking advantage of the longitudinal data structure in the TCHAD sample we were able to identify a behavioral domain (social problems) in young girls associated with pair-bonding later in life. This finding provides an interesting link to pair-bonding related behavior in childhood, by suggesting that women shown to bond less strongly with their partner in young adulthood also display less affiliative behavior during childhood. Interestingly, this is in line with vole studies showing that non-monogamous voles species show less affiliative behaviors than monogamous voles species already in their first days of life (72). In our further analyses a significant association between rs7632287 and the social problem measure in childhood was revealed and this association could be replicated in a third sample (CATSS) using a measure of autism related social problems. Based on findings from rodents showing that non-monogamous vole species with lower OTR expression in specific brain regions (12) and mice deficient of OT or OTR characterized by social amnesia in adulthood all display decreased social communication during early life (72–76), it may be suggested that the A-allele is associated with a decreased OT function in women which may influence social behaviors throughout life. The effect of the rs7632287 SNP on childhood social problems may also indicate that early precursor traits mediate the association between the rs7632287 SNP and pair-bonding. The limited sample size determined by the number of girls in the TCHAD sample having a romantic relationship, however did not provide enough power to directly test this hypothesis. Nevertheless, our findings suggest that OXTR plays a significant role in relationships across the life span. As described previously, human research suggests that OXTR is associated with a variety of prosocial behaviors during adulthood, behaviors possibly enhancing women’s social competence and the quality of their social relationships through eliciting more positive responses from social partners or through fostering more opportunities for social interactions. Our longitudinal analyses suggest that these effects are present by middle childhood, persist through adolescence, and possibly, through adulthood. These findings are consistent with previous studies that report similarities in relationship quality across different partners and over time (77), and with previous reports of associations between prosocial personality characteristics and marital quality and/or marital status (78–81). Importantly, the current study provides insight to the possible genetic sources of relationship quality and links between personality and relationship quality.
The effect size (d) for the influence of the studied SNP on the different measures of social behavior (d=0.13–0.28) is modest but comparable to what has been shown for the association between AVPR1A and pair-bonding related phenotypes (49), DRD4 and the personality trait novelty seeking (82) and that between a serotonin transporter polymorphism and neuroticism (83).
In the current study rs7632287, but none of the other SNPs, was associated with pair-bonding related behaviors using several measures and cohorts. The rs7632287 SNP is located in the 3′ region of OXTR and has recently been associated with autism in two previous studies (although not significant after multiple testing) (41, 43). Of the candidate gene studies of OXTR and risk of autism conducted so far, these two studies comprises the largest number of patients and are the only investigating the rs7632287 SNP. Interestingly, although both these studies, as well as ours, comprise most of the SNPs associated to autism in other studies the association to rs7632287 was the strongest. In comparison to our results it is however puzzling that these studies show associations between autism and the major allele (the G-allele) whereas our data suggest associations between increased number of symptoms related social problems and autism in childhood (as well as an decreased ability to bond to a partner in adulthood) and the minor allele (the A-allele). Although not making the reverse direction of association between studies less puzzling this phenomenon is rather common in genetic studies of complex traits, and may be explained by variations in study populations, such as differences in ethnicity and measures of social behaviors. Moreover, as the associations in the current investigation were restricted to females sex-specific analysis was not conducted in the two previous studies of the rs7632287. In silico analysis suggest that rs7632287 afflicts transcription factor binding to the 3′ end of the OXTR that may be important for gene expression levels (43). However, further investigations are needed before we know if this SNP has any importance for the protein function per se or if it is in linkage disequilibrium with a functional variant.
By demonstrating significant associations in several samples between variation in OXTR and phenotypes related to pair-bonding behavior in women and girls we provide compelling evidence that OTR, shown to be of great importance for these behaviors in female voles, could be of relevance also for humans.
Supplementary Material
Acknowledgments
Funding for the study was provided by grants from Swedish Research Council, Swedish Council for Working Life and Social Research, the National Institute of Mental Health Grant R01MH54610 and by The Bank of Sweden Tercentenary Foundation, J2004-0036:1. L.W. would like to thank Fredrik och Ingrid Thuringsstiftelse, Åke Wibergsstiftelse, Åhlén-stiftelsen, Jeanssons-stiftelsen, Magnus Bergvalls stiftelse, Söderström-Königska stiftelse and Märtha Lundqvist stiftelse.
Footnotes
Conflict of Interest
The authors report no biomedical financial interests or potential conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Lovejoy CO. The Origin of Man. Science. 1981;211:341–350. doi: 10.1126/science.211.4480.341. [DOI] [PubMed] [Google Scholar]
- 2.Marlowe FW. A critical period for provisioning by Hadza men - Implications for pair bonding. Evol Hum Behav. 2003;24:217–229. [Google Scholar]
- 3.Fuentes A. Patterns and trends in primate pair bonds. Int J Primatol. 2002;23:953–978. [Google Scholar]
- 4.Hawkes K. Mating, parenting and the evolution of human pair bonds. In: Chapais B, Berman C, editors. Kinship and Behavior in Primates. Oxford: Oxford University Press; 2004. pp. 443–473. [Google Scholar]
- 5.Chapais B. Primeval kinship: how pair-bonding gave birth to human society. Cambridge, Massachusetts: Harvard University Press; 2008. [Google Scholar]
- 6.Burbach JP, Young LJ, Russell J. Oxytocin: synthesis, secretion, and reproductive functions. In: Neill JD, editor. Knobil and Neill’s Physiology of Reproduction. Elsevier; 2006. pp. 3055–3128. [Google Scholar]
- 7.Lee HJ, Macbeth AH, Pagani JH, Young WS., 3rd Oxytocin: the great facilitator of life. Prog Neurobiol. 2009;88:127–151. doi: 10.1016/j.pneurobio.2009.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Neumann ID. Brain oxytocin: a key regulator of emotional and social behaviours in both females and males. J Neuroendocrinol. 2008;20:858–865. doi: 10.1111/j.1365-2826.2008.01726.x. [DOI] [PubMed] [Google Scholar]
- 9.Young LJ, Wang Z. The neurobiology of pair bonding. Nat Neurosci. 2004;7:1048–1054. doi: 10.1038/nn1327. [DOI] [PubMed] [Google Scholar]
- 10.Williams JR, Insel TR, Harbaugh CR, Carter CS. Oxytocin administered centrally facilitates formation of a partner preference in female prairie voles (Microtus ochrogaster) J Neuroendocrinol. 1994;6:247–250. doi: 10.1111/j.1365-2826.1994.tb00579.x. [DOI] [PubMed] [Google Scholar]
- 11.Insel TR, Hulihan TJ. A gender-specific mechanism for pair bonding: oxytocin and partner preference formation in monogamous voles. Behav Neurosci. 1995;109:782–789. doi: 10.1037//0735-7044.109.4.782. [DOI] [PubMed] [Google Scholar]
- 12.Insel TR, Shapiro LE. Oxytocin receptor distribution reflects social organization in monogamous and polygamous voles. Proc Natl Acad Sci U S A. 1992;89:5981–5985. doi: 10.1073/pnas.89.13.5981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Young LJ, Lim MM, Gingrich B, Insel TR. Cellular mechanisms of social attachment. Horm Behav. 2001;40:133–138. doi: 10.1006/hbeh.2001.1691. [DOI] [PubMed] [Google Scholar]
- 14.Ross HE, Freeman SM, Spiegel LL, Ren XH, Terwilliger EF, Young LJ. Variation in Oxytocin Receptor Density in the Nucleus Accumbens Has Differential Effects on Affiliative Behaviors in Monogamous and Polygamous Voles. Journal of Neuroscience. 2009;29:1312–1318. doi: 10.1523/JNEUROSCI.5039-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Young LJ, Huot B, Nilsen R, Wang Z, Insel TR. Species differences in central oxytocin receptor gene expression: comparative analysis of promoter sequences. J Neuroendocrinol. 1996;8:777–783. doi: 10.1046/j.1365-2826.1996.05188.x. [DOI] [PubMed] [Google Scholar]
- 16.Smith AS, Agmo A, Birnie AK, French JA. Manipulation of the oxytocin system alters social behavior and attraction in pair-bonding primates, Callithrix penicillata. Horm Behav. 2009;57:255–262. doi: 10.1016/j.yhbeh.2009.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kosfeld M, Heinrichs M, Zak PJ, Fischbacher U, Fehr E. Oxytocin increases trust in humans. Nature. 2005;435:673–676. doi: 10.1038/nature03701. [DOI] [PubMed] [Google Scholar]
- 18.Zak PJ, Stanton AA, Ahmadi S. Oxytocin increases generosity in humans. PLoS One. 2007;2:e1128. doi: 10.1371/journal.pone.0001128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Theodoridou A, Rowe AC, Penton-Voak IS, Rogers PJ. Oxytocin and social perception: oxytocin increases perceived facial trustworthiness and attractiveness. Horm Behav. 2009;56:128–132. doi: 10.1016/j.yhbeh.2009.03.019. [DOI] [PubMed] [Google Scholar]
- 20.Rimmele U, Hediger K, Heinrichs M, Klaver P. Oxytocin makes a face in memory familiar. J Neurosci. 2009;29:38–42. doi: 10.1523/JNEUROSCI.4260-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.De Dreu CK, Greer LL, Handgraaf MJ, Shalvi S, Van Kleef GA, Baas M, et al. The neuropeptide oxytocin regulates parochial altruism in intergroup conflict among humans. Science. 2010;328:1408–1411. doi: 10.1126/science.1189047. [DOI] [PubMed] [Google Scholar]
- 22.Domes G, Heinrichs M, Michel A, Berger C, Herpertz SC. Oxytocin improves “mind-reading” in humans. Biol Psychiatry. 2007;61:731–733. doi: 10.1016/j.biopsych.2006.07.015. [DOI] [PubMed] [Google Scholar]
- 23.Andari E, Duhamel JR, Zalla T, Herbrecht E, Leboyer M, Sirigu A. Promoting social behavior with oxytocin in high-functioning autism spectrum disorders. Proc Natl Acad Sci U S A. 2010;107:4389–4394. doi: 10.1073/pnas.0910249107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Guastella AJ, Einfeld SL, Gray KM, Rinehart NJ, Tonge BJ, Lambert TJ, et al. Intranasal oxytocin improves emotion recognition for youth with autism spectrum disorders. Biol Psychiatry. 2010;67:692–694. doi: 10.1016/j.biopsych.2009.09.020. [DOI] [PubMed] [Google Scholar]
- 25.Ditzen B, Schaer M, Gabriel B, Bodenmann G, Ehlert U, Heinrichs M. Intranasal oxytocin increases positive communication and reduces cortisol levels during couple conflict. Biol Psychiatry. 2009;65:728–731. doi: 10.1016/j.biopsych.2008.10.011. [DOI] [PubMed] [Google Scholar]
- 26.Gordon I, Zagoory-Sharon O, Schneiderman I, Leckman JF, Weller A, Feldman R. Oxytocin and cortisol in romantically unattached young adults: associations with bonding and psychological distress. Psychophysiology. 2008;45:349–352. doi: 10.1111/j.1469-8986.2008.00649.x. [DOI] [PubMed] [Google Scholar]
- 27.Domes G, Heinrichs M, Glascher J, Buchel C, Braus DF, Herpertz SC. Oxytocin attenuates amygdala responses to emotional faces regardless of valence. Biol Psychiatry. 2007;62:1187–1190. doi: 10.1016/j.biopsych.2007.03.025. [DOI] [PubMed] [Google Scholar]
- 28.Petrovic P, Kalisch R, Singer T, Dolan RJ. Oxytocin attenuates affective evaluations of conditioned faces and amygdala activity. J Neurosci. 2008;28:6607–6615. doi: 10.1523/JNEUROSCI.4572-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kirsch P, Esslinger C, Chen Q, Mier D, Lis S, Siddhanti S, et al. Oxytocin modulates neural circuitry for social cognition and fear in humans. J Neurosci. 2005;25:11489–11493. doi: 10.1523/JNEUROSCI.3984-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rodrigues SM, Saslow LR, Garcia N, John OP, Keltner D. Oxytocin receptor genetic variation relates to empathy and stress reactivity in humans. Proc Natl Acad Sci U S A. 2009;106:21437–21441. doi: 10.1073/pnas.0909579106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Costa B, Pini S, Gabelloni P, Abelli M, Lari L, Cardini A, et al. Oxytocin receptor polymorphisms and adult attachment style in patients with depression. Psychoneuroendocrinology. 2009;34:1506–1514. doi: 10.1016/j.psyneuen.2009.05.006. [DOI] [PubMed] [Google Scholar]
- 32.Park J, Willmott M, Vetuz G, Toye C, Kirley A, Hawi Z, et al. Evidence that genetic variation in the oxytocin receptor (OXTR) gene influences social cognition in ADHD. Prog Neuropsychopharmacol Biol Psychiatry. 2010;34:697–702. doi: 10.1016/j.pnpbp.2010.03.029. [DOI] [PubMed] [Google Scholar]
- 33.Kim HS, Sherman DK, Sasaki JY, Xu J, Chu TQ, Ryu C, et al. Culture, distress, and oxytocin receptor polymorphism (OXTR) interact to influence emotional support seeking. Proc Natl Acad Sci U S A. 2010;107:15717–15721. doi: 10.1073/pnas.1010830107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tost H, Kolachana B, Hakimi S, Lemaitre H, Verchinski BA, Mattay VS, et al. A common allele in the oxytocin receptor gene (OXTR) impacts prosocial temperament and human hypothalamic-limbic structure and function. Proc Natl Acad Sci U S A. 2010;107:13936–13941. doi: 10.1073/pnas.1003296107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bakermans-Kranenburg MJ, van Ijzendoorn MH. Oxytocin receptor (OXTR) and serotonin transporter (5-HTT) genes associated with observed parenting. Soc Cogn Affect Neurosci. 2008;3:128–134. doi: 10.1093/scan/nsn004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Israel S, Lerer E, Shalev I, Uzefovsky F, Riebold M, Laiba E, et al. The oxytocin receptor (OXTR) contributes to prosocial fund allocations in the dictator game and the social value orientations task. PLoS One. 2009;4:e5535. doi: 10.1371/journal.pone.0005535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wu S, Jia M, Ruan Y, Liu J, Guo Y, Shuang M, et al. Positive association of the oxytocin receptor gene (OXTR) with autism in the Chinese Han population. Biol Psychiatry. 2005;58:74–77. doi: 10.1016/j.biopsych.2005.03.013. [DOI] [PubMed] [Google Scholar]
- 38.Jacob S, Brune CW, Carter CS, Leventhal BL, Lord C, Cook EH., Jr Association of the oxytocin receptor gene (OXTR) in Caucasian children and adolescents with autism. Neurosci Lett. 2007;417:6–9. doi: 10.1016/j.neulet.2007.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lerer E, Levi S, Salomon S, Darvasi A, Yirmiya N, Ebstein RP. Association between the oxytocin receptor (OXTR) gene and autism: relationship to Vineland Adaptive Behavior Scales and cognition. Mol Psychiatry. 2008;13:980–988. doi: 10.1038/sj.mp.4002087. [DOI] [PubMed] [Google Scholar]
- 40.Yrigollen CM, Han SS, Kochetkova A, Babitz T, Chang JT, Volkmar FR, et al. Genes controlling affiliative behavior as candidate genes for autism. Biol Psychiatry. 2008;63:911–916. doi: 10.1016/j.biopsych.2007.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tansey KE, Brookes KJ, Hill MJ, Cochrane LE, Gill M, Skuse D, et al. Oxytocin receptor (OXTR) does not play a major role in the aetiology of autism: genetic and molecular studies. Neurosci Lett. 2010;474:163–167. doi: 10.1016/j.neulet.2010.03.035. [DOI] [PubMed] [Google Scholar]
- 42.Wang K, Zhang H, Ma D, Bucan M, Glessner JT, Abrahams BS, et al. Common genetic variants on 5p14.1 associate with autism spectrum disorders. Nature. 2009;459:528–533. doi: 10.1038/nature07999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Campbell DB, Datta D, Jones ST, Batey Lee E, Sutcliffe JS, Hammock EA, et al. Association of oxytocin receptor (OXTR) gene variants with multiple phenotype domains of autism spectrum disorder. J Neurodev Disord. 2011;3:101–112. doi: 10.1007/s11689-010-9071-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Liu X, Kawamura Y, Shimada T, Otowa T, Koishi S, Sugiyama T, et al. Association of the oxytocin receptor (OXTR) gene polymorphisms with autism spectrum disorder (ASD) in the Japanese population. J Hum Genet. 2010;55:137–141. doi: 10.1038/jhg.2009.140. [DOI] [PubMed] [Google Scholar]
- 45.Wermter AK, Kamp-Becker I, Hesse P, Schulte-Korne G, Strauch K, Remschmidt H. Evidence for the involvement of genetic variation in the oxytocin receptor gene (OXTR) in the etiology of autistic disorders on high-functioning level. Am J Med Genet B Neuropsychiatr Genet. 2010;153B:629–639. doi: 10.1002/ajmg.b.31032. [DOI] [PubMed] [Google Scholar]
- 46.Chen FS, Johnson SC. An Oxytocin Receptor Gene Variant Predicts Attachment Anxiety in Females and Autism-Spectrum Traits in Males. Social Psychological and Personality Science 2011 [Google Scholar]
- 47.Inoue H, Yamasue H, Tochigi M, Abe O, Liu X, Kawamura Y, et al. Association Between the Oxytocin Receptor Gene and Amygdala Volume in Healthy Adults. Biol Psychiatry. 2010 doi: 10.1016/j.biopsych.2010.07.019. [DOI] [PubMed] [Google Scholar]
- 48.Furman DJ, Chen MC, Gotlib IH. Variant in oxytocin receptor gene is associated with amygdala volume. Psychoneuroendocrinology. 2011;36:891–897. doi: 10.1016/j.psyneuen.2010.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Walum H, Westberg L, Henningsson S, Neiderhiser JM, Reiss D, Igl W, et al. Genetic variation in the vasopressin receptor 1a gene (AVPR1A) associates with pair-bonding behavior in humans. Proc Natl Acad Sci U S A. 2008;105:14153–14156. doi: 10.1073/pnas.0803081105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Neiderhiser JM, Reiss D, Lichtenstein P, Spotts EL, Ganiban J. Father-adolescent relationships and the role of genotype-environment correlation. J Fam Psychol. 2007;21:560–571. doi: 10.1037/0893-3200.21.4.560. [DOI] [PubMed] [Google Scholar]
- 51.Holmes TH, Rahe RH. The Social Readjustment Rating Scale. J Psychosom Res. 1967;11:213–218. doi: 10.1016/0022-3999(67)90010-4. [DOI] [PubMed] [Google Scholar]
- 52.Spanier GB. Measuring Dyadic Adjustment: New scales for Assessing the Quality of Marriage and Similar Dyads. Journal of Marriage and the Family. 1976;38:15–28. [Google Scholar]
- 53.Lichtenstein P, Tuvblad C, Larsson H, Carlstrom E. The Swedish Twin study of CHild and Adolescent Development: the TCHAD-study. Twin Res Hum Genet. 2007;10:67–73. doi: 10.1375/twin.10.1.67. [DOI] [PubMed] [Google Scholar]
- 54.Hannelius U, Gherman L, Makela VV, Lindstedt A, Zucchelli M, Lagerberg C, et al. Large-scale zygosity testing using single nucleotide polymorphisms. Twin Res Hum Genet. 2007;10:604–625. doi: 10.1375/twin.10.4.604. [DOI] [PubMed] [Google Scholar]
- 55.Hansson SL, Svanstrom Rojvall A, Rastam M, Gillberg C, Anckarsater H. Psychiatric telephone interview with parents for screening of childhood autism - tics, attention-deficit hyperactivity disorder and other comorbidities (A-TAC): preliminary reliability and validity. Br J Psychiatry. 2005;187:262–267. doi: 10.1192/bjp.187.3.262. [DOI] [PubMed] [Google Scholar]
- 56.Simpson JA. Influence of attachment styles on romantic relationships. Journal of Personality and Social Psychology. 1990;59:971–980. [Google Scholar]
- 57.Booth A, Johnson D, Edwards JN. Measuring Marital Instability. Journal of Marriage and the Family. 1983;45:387–394. [Google Scholar]
- 58.Hetherington EM, Clingempeel WG, Anderson ER, Deal JE, Hagan MS, Hollier EA, et al. Coping with Marital Transitions - a Family Systems Perspective. Monogr Soc Res Child. 1992;57:R5. [Google Scholar]
- 59.Plomin R, Reiss D, Hetherington EM, Howe GW. Nature and Nurture - Genetic Contributions to Measures of the Family Environment. Dev Psychol. 1994;30:32–43. [Google Scholar]
- 60.Anckarsäter H, Larson T, Hansson SL, Carlstrom E, Ståhlberg O, Gillberg C, et al. Child Neurodevelopmental and Behavioural Problems are Intercorrelated and Dimensionally Distributed in the General Population. The Open Psychiatry Journal. 2008;19:5–11. [Google Scholar]
- 61.Ronald A, Larsson H, Anckarsater H, Lichtenstein P. A twin study of autism symptoms in Sweden. Mol Psychiatry. 2010 doi: 10.1038/mp.2010.82. [DOI] [PubMed] [Google Scholar]
- 62.Furman W, Buhrmester D. Children’s perceptions of the qualities of sibling relationships. Child Dev. 1985;56:448–461. [PubMed] [Google Scholar]
- 63.Fisher RA. Statistical Methods for Research Workers. 13. New York: Hafner; 1958. [Google Scholar]
- 64.Achenbach TM. Manual for the Child Behavior Checklist/ 4–18 and 1991 Profile. University of Vermont; Burlington: 1993. [Google Scholar]
- 65.Hallerod SL, Larson T, Stahlberg O, Carlstrom E, Gillberg C, Anckarsater H, et al. The Autism--Tics, AD/HD and other Comorbidities (A-TAC) telephone interview: convergence with the Child Behavior Checklist (CBCL) Nord J Psychiatry. 2010;64:218–224. doi: 10.3109/08039480903514443. [DOI] [PubMed] [Google Scholar]
- 66.Lichtenstein P, Sullivan PF, Cnattingius S, Gatz M, Johansson S, Carlstrom E, et al. The Swedish Twin Registry in the third millennium: an update. Twin Res Hum Genet. 2006;9:875–882. doi: 10.1375/183242706779462444. [DOI] [PubMed] [Google Scholar]
- 67.Spotts EL, Neiderhiser JM, Towers H, Hansson K, Lichtenstein P, Cederblad M, et al. Genetic and environmental influences on marital relationships. J Fam Psychol. 2004;18:107–119. doi: 10.1037/0893-3200.18.1.107. [DOI] [PubMed] [Google Scholar]
- 68.Edelbrock C, Rende R, Plomin R, Thompson LA. A twin study of competence and problem behavior in childhood and early adolescence. J Child Psychol Psychiatry. 1995;36:775–785. doi: 10.1111/j.1469-7610.1995.tb01328.x. [DOI] [PubMed] [Google Scholar]
- 69.Lichtenstein P, Carlstrom E, Rastam M, Gillberg C, Anckarsater H. The genetics of autism spectrum disorders and related neuropsychiatric disorders in childhood. Am J Psychiatry. 2010;167:1357–1363. doi: 10.1176/appi.ajp.2010.10020223. [DOI] [PubMed] [Google Scholar]
- 70.Insel TR. The challenge of translation in social neuroscience: a review of oxytocin, vasopressin, and affiliative behavior. Neuron. 2010;65:768–779. doi: 10.1016/j.neuron.2010.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Ebstein RP, Israel S, Chew SH, Zhong S, Knafo A. Genetics of human social behavior. Neuron. 2010;65:831–844. doi: 10.1016/j.neuron.2010.02.020. [DOI] [PubMed] [Google Scholar]
- 72.Shapiro LE, Insel TR. Infant’s response to social separation reflects adult differences in affiliative behavior: a comparative developmental study in prairie and montane voles. Dev Psychobiol. 1990;23:375–393. doi: 10.1002/dev.420230502. [DOI] [PubMed] [Google Scholar]
- 73.Winslow JT, Hearn EF, Ferguson J, Young LJ, Matzuk MM, Insel TR. Infant vocalization, adult aggression, and fear behavior of an oxytocin null mutant mouse. Horm Behav. 2000;37:145–155. doi: 10.1006/hbeh.1999.1566. [DOI] [PubMed] [Google Scholar]
- 74.Ferguson JN, Young LJ, Hearn EF, Matzuk MM, Insel TR, Winslow JT. Social amnesia in mice lacking the oxytocin gene. Nat Genet. 2000;25:284–288. doi: 10.1038/77040. [DOI] [PubMed] [Google Scholar]
- 75.Higashida H, Lopatina O, Yoshihara T, Pichugina YA, Soumarokov AA, Munesue T, et al. Oxytocin Signal and Social Behaviour: Comparison among Adult and Infant Oxytocin, Oxytocin Receptor and CD38 Gene Knockout Mice. Journal of Neuroendocrinology. 2010;22:373–379. doi: 10.1111/j.1365-2826.2010.01976.x. [DOI] [PubMed] [Google Scholar]
- 76.Takayanagi Y, Yoshida M, Bielsky IF, Ross HE, Kawamata M, Onaka T, et al. Pervasive social deficits, but normal parturition, in oxytocin receptor-deficient mice. P Natl Acad Sci USA. 2005;102:16096–16101. doi: 10.1073/pnas.0505312102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Robins RW, Caspi A, Moffitt TE. It’s not just who you’re with, it’s who you are: personality and relationship experiences across multiple relationships. J Pers. 2002;70:925–964. doi: 10.1111/1467-6494.05028. [DOI] [PubMed] [Google Scholar]
- 78.Blum JS, Mehrabian A. Personality and temperament correlates of marital satisfaction. J Pers. 1999;67:93–125. [Google Scholar]
- 79.Caspi A, Elder GH, Bem DJ. Moving Away from the World - Life-Course Patterns of Shy Children. Dev Psychol. 1988;24:824–831. [Google Scholar]
- 80.Ganiban JM, Ulbricht J, Spotts EL, Lichtenstein P, Reiss D, Neiderhiser JM. Can parent personality explain links between marital satisfaction and parenting? Journal of Family Psychology. 2009;23:646–660. doi: 10.1037/a0016091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Johnson W, McGue M, Krueger RF, Bouchard TJ., Jr Marriage and personality: a genetic analysis. J Pers Soc Psychol. 2004;86:285–294. doi: 10.1037/0022-3514.86.2.285. [DOI] [PubMed] [Google Scholar]
- 82.Munafo MR, Yalcin B, Willis-Owen SA, Flint J. Association of the dopamine D4 receptor (DRD4) gene and approach-related personality traits: meta-analysis and new data. Biol Psychiatry. 2008;63:197–206. doi: 10.1016/j.biopsych.2007.04.006. [DOI] [PubMed] [Google Scholar]
- 83.Schinka JA, Busch RM, Robichaux-Keene N. A meta-analysis of the association between the serotonin transporter gene polymorphism (5-HTTLPR) and trait anxiety. Mol Psychiatr. 2004;9:197–202. doi: 10.1038/sj.mp.4001405. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.