Abstract
Background
Although the severity of inducible ischemia provides incremental prognostic information in persons with known or suspected coronary artery disease (CAD), its significance for predicting long-term CAD outcomes in apparently healthy populations is unknown. This study was designed to evaluate the presence and degree of myocardial ischemia in asymptomatic siblings of persons with premature CAD <60 years of age and to determine its significance for predicting incident acute coronary syndromes (ACS) during follow-up of 5 to 25 years.
Methods
Siblings (n = 1,287, age 30-59 years, 55% female) were screened for traditional risk factors, underwent exercise treadmill testing with nuclear perfusion imaging, and were followed for the development of ACS (mean follow-up 11.6 ± 5.1 years). The severity of ischemia was assessed by semiquantitative methods using the standard 17-segment model and then categorized by the percent maximal summed stress score as none (0%), minimal (1% to <5%), mild (5% to 10%), moderate (10% to 15%), or severe (≥15%).
Results
ACS occurred in 132 subjects (10.3%) and included sudden cardiac death (n = 13), acute MI (n = 62), and unstable angina with revascularization (n = 57). The presence of no (88%), minimal (6%), mild (5%), and moderate/severe (1%) ischemia was associated with an ACS incidence of 8.3%, 19.7%, 25.0%, and 38.9%, respectively (P < .0001 for trend). Kaplan-Meier event-free survival analyses by myocardial ischemia severity categories showed that even minimal and mild myocardial ischemia were associated with greater ACS incidence detectable as early as 2 years after baseline. A Cox proportional hazard model, adjusted for risk factors and follow-up time, showed that each 5% increment in the severity of ischemia resulted in a 77% increase in the hazard of incident ACS (P < .001).
Conclusion
Inducible myocardial ischemia is prevalent in asymptomatic siblings of persons with early onset CAD. Most ischemia is minimal or mild in severity, and although the severity of ischemia is associated with the risk of ACS in a graded fashion, the presence of even minimal and mild perfusion defects predicts worse CAD outcomes in this population.
Keywords: SPECT, ischemia, myocardial, stress testing, acute coronary syndromes, outcomes research
INTRODUCTION
Coronary atherosclerosis begins long before the clinical onset of coronary artery disease (CAD).1 During this period of quiescent CAD progression, myocardial ischemia may be detected by exercise stress myocardial perfusion imaging even in the absence of symptoms.2 Its presence predicts CAD events in subjects without established CAD.2-4 Although the severity of inducible myocardial ischemia provides incremental prognostic information in persons with known or suspected CAD,5-7 its significance in asymptomatic populations is unknown.
Family history of CAD in a first-degree relative is a well-recognized risk factor for premature CAD; the younger the age of CAD onset in a proband, the higher the risk to other first-degree relatives.8-10 We have previously reported that apparently healthy siblings of persons with CAD events prior to 60 years of age have an excess prevalence of all CAD risk factors and also of subclinical CAD11,12 and a higher than predicted incidence of CAD events within a relatively short time after the proband CAD event.4,13,14 Early detection of the presence and characterization of the severity of inducible myocardial ischemia in this asymptomatic population may help identify individuals at highest risk, in whom aggressive preventive risk management would be most beneficial.
We therefore designed this study to examine whether the severity of inducible myocardial ischemia is useful for predicting clinical CAD outcomes in asymptomatic siblings of persons with premature CAD.
METHODS
Study Population
The study population consisted of 1,287 apparently healthy siblings of individuals with documented CAD prior to 60 years of age as part of the ongoing prospective Genetic Study of Atherosclerosis Risk (GeneSTAR) research program in families with early onset CAD. Index patients were identified at any of 10 Baltimore hospitals with documented acute myocardial infarction, coronary artery bypass surgery, percutaneous coronary intervention, or angina with angiographic evidence of a stenosis >50% diameter in at least one coronary artery. Siblings of the index patients were eligible if they were 30 to 60 years of age and had no known CAD, but were excluded if they had systemic autoimmune disease, life-threatening co-morbidity, motor function limitations precluding exercise testing, or were receiving glucocorticosteroid therapy, as previously described.4 The study was approved by the Johns Hopkins Medicine Institutional Review Board and all participants gave informed consent.
Risk Factor Screening
Following an overnight fast, siblings underwent baseline screening for traditional CAD risk factors and evaluation of inducible myocardial ischemia during maximal stress myocardial perfusion testing. Medical history and information on all medications were elicited. A physical examination was performed by an attending cardiologist. For anthropometric measures, height was determined using a stadiometer, and weight was measured with the subject wearing light clothing and no shoes. Body mass index (BMI) was calculated as weight in kilograms divided by height in meters squared. Cigarette smoking was assessed by self-report or by an expired carbon monoxide level.15 Participants who reported any cigarette smoking within the past month or who had 2 expired CO readings of ≥8 ppm were classified as current smokers. Blood pressure was measured according to American Heart Association guidelines three times over the course of an 8-hour day and the average of 3 measurements was used to characterize blood pressure.16 Hypertension was defined as an average blood pressure ≥140 mm Hg systolic, or ≥90 mm Hg diastolic, or by the patient taking an antihypertensive drug. Blood was drawn for determination of lipid and glucose levels. Total cholesterol, high-density lipoprotein (HDL) cholesterol, and triglyceride levels were measured using Centers for Disease Control standardized methods in the Johns Hopkins Clinical Chemistry Laboratory.17 Low-density lipoprotein (LDL) cholesterol was estimated using the Friedewald formula18 for persons with triglyceride levels up to 400 mg/dL. Glucose concentration was measured using the glucose oxidase method in The Johns Hopkins Clinical Chemistry Laboratory.19 Type 2 diabetes mellitus was defined as a fasting glucose level ≥126 mg/dL, ore self-report of a physician’s diagnosis and use of hypoglycemic medication. We calculated the 10-year Framingham Risk Score (FRS) to identify siblings with a low annual risk (<1% per year), intermediate risk (1% to 2% per year), and high risk (≥2% per year) for total CAD events based on their baseline risk factor levels.20
Maximal Graded Exercise with Stress Thallium Scintigraphy
All siblings underwent a maximal graded treadmill test using a modified Bruce protocol.4 Exercise was continued until maximal effort (n = 967), fatigue and/or musculoskeletal pain (n = 104), dyspnea (n = 78), dizziness (n = 4), chest pain (n = 6), leg weakness or pain (n = 76), panic (n = 3), severe pallor and diaphoresis (n = 4), hypertensive response (n = 21), serious arrhythmias (n = 2), or marked ST-segment changes (n = 22).
Thallium-201 scintigraphy was performed immediately following the maximal treadmill test. One minute before the end of exercise, 3 mCi thallium-201 was injected intravenously and tomographic imaging was performed as previously described.4 Three hours later, delayed imaging was performed without reinjection of thallium. Images were reconstructed by filtered back projection using a ramp filter after prefiltering of the projection images with a two-dimensional Fourier (Weiner) filter and correction for translational motion.21,22 Image interpretation was performed by two experienced nuclear cardiologists, blinded to the subject’s risk factor profile, identity or exercise test results, and in the case of disagreement, the images were discussed until consensus. Inducible ischemia was defined as a perfusion defect on the immediate post-exercise images in at least two contiguous tomographic slices and two image orientations, with definite improvement or normalization on delayed images.23
Semiquantitative visual interpretation of perfusion images using the standard 17-segment model24 as recommended by the American Heart Association25 was performed by the two readers. All segments were scored to consensus using a five-point scoring system for reversible perfusion defects (0 = normal; 1 = mild; 2 = moderate; 3 = severe; and 4 = absence of detectable radiotracer uptake). A summed stress score (SSS) was calculated as the summed score of all individual segments. The percent severity of ischemia was expressed as the summed stress score divided by the maximum possible score of 6824 and categorized as normal (0%), minimal (1 to <5%), mild (5 to <10%), or moderate to severe (≥10%).26
Definition and Adjudication of Incident CAD
Participants were followed at 5-year intervals up to 25 years after baseline screening for incident CAD events. A standardized instrument was administered via telephone by a trained interviewer to elicit a history of cardiac-related procedures or symptoms, the use of any coronary disease-related medications, and any history of CAD. In the event of a death, the closest family member served as a proxy. All reported CAD events were verified from physician records, hospital records, death records, and/or autopsy records. Data were also recorded on all CAD diagnostic tests and results. The “hardest” first CAD event during the follow-up period was recorded in the following priority order: sudden cardiac death, myocardial infarction (MI), unstable angina with coronary bypass surgery (CABG), unstable angina with percutaneous coronary intervention (PCI), stable angina with CABG or PCI, and medically treated angina with documented stenosis ≥50%, but no coronary intervention. Thus, each person could only enter CAD event modeling once.
The primary endpoint was defined as acute coronary syndrome (ACS), including sudden death, acute myocardial infarction, and unstable angina requiring hospitalization with revascularization. Participants with any event occurring within the first 3 months of follow-up (n = 2, one acute MI and one with new onset angina with revascularization) were censored to remove any potential screening bias introduced by early post-screening events.
All records were reviewed independently by two cardiologists and one epidemiologist, each blinded to the classification assignments of the other reviewers. Whenever there was a discordant classification by any single member of this team, an External Adjudication Committee consisting of at least one non-study cardiologist from Johns Hopkins and one from an extrinsic academic institution reviewed the records and adjudicated the final event classification using the same standardized coding schema.
Statistical Analyses
All risk factors were examined for distributions using standard univariate techniques. For continuous variables, frequencies, means, and standard deviations were examined. For bivariable analyses among continuous variables, the t test and ANOVA were used; contingency table arrays and the χ2 statistic were used for the examination of relationships between categorical variables. Kaplan-Meier CAD event-free survival curves were generated for the absence and severity classification of baseline exercise-induced ischemia. We analyzed the annual incidence of total and ACS events at 5 years of follow-up, by ischemia absence or severity stratified by Framingham risk categories. For multivariable analyses, Cox proportional hazards modeling predicting incident ACS events and accounting for familial clustering was used for the full duration of follow-up with adjustment for baseline traditional CAD risk factors. Potential covariate relationships were examined between inducible ischemia and age, sex, race, and other risk factors. The final and best fitting model allowed for different underlying hazards according to sex and race. Hazards ratios, 95% confidence intervals, and statistical significance for each variable in the models were determined. Thus in the final model, hazard ratios were standardized for the severity of inducible ischemia and all modifiable risk factors, and reflect the increased risk above stratified sex and race specific baseline hazards. Since the occurrence of non-ACS cardiac events with subsequent treatment competes with the risk of ACS, to estimate the ACS-specific relative hazards, we used the modeling for cause-specific hazards of competing events as described by Lau et al.27 In these models the time to each of the competing events is censored when the other event occurs, but covariate adjustment for both ACS and non-ACS events is done in the same model.
RESULTS
Baseline Sample Characteristics
The 1,287 siblings were identified from 699 families (one index case per family, 1.84 ± 1.1 siblings per proband). Index cases were 67% male, with a mean age of 46.5 ± 7.3 years for first CAD event, and had the following diagnoses: MI (48.2%), unstable angina with revascularization (31.8%), and stable angina with revascularization or managed medically (19.8%). Their siblings, the subjects of this study, were 55% female and 47% African American, and all were asymptomatic, without chest pain or angina-equivalent symptoms at the time of baseline screening. No subjects had a history of CAD or evidence of silent myocardial infarction on the baseline resting electrocardiogram.
Baseline population characteristics are shown by ischemia severity classification in Table 1. Increasing severity of inducible ischemia was associated with older age, male sex, hypertension, higher levels of low-density lipoprotein cholesterol and triglycerides, and lower levels of high-density lipoprotein cholesterol. There was a significantly higher incidence of ACS across worsening categories of ischemia severity.
Table 1.
Population characteristics and CAD events by classification of ischemia severity (N = 1,287)
| Characteristics | None (n = 1129) | Minimal (n = 76) | Mild (n = 64) | Moderate or severe (n = 18) |
P value |
|---|---|---|---|---|---|
| Age, years† | 46.1 ± 7.0 | 47.8 ± 7.3 | 50.0 ± 5.9 | 47.5 ± 8.0 | <.0001 |
| Male sex (%) | 40.8 | 80.3 | 76.6 | 83.3 | <.0001 |
| African American (%) | 48.0 | 38.2 | 37.5 | 44.4 | .16 |
| Hypertensive (%) | 47.7 | 56.6 | 60.9 | 77.8 | .007 |
| Diabetic (%) | 9.2 | 10.5 | 7.8 | 27.8 | .06 |
| Current smoking (%) | 31.3 | 39.5 | 29.7 | 38.9 | .43 |
| Low-density lipoprotein cholesterol, mmol/L (mg/dL)† |
3.68 ± 1.14 (142.1 ± 44.0) | 4.07 ± 1.09 (157.0 ± 42.2) | 4.2 ± 1.29 (163.0 ± 49.8) | 4.11 ± 1.13 (158.7 ± 43.5) | .0001 |
| High-density lipoprotein cholesterol, mmol/L (mg/dL)† |
1.36 ± 0.42 (52.4 ± 16.3) | 1.25 ± 0.34 (48.1 ± 13.2) | 1.21 ± 0.38 (46.8 ± 14.8) | 1.19 ± 0.33 (45.8 ± 12.9) | .003 |
| Triglycerides, mmol/L (mg/dL)† |
1.58 ± 1.33 (139.5 ± 118.1) | 1.77 ± 1.10 (157.0 ± 97.2) | 2.02 ± 1.36 (178.6 ± 120.2) | 1.76 ± 1.00 (156.1 ± 88.4) | .04 |
| Body-mass index (kg/m2)† |
29.3 ± 6.4 | 28.9 ± 5.1 | 29.1 ± 4.6 | 29.9 ± 4.7 | .91 |
| MET level achieved | 11.1 ± 3.2 | 11.2 ± 3.1 | 11.2 ± 3.0 | 11.1 ± 3.3 | .99 |
| Incident CAD event rates Incident ACS event (%)‡ |
8.4 | 19.7 | 25 | 38.9 | <.0001 |
P value across categories of ischemia severity as measured by 17-segment semi-quantitative analysis [none (0%), minimal (1% to 5%), mild (5% to 10%), moderate (10% to 15%), and severe (≥15%)].
Mean ± 1 standard deviation.
Acute coronary syndromes (ACS) defined as sudden cardiac death, acute myocardial infarction, or unstable angina requiring revascularization.
Results of Perfusion Imaging
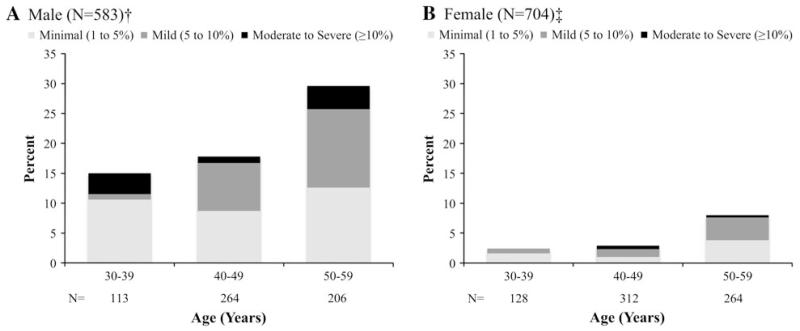
Inducible ischemia, defined by the presence of a reversible perfusion defect, was present at baseline in a total of 158 siblings (12.3%) (Figure 1). Notably, the prevalence of any ischemia was strongly associated with increasing age decade in both males and females (P = .002 and .008, respectively). Males had a higher prevalence of inducible ischemia than females across all age strata (21.5% vs 4.8%) with the highest prevalence >30% in males 50-59 years of age. Using 17-segment semiquantitative analysis for those siblings with perfusion defects, the mean severity of ischemia was 6.0% ± 3.3%, consistent with mild ischemia. Overall classification of perfusion defects showed that 48% were minimal, 40% were mild, and 12% were moderate or severe.
Figure 1.
Prevalence of inducible ischemia and ischemia severity (based as measured by 17-segment semi-quantitative analysis [none (0%), minimal (1% to 5%), mild (5% to 10%), moderate (10% to 15%), and severe (≥15%)]) by sex and age group. A Male (N = 583), P = .002 for the presence of ischemia across age decade and B female (N = 704), P = .008 for the presence of ischemia across age decade.
CAD Events During Follow-up
The overall mean follow-up time was 11.6 ± 5.1 years, with a maximum of 25 years. Follow-up was conducted in regular intervals with censoring at the time of occurrence of an incident event. For those without events, 38.3% were followed for a maximum of 5 years, 34.8% for 10 years, 15.9% for 15 years, 7.9% for 20 years, and 3.1% for 25 years. During follow-up there were 195 people with newly documented CAD events, including 132 with ACS [90 males (15.4%) and 42 females (6.0%)], and an additional 63 with the onset of stable CAD. Acute coronary syndromes, including sudden death (7%), acute myocardial infarction (31%), or unstable angina with revascularization (29%), was the predominant event classification and accounted for 68% of all events. Stable symptomatic CAD, including angina with revascularization and medically treated angina with documented angiographic CAD accounted for 32% of all CAD events. After censoring events in the first 3 months, the mean time to an ACS event following baseline screening was similar in siblings with mild compared to moderate/severe ischemia (5.5 vs 5.1 years, respectively) but less than those with absent or minimal ischemia (8.7 vs 7.3 years, respectively; P = .06 for time to event across categories of ischemia severity).
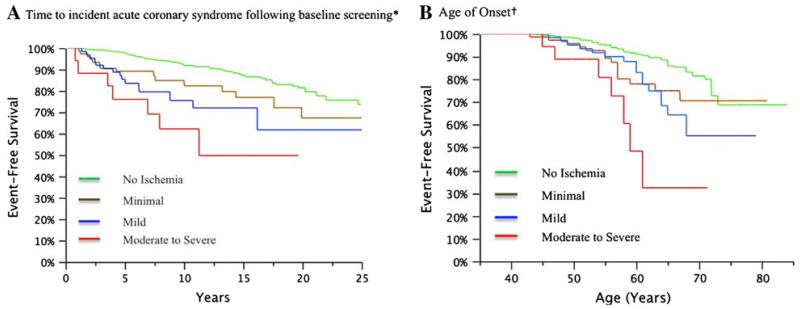
Kaplan-Meier ACS event-free survival analyses were performed for the entire length of follow-up for each individual up to 25 years by the categories of ischemia severity (Figure 2). Overall, siblings with any inducible ischemia had a worse event-free survival than those without ischemia. For increasing categories of ischemia severity, event-free survival decreased progressively (P < 0.0001) (Panel A). The divergence in event-free survival between categories of ischemia was most pronounced during the first 5 years but continued to increase throughout the entire follow-up period. Decreased event-free survival by age of onset was also observed for increasing categories of ischemia severity (P < .0001) (Panel B). For those siblings followed until 65 years of age, event-free survival was only 75% in those with minimal or mild reversible perfusion defects and 30% in those with moderate or severe defects at baseline screening.
Figure 2.
Kaplan-Meier event-free survival curves by categories of ischemia severity. A Time to incident acute coronary syndrome following baseline screening and B age of onset, P < .0001 for increasing categories of ischemia severity.
Annual Incident ACS Risk at Five Years of Follow-up
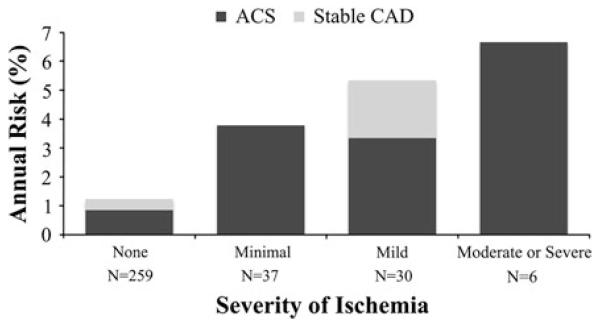
After 5 years of follow-up, there were 61 total CAD events, of which 46 were ACS. Increasing categories of ischemia severity were associated with higher 5-year incident total CAD and ACS events (for both, P < .0001 for trend overall). It was notable that siblings with minimal or mild ischemia had an annual ACS incidence of 2.1% and 2.8%, respectively, compared to a 0.4% incidence in those with no ischemia. The distribution of siblings with low, intermediate, and high Framingham risk scores was 67%, 26%, and 7%, respectively. Only 7.2% of siblings with low Framingham risk scores had any inducible ischemia at baseline and also had a relatively low annual incidence of ACS events in those without or with ischemia (0.2% and 1.0%, respectively). Conversely, 25.0% of high-Framingham risk siblings had inducible ischemia with annual incidence of ACS of 3.5%, compared to 1.7% in those without ischemia. The annual incidence of total and ACS events at 5 years of follow-up in intermediate risk siblings is shown in Figure 3. The prevalence of inducible ischemia in this group was 21.9%, and most of the ischemia that was present was minimal or mild in severity. Overall, only 1.8% had moderate or high severity ischemia. Annual incidence of CAD increased progressively with the severity of ischemia, and all events in this group were ACS, with the exception of 3 individuals with stable CAD in those with mild ischemia.
Figure 3.
Annual risk of incident acute coronary syndromes and stable coronary artery disease at 5 years of follow-up by the absence or the severity of myocardial ischemia in siblings considered intermediate risk (Intermediate risk defined as annual risk of an incident event of 1% to 2% based on the Framingham 10-year total CAD risk equation.) (N = 332), P < .0001 for annual risk of total CAD and ACS events across categories of ischemia severity.
Cox Proportional Hazard Analyses of ACS Risk
Cox proportional hazard modeling using a competitive risk model, demonstrated that all traditional risk factors were strong independent predictors of ACS events (Table 2). In addition, for each 5% increase in percent severity of ischemia (summed stress score divided by the maximum possible score of 68), there was a statistically significant relative hazard of 1.61 compared to those without ischemia.
Table 2.
Cox proportional hazard analysis of incident acute coronary syndromes defined by severity of inducible silent myocardial ischemia
| Severity of ischemia (%) | Hazard ratioδ | 95% confidence intervalδ | P valueδ |
|---|---|---|---|
| 5 | 1.61 | 1.22-2.01 | <.001 |
| 10 | 2.59 | 1.49-4.04 | |
| 15 | 4.17 | 1.81-8.12 |
Model adjusted for age, hypertension, diabetes, LDL cholesterol, and current smoking and assumes different underlying hazards for sex and race.
Severity of ischemia = Summed Stress Score divided by 68 (maximum possible) × 100%.
Reflects an underlying hazard of 1.1 (95% CI 1.04-1.15) for each 1% increase in severity of ischemia as a continuous variable in the model.
DISCUSSION
This is the first study to examine the severity of inducible ischemia in an asymptomatic population of individuals with a sibling family history of premature CAD. We demonstrate that the presence and the severity of inducible ischemia at the time of screening are strongly and independently associated with long-term prognosis and the development of ACS. The prevalence of inducible ischemia was notably high in male siblings, including >30% after 50 years of age. Most siblings with reversible perfusion abnormalities had only minimal or mild severity ischemia but that still conveyed significant excess risk of subsequent ACS events. The presence and the severity of ischemia were particularly significant in predicting ACS events in siblings at intermediate risk by traditional risk factor assessment.
Very little is known about ischemia severity and CAD outcomes in asymptomatic populations. Few studies have been constituted entirely of asymptomatic persons who were not referred for testing based on risk assessment. Khandaker et al7 retrospectively evaluated 260 asymptomatic patients who were referred for nuclear perfusion imaging without known CAD but with an increased index of suspicion; all were at intermediate Framingham risk. Low and severe risk scans were defined by corresponding percent summed stress score as <5.5% and ≥14.0%, respectively, as per the Cedars-Sinai criteria.6 The prevalence of low, moderate, and severe ischemia was 22%, 20%, and 13%, respectively. Over 10 years of follow-up, little difference was seen in annual mortality in absent, low, and moderate severity ischemia but high severity subjects had an annual mortality rate ≥3%. Recently, Zellweger et al3 retrospectively examined an asymptomatic population referred for exercise treadmill stress myocardial perfusion imaging. High severity ischemia (≥7.5%) was significantly predictive for MI or cardiac death (annual event rate ≥3.0%). For other ischemia severity groups, the rate of incident CAD was very low, 0.4%. Thus, in a referred population, ischemia severity was not really predictive of CAD events, likely because the group was relatively homogenous for CAD events a priori. In contrast, our study did not select subjects based on any a priori suspicion or concern about possible CAD, except for family history. Fleg et al2 found the prevalence of ischemia by stress thallium perfusion imaging to be <5% in apparently healthy individuals 40 to 60 years of age in the Baltimore Longitudinal Study of Aging (BLSA), in contrast to the higher prevalence observed in our higher risk population with a family history of CAD. Annual ACS event rates were lower than seen in our study.
Recent position statements by the American College of Cardiology/American Heart Association28 and the American Society of Nuclear Cardiology29 indicate that myocardial perfusion imaging may be considered in asymptomatic adults with a strong family history of CAD, although there are no prior studies that support this and no guidelines currently exist. Our findings indicate that stress myocardial perfusion imaging in asymptomatic persons with a sibling history of premature CAD further risk stratifies persons in the Framingham intermediate risk category.
Other noninvasive imaging modalities exist for identifying occult coronary atherosclerosis. Coronary artery calcification (CAC) as determined by electron beam computed tomography or multidetector helical computed tomography (MDCT) is a popular and rapid means of detection of subclinical CAD. Higher coronary calcium scores offer incremental predictive value for CAD events over the Framingham Risk Score, especially among those at intermediate Framingham risk.30 However, CAC is an indirect measurement of anatomical CAD and does not necessarily reflect the severity of CAD stenoses,31 or more dynamic functional ischemic abnormalities on perfusion imaging.12,32 Some investigators have suggested that CAC could be used as a first-step screening tool in asymptomatic subjects to identify individuals with a higher pretest likelihood of hemodynamically significant CAD on stress perfusion testing.33,34 However, the specificity of CAC is very low in young persons, especially those <50 years of age.35 We have previously demonstrated discordance between CAC on MDCT and inducible ischemia on exercise thallium tomography.12 Recent advances in MDCT angiography now enable direct anatomical quantification of plaque severity and composition but the technique does not routinely detect perfusion abnormalities, and true utility for risk prediction in asymptomatic populations has yet to be determined.36 Thus, perfusion imaging may improve CAD risk prediction in this population with a strong family history of premature CAD by possibly capturing the early dynamic vascular pathophysiology of subclinical CAD.37 It is also possible that a tiered or hybrid approach for CAD detection using a combination of stress perfusion imaging with helical CT for CAC or MDCT angiography could provide improved prognostic assessment in higher risk asymptomatic populations, as has been suggested by some investigators for patients with known or suspected CAD.38
CONCLUSION
Inducible silent myocardial ischemia is common in asymptomatic persons with a sibling family history of premature CAD, particularly in men over 50 years of age. The degree of ischemic burden is a potent independent predictor of subsequent acute coronary syndromes in this population. Most siblings with ischemia have perfusion defects of only minimal or mild severity, but even this finding conveys an excess risk and may represent an early high-risk pathophysiologic substrate for subsequent coronary plaque rupture and thrombosis.
Acknowledgments
Funding: This work was supported by grants from The Johns Hopkins General Clinical Research Center (Grant M01-RR000052 from the National Center for Research Resources, National Institutes of Health), the National Institute of Nursing Research (Grant RO1 NR08153), and the National Heart, Lung, and Blood Institute (Grants R01 HL59684 and R01 HL071025).
References
- 1.Ross R. The pathogenesis of atherosclerosis: A perspective for the 1990s. Nature. 1993;362:801–9. doi: 10.1038/362801a0. [DOI] [PubMed] [Google Scholar]
- 2.Fleg JL, Gerstenblith G, Zonderman AB, Becker LC, Weisfeldt ML, Costa PT, Jr, et al. Prevalence and prognostic significance of exercise-induced silent myocardial ischemia detected by thallium scintigraphy and electrocardiography in asymptomatic volunteers. Circulation. 1990;81:428–36. doi: 10.1161/01.cir.81.2.428. [DOI] [PubMed] [Google Scholar]
- 3.Zellweger MJ, Hachamovitch R, Kang X, Hayes SW, Friedman JD, Germano G, et al. Threshold, incidence, and predictors of prognostically high-risk silent ischemia in asymptomatic patients without prior diagnosis of coronary artery disease. J Nucl Cardiol. 2009;16:193–200. doi: 10.1007/s12350-008-9016-2. [DOI] [PubMed] [Google Scholar]
- 4.Blumenthal RS, Becker DM, Moy TF, Coresh J, Wilder LB, Becker LC. Exercise thallium tomography predicts future clinically manifest coronary heart disease in a high-risk asymp-tomatic population. Circulation. 1996;93:915–23. doi: 10.1161/01.cir.93.5.915. [DOI] [PubMed] [Google Scholar]
- 5.Berman DS, Hachamovitch R, Kiat H, Cohen I, Cabico JA, Wang FP, et al. Incremental value of prognostic testing in patients with known or suspected ischemic heart disease: A basis for optimal utilization of exercise technetium-99m sestamibi myocardial per-fusion single-photon emission computed tomography. J Am Coll Cardiol. 1995;26:639–47. doi: 10.1016/0735-1097(95)00218-S. [DOI] [PubMed] [Google Scholar]
- 6.Hachamovitch R, Berman DS, Kiat H, Cohen I, Cabico JA, Friedman J, et al. Exercise myocardial perfusion SPECT in patients without known coronary artery disease: Incremental prognostic value and use in risk stratification. Circulation. 1996;93:905–14. doi: 10.1161/01.cir.93.5.905. [DOI] [PubMed] [Google Scholar]
- 7.Khandaker MH, Miller TD, Chareonthaitawee P, Askew JW, Hodge DO, Gibbons RJ. Stress single photon emission computed tomography for detection of coronary artery disease and risk stratification of asymptomatic patients at moderate risk. J Nucl Cardiol. 2009;16:516–23. doi: 10.1007/s12350-009-9085-x. [DOI] [PubMed] [Google Scholar]
- 8.Horne BD, Camp NJ, Muhlestein JB, Cannon-Albright LA. Identification of excess clustering of coronary heart diseases among extended pedigrees in a genealogical population database. Am Heart J. 2006;152:305–11. doi: 10.1016/j.ahj.2005.12.028. [DOI] [PubMed] [Google Scholar]
- 9.Rose G. Familial patterns in ischaemic heart disease. Br J Prev Soc Med. 1964;18:75–80. doi: 10.1136/jech.18.2.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Snowden CB, McNamara PM, Garrison RJ, Feinleib M, Kannel WB, Epstein FH. Predicting coronary heart disease in siblings—a multivariate assessment: The Framingham Heart Study. Am J Epidemiol. 1982;115:217–22. doi: 10.1093/oxfordjournals.aje.a113293. [DOI] [PubMed] [Google Scholar]
- 11.Blumenthal RS, Becker DM, Yanek LR, Aversano TR, Moy TF, Kral BG, et al. Detecting occult coronary disease in a high-risk asymptomatic population. Circulation. 2003;107:702–7. doi: 10.1161/01.cir.0000048127.93169.88. [DOI] [PubMed] [Google Scholar]
- 12.Blumenthal RS, Becker DM, Yanek LR, Moy TF, Michos ED, Fishman EK, et al. Comparison of coronary calcium and stress myocardial perfusion imaging in apparently healthy siblings of individuals with premature coronary artery disease. Am J Cardiol. 2006;97:328–33. doi: 10.1016/j.amjcard.2005.08.048. [DOI] [PubMed] [Google Scholar]
- 13.Becker DM, Yook RM, Moy TF, Blumenthal RS, Becker LC. Markedly high prevalence of coronary risk factors in apparently healthy African-American and white siblings of persons with premature coronary heart disease. Am J Cardiol. 1998;82:1046–51. doi: 10.1016/s0002-9149(98)00553-0. [DOI] [PubMed] [Google Scholar]
- 14.Vaidya D, Yanek LR, Moy TF, Pearson TA, Becker LC, Becker DM. Incidence of coronary artery disease in siblings of patients with premature coronary artery disease: 10 years of follow-up. Am J Cardiol. 2007;100:1410–5. doi: 10.1016/j.amjcard.2007.06.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Murray RP, Connett JE, Lauger GG, Voelker HT, The Lung Health Study Research Group Error in smoking measures: Effects of intervention on relations of cotinine and carbon monoxide to self-reported smoking. Am J Public Health. 1993;83:1251–7. doi: 10.2105/ajph.83.9.1251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Frohlich ED. Recommendations for blood pressure determination by sphygmomanometry. Ann Intern Med. 1988;109:612. doi: 10.7326/0003-4819-109-8-612. [DOI] [PubMed] [Google Scholar]
- 17.Bachorik PS, Cloey TA, Finney CA, Lowry DR, Becker DM. Lipoprotein-cholesterol analysis during screening: Accuracy and reliability. Ann Intern Med. 1991;114:741–7. doi: 10.7326/0003-4819-114-9-741. [DOI] [PubMed] [Google Scholar]
- 18.Friedewald WT, Levy RI, Fredrickson DS. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem. 1972;18:499–502. [PubMed] [Google Scholar]
- 19.Pesce AJ, Kaplan LA. Methods in clinical chemistry. St. Louis; Mosby: 1987. [Google Scholar]
- 20.Wilson PW, D’Agostino RB, Levy D, Belanger AM, Silbershatz H, Kannel WB. Prediction of coronary heart disease using risk factor categories. Circulation. 1998;97:1837–47. doi: 10.1161/01.cir.97.18.1837. [DOI] [PubMed] [Google Scholar]
- 21.Geckle WJ, Frank TL, Links JM, Becker LC. Correction for patient and organ movement in SPECT: Application to exercise thallium-201 cardiac imaging. J Nucl Med. 1988;29:441–50. [PubMed] [Google Scholar]
- 22.Links JM, Jeremy RW, Dyer SM, Frank TL, Becker LC. Wiener filtering improves quantification of regional myocardial perfusion with thallium-201 SPECT. J Nucl Med. 1990;31:1230–6. [PubMed] [Google Scholar]
- 23.Hansen CL, Goldstein RA, Akinboboye OO, Berman DS, Botvinick EH, Churchwell KB, et al. Myocardial perfusion and function: Single photon emission computed tomography. J Nucl Cardiol. 2007;14:e39–60. doi: 10.1016/j.nuclcard.2007.09.023. [DOI] [PubMed] [Google Scholar]
- 24.Germano G, Berman DS. Clinical gated cardiac SPECT. 2nd ed. Blackwell Pub.; Malden, Mass.: 2006. [Google Scholar]
- 25.Cerqueira MD, Weissman NJ, Dilsizian V, Jacobs AK, Kaul S, Laskey WK, et al. Standardized myocardial segmentation and nomenclature for tomographic imaging of the heart: A statement for healthcare professionals from the Cardiac Imaging Committee of the Council on Clinical Cardiology of the American Heart Association. Circulation. 2002;105:539–42. doi: 10.1161/hc0402.102975. [DOI] [PubMed] [Google Scholar]
- 26.Shaw LJ, Hendel RC, Heller GV, Borges-Neto S, Cerqueira M, Berman DS. Prognostic estimation of coronary artery disease risk with resting perfusion abnormalities and stress ischemia on myocardial perfusion SPECT. J Nucl Cardiol. 2008;15:762–73. doi: 10.1007/BF03007357. [DOI] [PubMed] [Google Scholar]
- 27.Lau B, Cole SR, Gange SJ. Competing risk regression models for epidemiologic data. Am J Epidemiol. 2009;170:244–56. doi: 10.1093/aje/kwp107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Greenland P, Alpert JS, Beller GA, Benjamin EJ, Budoff MJ, Fayad ZA, et al. ACCF/AHA guideline for assessment of cardiovascular risk in asymptomatic adults: Executive summary: A report of the American College of Cardiology Foundation/ American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2010;56:2182–99. doi: 10.1016/j.jacc.2010.09.001. 2010. [DOI] [PubMed] [Google Scholar]
- 29.Hendel RC, Abbott BG, Bateman TM, Blankstein R, Calnon DA, Leppo JA, et al. The role of radionuclide myocardial perfusion imaging for asymptomatic individuals. J Nucl Cardiol. 2011;18:3–15. doi: 10.1007/s12350-010-9320-5. [DOI] [PubMed] [Google Scholar]
- 30.Greenland P, LaBree L, Azen SP, Doherty TM, Detrano RC. Coronary artery calcium score combined with Framingham score for risk prediction in asymptomatic individuals. JAMA. 2004;291:210–5. doi: 10.1001/jama.291.2.210. [DOI] [PubMed] [Google Scholar]
- 31.Schmermund A, Baumgart D, Adamzik M, Ge J, Gronemeyer D, Seibel R, et al. Comparison of electron-beam computed tomography and intracoronary ultrasound in detecting calcified and noncalcified plaques in patients with acute coronary syndromes and no or minimal to moderate angiographic coronary artery disease. Am J Cardiol. 1998;81:141–6. doi: 10.1016/s0002-9149(97)00890-4. [DOI] [PubMed] [Google Scholar]
- 32.Rozanski A, Gransar H, Wong ND, Shaw LJ, Miranda-Peats R, Polk D, et al. Clinical outcomes after both coronary calcium scanning and exercise myocardial perfusion scintigraphy. J Am Coll Cardiol. 2007;49:1352–61. doi: 10.1016/j.jacc.2006.12.035. [DOI] [PubMed] [Google Scholar]
- 33.He ZX, Hedrick TD, Pratt CM, Verani MS, Aquino V, Roberts R, et al. Severity of coronary artery calcification by electron beam computed tomography predicts silent myocardial ischemia. Circulation. 2000;101:244–51. doi: 10.1161/01.cir.101.3.244. [DOI] [PubMed] [Google Scholar]
- 34.Anand DV, Lim E, Raval U, Lipkin D, Lahiri A. Prevalence of silent myocardial ischemia in asymptomatic individuals with subclinical atherosclerosis detected by electron beam tomography. J Nucl Cardiol. 2004;11:450–7. doi: 10.1016/j.nuclcard.2004.06.125. [DOI] [PubMed] [Google Scholar]
- 35.Fallavollita JA, Brody AS, Bunnell IL, Kumar K, Canty JM., Jr. Fast computed tomography detection of coronary calcification in the diagnosis of coronary artery disease. Comparison with angiography in patients <50 years old. Circulation. 1994;89:285–90. doi: 10.1161/01.cir.89.1.285. [DOI] [PubMed] [Google Scholar]
- 36.Berman DS, Hachamovitch R, Shaw LJ, Friedman JD, Hayes SW, Thomson LE, et al. Roles of nuclear cardiology, cardiac computed tomography, and cardiac magnetic resonance: Assessment of patients with suspected coronary artery disease. J Nucl Med. 2006;47:74–82. [PubMed] [Google Scholar]
- 37.Kral BG, Becker LC, Blumenthal RS, Aversano T, Fleisher LA, Yook RM, et al. Exaggerated reactivity to mental stress is associated with exercise-induced myocardial ischemia in an asymptomatic high-risk population. Circulation. 1997;96:4246–53. doi: 10.1161/01.cir.96.12.4246. [DOI] [PubMed] [Google Scholar]
- 38.Yerramasu A, Lahiri A, Chua T. Comparative roles of cardiac CT and myocardial perfusion scintigraphy in the evaluation of patients with coronary artery disease: Competitive or complementary. J Nucl Cardiol. 2010;17:761–70. doi: 10.1007/s12350-010-9260-0. [DOI] [PubMed] [Google Scholar]