Abstract
We report the total synthesis of (–)-Nmethylwelwitindolinone C isonitrile, in addition to the total syntheses of the 3-hydroxylated welwitindolinones. Our routes to these elusive natural products feature the strategic use of a deuterium kinetic isotope effect to improve the efficiency of a late-stage nitrene insertion reaction. We also provide a computational prediction for the stereochemical configuration at C3 of the hydroxylated welwitindolinones, which was confirmed by experimental studies.
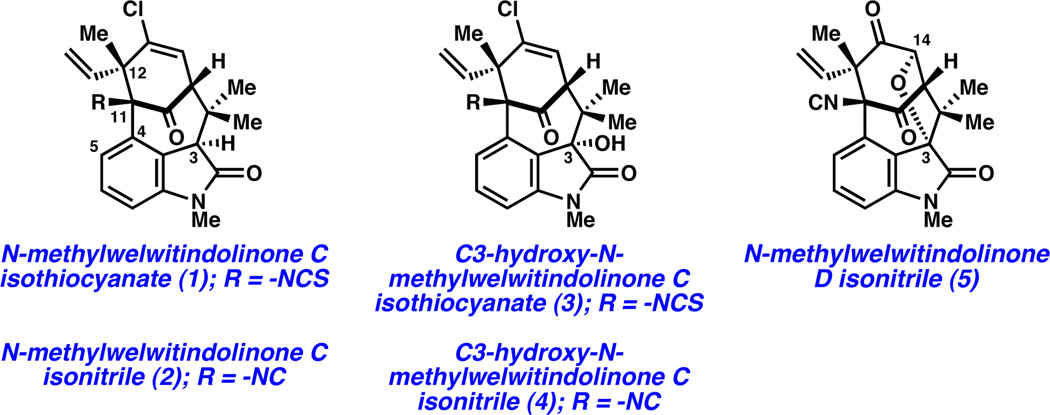
Since their isolation reports in 1994 and 1999,1 the welwitindolinone natural products have captivated synthetic chemists worldwide.2 To date, nine welwitindolinones with [4.3.1]-bicyclic frameworks have been discovered (e.g., 1–5, Figure 1), some of which show promising activity for the treatment of drug resistant cancer cells.3 The dense array of functional groups that decorate the compact structure of these targets has taunted chemists for nearly two decades. More than fifteen laboratories have reported progress toward these intriguing natural products, resulting in many elegant approaches to the bicyclic core.4,5 The strategies used by our laboratory and Rawal’s, respectively, have recently facilitated the first two syntheses of these elusive natural products.6,7 However, syntheses of several challenging members of the welwitindolinone family of natural products have not been reported.8
Figure 1.
Welwitindolinones 1–5.
In this communication, we report the total syntheses of three natural products in the welwitindolinone C series: (–)-2, (–)-3 and (–)-4. The latter two of these targets represent the so-called "oxidized welwitindolinones", whose configuration at C3 had not been unambiguously defined. We also describe the strategic manipulation of a kinetic isotope effect to improve the efficiency of a challenging C–H activation / nitrene-insertion reaction, which takes place late-stage in the total syntheses to forge a critical C–N bond.
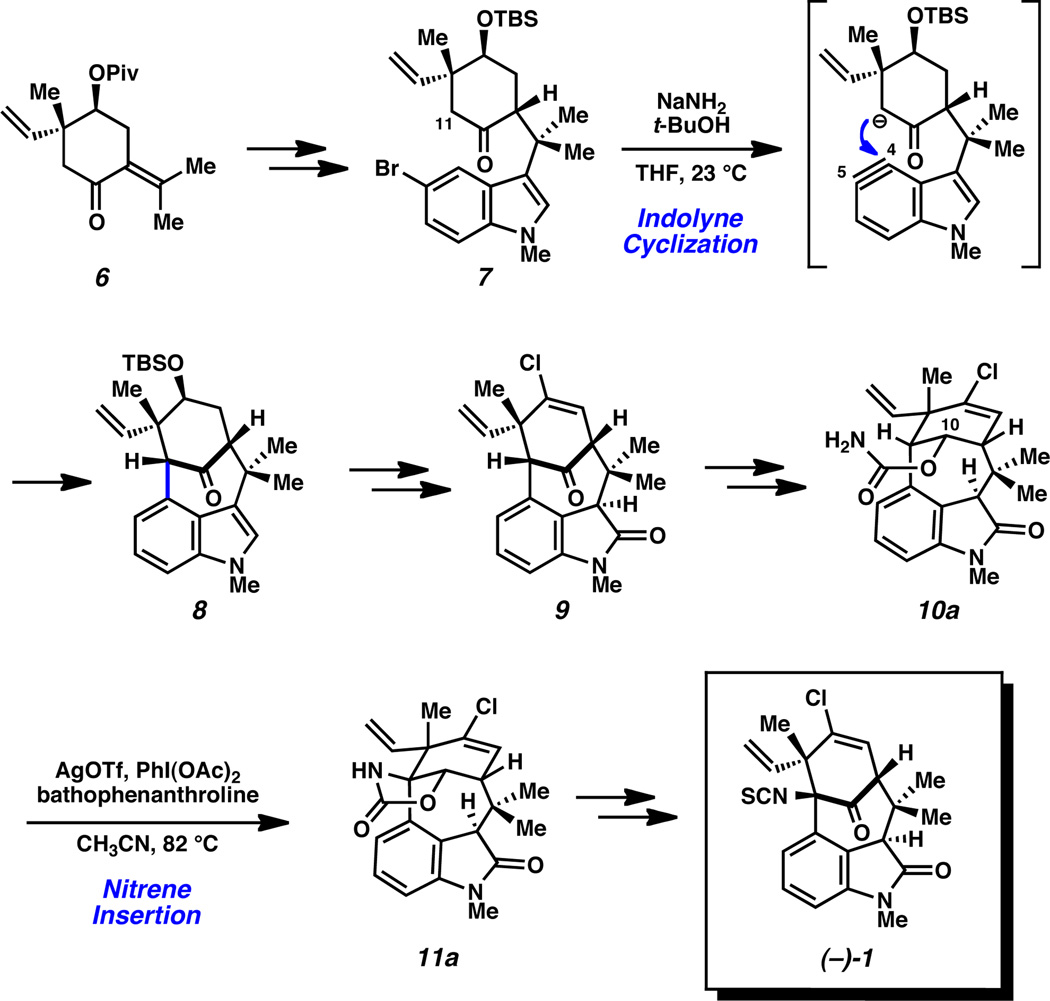
A summary of our recent total synthesis of (–)-17 is shown in Scheme 1. Known carvone derivative 6 was elaborated to bromoindole 7 over three synthetic steps. Subsequent treatment of 7 with NaNH2 and t-BuOH in THF facilitated an indolyne cyclization to afford 8, which possesses the desired [4.3.1]-bicycle. Bicycle 8 was elaborated to ketone 9, which lacked only the isothiocyanate functional group. Thus, ketone 9 was readily converted to carbamate 10a the substrate for a critical nitrene C–H insertion reaction.9,10,11 We were delighted to find that the desired C–H functionalization took place to afford 11a upon exposure of substrate 10a to the Ag-promoted conditions described by He.11b,c Insertion product 11a could be elaborated to the elusive natural product (–)-1 over three additional transformations.
Scheme 1.
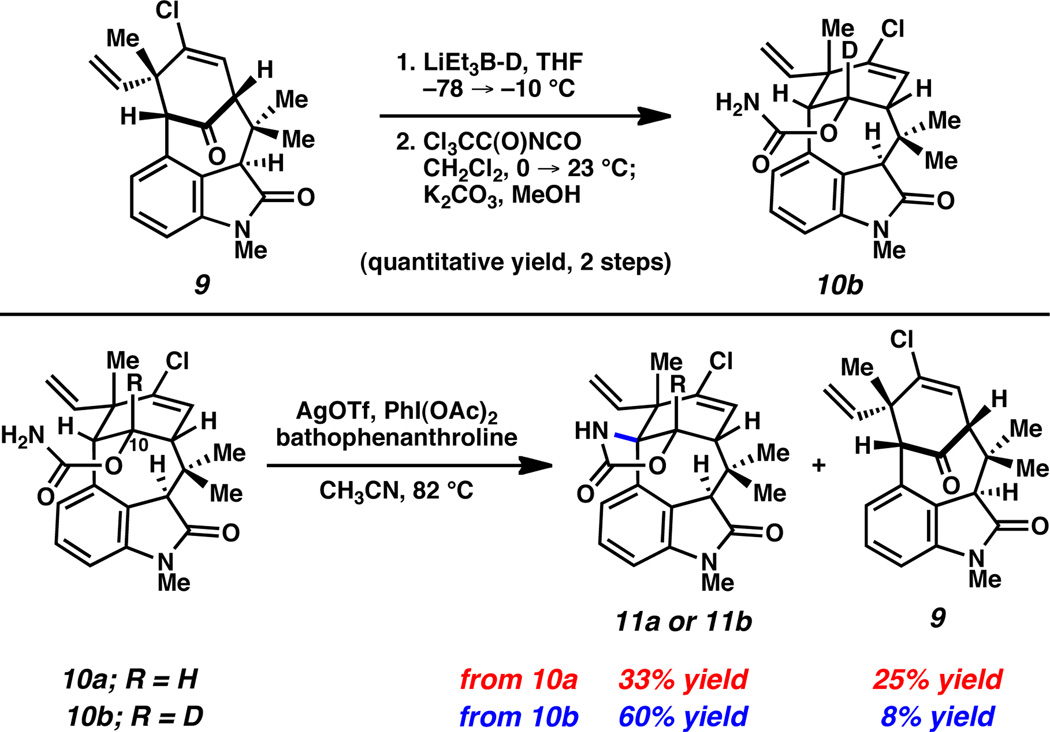
In order to facilitate syntheses of the remaining natural products in the welwitindolinone C series, we sought to first improve the efficiency of the late-stage nitrene insertion reaction (i.e., 10a→11a, Scheme 1), which had proceeded in a modest 33% yield. It was noted that a major byproduct of the insertion step was ketone 9, which presumably formed through the undesired insertion of the intermediate nitrene species into the C10 C–H bond.12 We hypothesized that replacing the problematic hydrogen with deuterium would subdue the undesired insertion process, thereby favoring the desired functionalization event.13 The deuterated substrate 10b was readily prepared by a sequence involving reduction of ketone 9 with super deuteride, followed by carbamoylation (Figure 2). We were delighted to find that exposure of this substrate to our optimal reaction conditions for nitrene insertion furnished the desired product 11b in 60% yield, while the formation of ketone 9 was diminished. The strategic use of a deuterium kinetic isotope effect in total synthesis is rare,14 and the present study marks the first use of this approach to facilitate a C–H functionalization event en route to natural products.
Figure 2.
Nitrene insertion of substrates 10a and 10b.
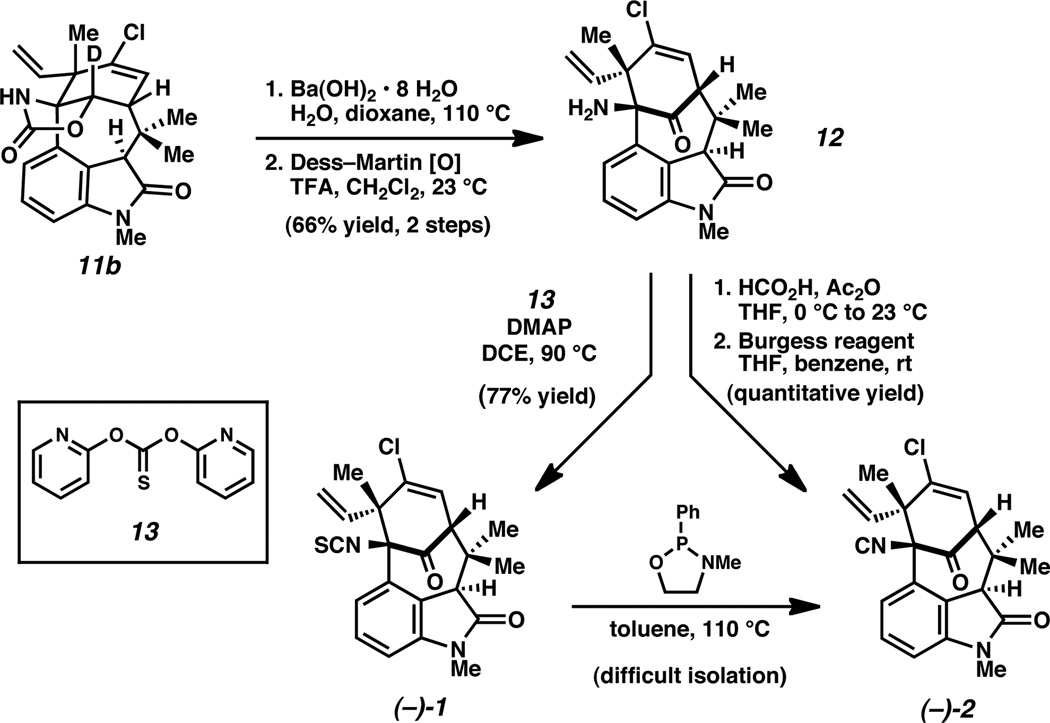
With improved access to a C11 N-functionalized product, we explored elaboration of 11b to several welwitindolinone natural products. Hydrolysis of the carbamate, followed by Dess–Martin oxidation, proceeded smoothly to furnish aminoketone 12 (Scheme 2). Subsequent elaboration of 12 delivered N-methylwelwitindolinone C isothiocyanate (–)-1 as we have shown previously.6 Subsequently, exposure of this natural product to Rawal’s desulfurization conditions provided (–)-N-methylwelwitindolinone isonitrile (2) as the major product.6 Unfortunately, purification of the crude natural product proved difficult.15 As a workaround, aminoketone 12 was subjected to sequential formylation4s and dehydration4m to afford the desired natural product (–)-2 in 88% yield.16 Spectral data for synthetic (–)-2 was in accord with that provided for natural (–)-2 in the isolation report.1a
Scheme 2.
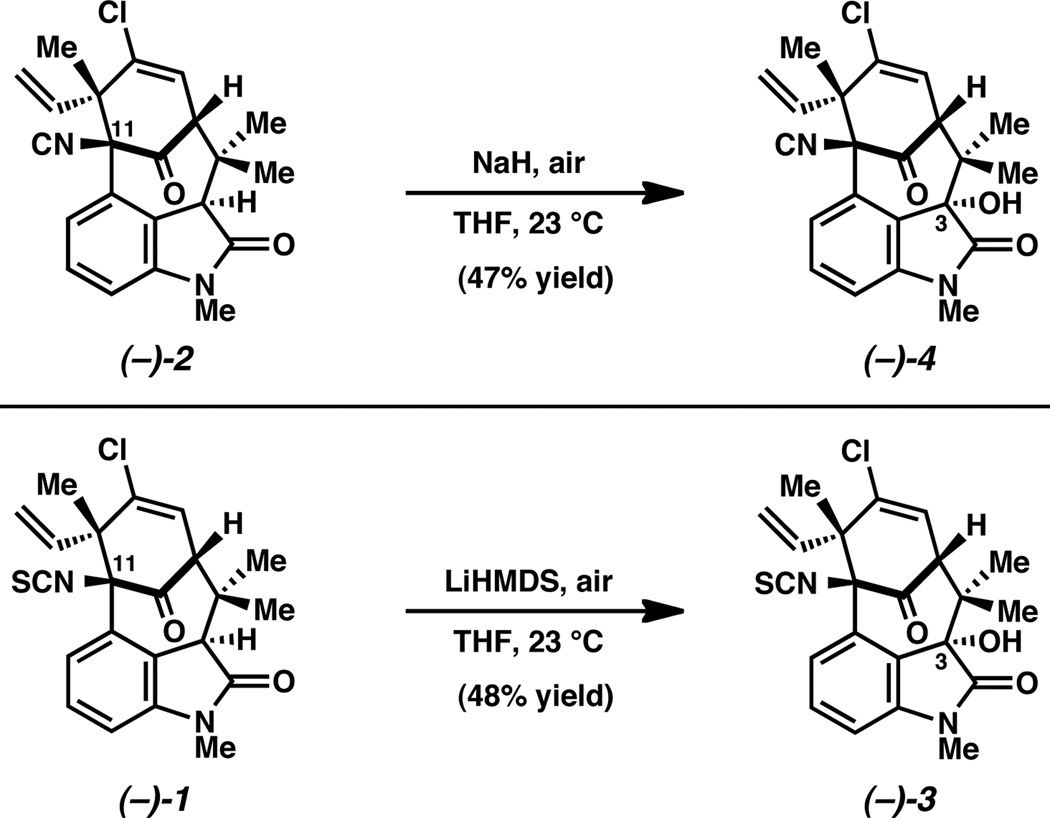
We next pursued total syntheses of the C3-hydroxylated welwitindolinones, the two oxidized welwitindolinones that had not been synthesized previously. Furthermore, the stereochemical configuration of these natural products at C3 had not been rigorously established spectroscopically, but rather, had been assigned based on analogy to the non-hydroxylated welwitindolinone natural products.17 In our first attempts toward these natural products, aminoketone 12 was treated with various bases, with the reaction vessels being under standard atmospheric conditions to allow for air-oxidation. Although the corresponding C3 oxidized product was formed and could be manipulated further, low yields and irreproducibility hampered our efforts. However, direct oxidation of the non-hydroxylated natural products was found to be a more fruitful strategy (Figure 3). It should be noted that related aerobic oxidations of oxindoles have been reported,18 including an impressive example in the context of the welwitindolinones.19 Treatment of (–)-N-methylwelwitindolinone C isonitrile (2) with NaH in the presence of air, provided (–)-3-hydroxy-Nmethylwelwitindolinone C isonitrile (4). Similarly, oxidation of (–)-N-methylwelwitindolinone C isothiocyanate (1) delivered (–)-3-hydroxy-N-methylwelwitindolinone C isonitrile (3). Both oxidations occurred selectively to furnish single diastereomers of hydroxylated products, while leaving the sensitive C11 functional groups undisturbed.
Figure 3.
Total synthesis of oxidized welwitindolinones 3 and 4.
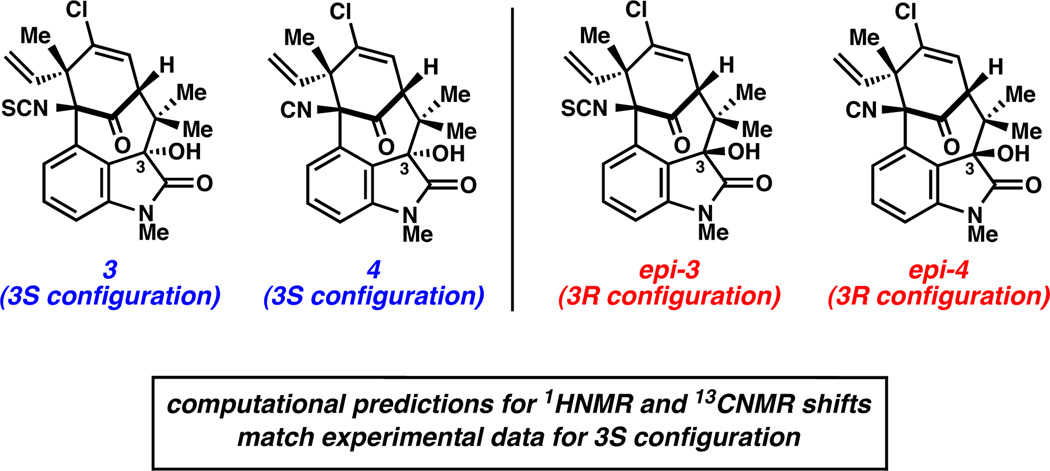
For each of the natural products synthesized, our synthetic samples matched the natural materials by spectroscopic means.1b,20 However, for the hydroxylated welwitindolinones, the C3 stereochemistry remained to be unambiguously established. Since computational predictions for 1H and 13C NMR chemical shifts have proven valuable in elucidating stereochemical configurations of natural products,21,22 we calculated the 1H and 13C NMR chemical shifts for the C3 epimers of welwitindolinones 3 and 4.23 In both cases, the computed chemical shifts for the C3(S) diastereomer matched the experimental data better than did the computed shifts for the C3(R) diastereomer. For example, although computed 13C shifts for 4 and epi-4 deviated from the experimental shifts by similar amounts (mean absolute deviations (MADs) of 2.13 and 2.69 ppm, with largest outliers off by 5.59 and 5.34 ppm for 4 and epi-4, respectively), computed 1H shifts for 4 matched the experimental values much more closely than did computed shifts for epi-4 (MADs of 0.08 (0.05 without the OH proton included) and 0.36 ppm (0.34 without the OH proton included), with largest C–H outliers off by 0.13 and 0.79 ppm for 4 and epi-4, respectively). Similar results were obtained for 3.23 We therefore propose that the stereochemical configuration at C3 is S in 3 and 4, in accord with the hypothesis made by the isolation chemists.1
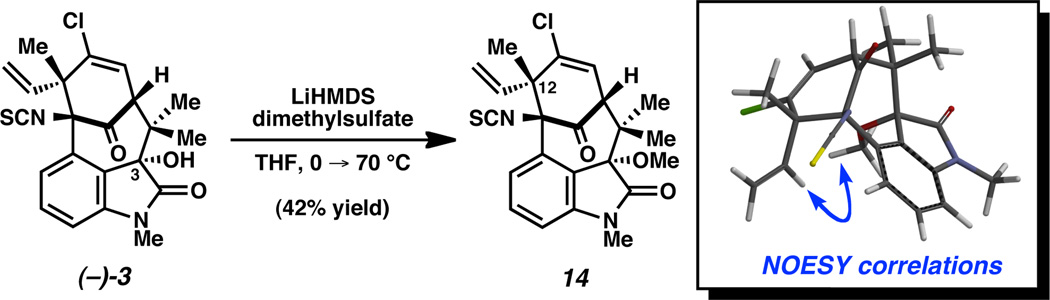
To provide evidence for this stereochemical assignment, (–)-3-hydroxy-N-methylwelwitindolinone C isothiocyanate (3) was treated with LiHMDS and dimethylsulfate (Scheme 3). Despite the severely hindered nature of the tertiary alcohol, methylation proceeded to provide ether 14. 2D-NOESY experiments of 14 showed correlations between the methoxy protons and the protons of the vinyl group at C12, thus supporting the proposed C3(S) configuration.24 This result further validates the promising use of computational chemistry to establish stereochemical assignments on complex molecules.21,22
Scheme 3.
In summary, we have completed the total syntheses of several elusive welwitindolinone natural products. Our routes to these natural products feature the strategic use of a deuterium kinetic isotope effect to improve the efficiency of a late-stage nitrene insertion reaction. We also provide a computational prediction for the stereochemical configuration at C3 of the hydroxylated welwitindolinones 3 and 4. This prediction was confirmed by experimental studies. Our findings are expected to facilitate the total syntheses of other welwitindolinone natural products, while demonstrating the utility of computational chemistry in elucidating stereochemical assignments and the strategic manipulation of kinetic isotope effects in total synthesis.
Supplementary Material
Figure 4.
Structures of 3 and 4, in addition to C3 epimers, and summary of computational findings.
ACKNOWLEDGMENT
The authors are grateful to the NIH-NIGMS (R01 GM090007), the NSF (CHE-0957416), Boehringer Ingelheim, DuPont, Eli Lilly, Amgen, AstraZeneca, the University of California, Los Angeles, the ACS Organic Division (fellowship to K.W.Q.), Bristol–Myers Squibb (fellowship to K.W.Q.), and the Foote Family (fellowships to A.D.H. and K.W.Q.), for financial support. We thank the Garcia–Garibay laboratory (UCLA) for access to instrumentation, Dr. John Greaves (UC Irvine) for mass spectra, and Professor Philip Williams and Dr. Wesley Yoshida (University of Hawaii) for NMR spectra of natural products.
Footnotes
Supporting Information Available. Complete authorship for Ref. 4s, detailed experimental and theoretical procedures, compound characterization data, and computed coordinates, energies and chemical shift data for 3, epi-3, 4 and epi-4 (PDF). This material is available free of charge via the Internet at http://pubs.acs.org.
Contributor Information
Kyle W. Quasdorf, Department of Chemistry and Biochemistry, University of California, Los Angeles, California 90095, United States
Alexander D. Huters, Department of Chemistry and Biochemistry, University of California, Los Angeles, California 90095, United States
Michael W. Lodewyk, Department of Chemistry, University of California, Davis, One Shields Avenue, Davis, California 95616, United States
Dean J. Tantillo, Department of Chemistry, University of California, Davis, One Shields Avenue, Davis, California 95616, United States
Neil K. Garg, Email: neilgarg@chem.ucla.edu, Department of Chemistry and Biochemistry, University of California, Los Angeles, California 90095, United States.
REFERENCES
- 1.(a) Stratmann K, Moore RE, Bonjouklian R, Deeter JB, Patterson GML, Shaffer S, Smith CD, Smitka TA. J. Am. Chem. Soc. 1994;116:9935–9942. [Google Scholar]; (b) Jimenez JL, Huber U, Moore RE, Patterson GML. J. Nat. Prod. 1999;62:569–572. doi: 10.1021/np980485t. [DOI] [PubMed] [Google Scholar]
- 2.Welwitindolinone A isonitrile a unique welwitindolinone that possesses a C3 spirooxindoline core has been synthesized independently by the Baran and Wood groups see: Baran PS, Richter JM. J. Am. Chem. Soc. 2005;127:15394–15396. doi: 10.1021/ja056171r. Reisman SE, Ready JM, Hasuoka A, Smith CJ, Wood JL. J. Am. Chem. Soc. 2006;128:1448–1449. doi: 10.1021/ja057640s.
- 3.(a) Smith CD, Zilfou JT, Stratmann K, Patterson GML, Moore RE. Mol. Pharmacol. 1995;47:241–247. [PubMed] [Google Scholar]; (b) Zhang X, Smith CD. Mol. Pharmacol. 1996;49:288–294. [PubMed] [Google Scholar]
- 4.(a) Konopelski JP, Deng H, Schiemann K, Keane JM, Olmstead MM. Synlett. 1998:1105–1107. [Google Scholar]; (b) Wood JL, Holubec AA, Stoltz BM, Weiss MM, Dixon JA, Doan BD, Shamji MF, Chen JM, Heffron TP. J. Am. Chem. Soc. 1999;121:6326–6327. [Google Scholar]; (c) Kaoudi T, Ouiclet-Sire B, Seguin S, Zard SZ. Angew. Chem. Int. Ed. 2000;39:731–733. [PubMed] [Google Scholar]; (d) Deng H, Konopelski JP. Org. Lett. 2001;3:3001–3004. doi: 10.1021/ol016379r. [DOI] [PubMed] [Google Scholar]; (e) Jung ME, Slowinski F. Tetrahedron Lett. 2001;42:6835–6838. [Google Scholar]; (f) López-Alvarado P, García-Granda S, Ivarez-Rúa C, Avendaño C. Eur. J. Org. Chem. 2002:1702–1707. [Google Scholar]; (g) MacKay JA, Bishop RL, Rawal VH. Org. Lett. 2005;7:3421–3424. doi: 10.1021/ol051043t. [DOI] [PubMed] [Google Scholar]; (h) Baudoux J, Blake AJ, Simpkins NS. Org. Lett. 2005;7:4087–4089. doi: 10.1021/ol051239t. [DOI] [PubMed] [Google Scholar]; (i) Greshock TJ, Funk RL. Org. Lett. 2006;8:2643–2645. doi: 10.1021/ol0608799. [DOI] [PMC free article] [PubMed] [Google Scholar]; (j) Lauchli R, Shea KJ. Org. Lett. 2006;8:5287–5289. doi: 10.1021/ol0620747. [DOI] [PubMed] [Google Scholar]; (k) Guthikonda K, Caliando BJ, Du Bois J. Abstracts of Papers. 232nd ACS National Meeting, September 2006, abstr ORGN-002. [Google Scholar]; (l) Xia J, Brown LE, Konopelski JP. J. Org. Chem. 2007;72:6885–6890. doi: 10.1021/jo071156l. [DOI] [PMC free article] [PubMed] [Google Scholar]; (m) Richter JM, Ishihara Y, Masuda T, Whitefield BW, Llamas T, Pohjakallio A, Baran PS. J. Am. Chem. Soc. 2008;130:17938–17945. doi: 10.1021/ja806981k. [DOI] [PMC free article] [PubMed] [Google Scholar]; (n) Boissel V, Simpkins NS, Bhalay G, Blake AJ, Lewis W. Chem. Commun. 2009:1398–1400. doi: 10.1039/b820674k. [DOI] [PubMed] [Google Scholar]; (o) Boissel V, Simpkins NS, Bhalay G. Tetrahedron Lett. 2009;50:3283–3286. [Google Scholar]; (p) Tian X, Huters AD, Douglas CJ, Garg NK. Org. Lett. 2009;11:2349–2351. doi: 10.1021/ol9007684. [DOI] [PubMed] [Google Scholar]; (q) Trost BM, McDougall PJ. Org. Lett. 2009;11:3782–3785. doi: 10.1021/ol901499b. [DOI] [PMC free article] [PubMed] [Google Scholar]; (r) Brailsford JA, Lauchli R, Shea KJ. Org. Lett. 2009;11:5330–5333. doi: 10.1021/ol902173g. [DOI] [PMC free article] [PubMed] [Google Scholar]; (s) Freeman DB, et al. Tetrahedron. 2010;66:6647–6655. doi: 10.1016/j.tet.2010.04.131. [DOI] [PMC free article] [PubMed] [Google Scholar]; (t) Heidebrecht RW, Jr, Gulledge B, Martin SF. Org. Lett. 2010;12:2492–2495. doi: 10.1021/ol1006373. [DOI] [PMC free article] [PubMed] [Google Scholar]; (u) Ruiz M, López-Alvarado P, Menéndez JC. Org. Biomol. Chem. 2010;8:4521–4523. doi: 10.1039/c0ob00382d. [DOI] [PubMed] [Google Scholar]; (v) Bhat V, Rawal VH. Chem. Comm. 2011;47:9705–9707. doi: 10.1039/c1cc13498a. [DOI] [PubMed] [Google Scholar]; (w) Bhat V, MacKay JA, Rawal VH. Org. Lett. 2011;13:3214–3217. doi: 10.1021/ol201122f. [DOI] [PMC free article] [PubMed] [Google Scholar]; (x) Bhat V, MacKay JA, Rawal VH. Tetrahedron. 2011;67:10097–10104. doi: 10.1016/j.tet.2011.09.088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.For pertinent reviews see: Brown LE, Konopelski JP. Org. Prep. Proc. Intl. 2008;40:411–445. Avendaño C, Menéndez JC. Curr. Org. Synth. 2004;1:65–82.
- 6.For Rawal’s breakthrough total synthesis of (±)-5 see: Bhat V, Allan KM, Rawal VH. J. Am. Chem. Soc. 2011;133:5798–5801. doi: 10.1021/ja201834u.
- 7.For the total synthesis of (–)-1, see: Huters AD, Quasdorf KW, Styduhar ED, Garg NK. J. Am. Chem. Soc. 2011;133:15797–15799. doi: 10.1021/ja206538k.
- 8.As described in the preceding communication, Rawal and co-workers have simultaneously achieved elegant asymmetric total syntheses of 1–3.
- 9.(a) Davies HML, Manning JR. Nature. 2008;451:417–424. doi: 10.1038/nature06485. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Collet F, Lescot C, Liang C, Dauban P. Dalton Trans. 2010;39:10401–10413. doi: 10.1039/c0dt00283f. [DOI] [PubMed] [Google Scholar]
- 10.For an elegant late-stage nitrene insertion in natural product total synthesis, see: Hinman A, Du Bois J. J. Am. Chem. Soc. 2003;125:11510–11511. doi: 10.1021/ja0368305.
- 11.For intramolecular nitrene C–H insertion reactions using carbamate substrates, see: Espino CG, Du Bois J. Angew. Chem. Int. Ed. 2001;40:598–600. Li Z, Capretto DA, Rahaman R, He C. Angew. Chem. Int. Ed. 2007;46:5184–5186. doi: 10.1002/anie.200700760. Cui Y, He C. Angew. Chem. Int. Ed. 2004;43:4210–4212. doi: 10.1002/anie.200454243.
- 12.Related oxidation processes have previously been observed; see: Hinman AW. Ph.D. Dissertation. Stanford CA: Stanford University; 2004.
- 13.For a study of the kinetic isotope effect in Rh-catalyzed nitrene insertion reactions, see: Fiori KW, Espino CG, Brodsky BH, Du Bois J. Tetrahedron. 2009;65:3042–3051.
- 14.For elegant examples involving the strategic use of deuterium in total synthesis see: Clive DLJ, Cantin M, Khodabocus A, Kong X, Tao Y. Tetrahedron. 1993;49:7917–7930. Vedejs E, Little J. J. Am. Chem. Soc. 2002;124:748–749. doi: 10.1021/ja0120835. Miyashita M, Sasaki M, Hattori I, Sakai M, Tanino K. Science. 2004;305:495–499. doi: 10.1126/science.1098851.
- 15.Rawal and coworkers have recently achieved this transformation; see reference 8.
- 16.Moore and coworkers have shown that (–)-2can be converted to (–)-4and (–)-5, albeit in low yield (5% and 3% yield, respectively) using a photooxidation procedure. Thus, the synthesis of (–)-2 also constitutes formal total syntheses of (–)-4and (–)-5.
- 17.The C3 stereochemical configuration of 4 was assigned based on this compound having similar 1HNMR and CD spectra in comparison to 2. Further support was obtained by the experiment described in reference 16. The C3 configuration of 3 was assigned by analogy to 4. See reference 2b.
- 18.For recent examples of the aerobic oxidation of oxindoles to C3-hydroxy oxindoles, see: Shen HC, Ding FX, Colletti SL. Org. Lett. 2006;8:1447–1450. doi: 10.1021/ol060246u. Durbin MJ, Willis MC. Org. Lett. 2008;10:1413–1415. doi: 10.1021/ol800141t. Sano D, Nagata K, Itoh T. Org. Lett. 2008;10:1593–1595. doi: 10.1021/ol800260r.
- 19.Holubec AA. Ph.D. Dissertation. New Haven CT: Yale University; 2000. [Google Scholar]
- 20.NMR data for synthetic 3 did not match the tabulated data provided in the original isolation report (see reference 1b). However, an authentic sample of 3 was recently located at the University of Hawaii, and subsequent NMR analysis revealed that the NMR data for 3 reported upon isolation was mis-tabulated. Indeed, synthetic 3 matched natural 3 by all spectroscopic means. We thank Philip Williams and Wesley Yoshida (University of Hawaii) for resolving this discrepancy. Of note, Rawal and coworkers have arrived at the same conclusion regarding the spectral data for 3 see reference 8.
- 21.Recent examples: Saielli G, Nicolaou KC, Ortiz A, Zhang H, Bagno A. J. Am. Chem. Soc. 2011;133:6072–6077. doi: 10.1021/ja201108a. Smith SG, Goodman JM. J. Am. Chem. Soc. 2010;132:12946–12959. doi: 10.1021/ja105035r. Lodewyk MW, Tantillo DJ. J. Nat. Prod. 2011;74:1339–1343. doi: 10.1021/np2000446. Schwartz BD, White LV, Banwell MG, Willis AC. J. Org. Chem. 2011;76:8560–8563. doi: 10.1021/jo2016899.
- 22.For a review on chemical shift calculations, see: Lodewyk MW, Siebert MR, Tantillo DJ. Chem. Rev. doi: 10.1021/cr200106v. ASAP, DOI:101021/cr200106v.
- 23.Calculated at the SMD(chloroform)-mPW1PW91/6-311+G(2d,p)//B3LYP/6-31+G(d,p) level with linear scaling (see http://cheshireNMR.info and Jain RJ, Bally T, Rablen PR. J. Org. Chem. 2009;74:4017–4023. doi: 10.1021/jo900482q. . A thorough conformational search was performed on 4 and computed shifts were averaged based on a Boltzman distribution. See Supporting Information for additional details.
- 24.The 3-dimensional structure shown in Scheme 3 was obtained by geometry optimization calculations (MMFF) using MacSpartan ’10 (Wavefunction, Inc. Irvine CA).
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.