Abstract
Because no organism lives in an unchanging environment, sensory processes must remain plastic so that in any context, they emphasize the most relevant signals. As the behavioral relevance of sociosexual signals changes along with reproductive state, the perception of those signals is altered by reproductive hormones such as estradiol (E2). We showed previously that in white-throated sparrows, immediate early gene responses in the auditory pathway of females are selective for conspecific male song only when plasma E2 is elevated to breeding-typical levels. In this study, we looked for evidence that E2-dependent modulation of auditory responses is mediated by serotonergic systems. In female nonbreeding white-throated sparrows treated with E2, the density of fibers immunoreactive for serotonin transporter innervating the auditory midbrain and rostral auditory forebrain increased compared with controls. E2 treatment also increased the concentration of the serotonin metabolite 5-HIAA in the caudomedial mesopallium of the auditory forebrain. In a second experiment, females exposed to 30 min of conspecific male song had higher levels of 5-HIAA in the caudomedial nidopallium of the auditory forebrain than birds not exposed to song. Overall, we show that in this seasonal breeder, (1) serotonergic fibers innervate auditory areas; (2) the density of those fibers is higher in females with breeding-typical levels of E2 than in nonbreeding, untreated females; and (3) serotonin is released in the auditory forebrain within minutes in response to conspecific vocalizations. Our results are consistent with the hypothesis that E2 acts via serotonin systems to alter auditory processing.
Keywords: 5-HT, 5-HIAA, estradiol, serotonin transporter (SERT), songbird
INTRODUCTION
Sensory systems must be adapted to emphasize the stimuli most important for reproduction and survival. No environment is constant, however, and the relative importance of some types of stimuli, particularly social signals, changes over time. As a consequence, the neural substrates that process and encode those stimuli must also change in order to facilitate context-appropriate behavioral responses. What mechanisms govern this plasticity? Recent evidence from fish, frogs, birds, and mammals suggests that one powerful mediator of context-dependent sensory processing is endocrine state; hormones can alter perception of social signals from the “bottom up” (reviewed by Maney, 2010; Maney & Pinaud, 2011). Despite the ubiquity of this phenomenon, the neural processes underlying it are not well understood.
Beach (1948) proposed that hormones act at the sensory periphery to alter how social signals are perceived. It has since become clear that hormones do have profound effects on sensory systems, to the extent that detection thresholds and discrimination can be altered. In both rats and humans, for example, the ability to detect odors is greater during periods of high estradiol (E2) than during other phases of the reproductive cycle, and E2 treatment improves olfactory sensitivity in ovariectomized rats and postmenopausal women (Caruso et al., 2001, 2004; Pietras & Moulton, 1974). Estrogen receptors can be found in sensory organs (Barni et al., 1999; Begay, Valotaire, Ravault, Collin, & Falcon, 1994; Maruska & Fernald, 2010; Noirot et al., 2009; Sisneros, Forlano, Deitcher, & Bass, 2004) and estradiol is likely to impact sensory processing at every level in the brain.
Researchers investigating hormone-modulated sensory function have made tremendous advances in the past five years using songbirds as model systems. For example, we have shown evidence that in seasonally breeding sparrows, systemic E2 treatment can dramatically alter the responsiveness and neurochemistry of auditory areas. In the auditory midbrain, thalamus, and forebrain, immediate early gene (IEG) responses are “selective”, meaning the response is greater to conspecific male song than to an irrelevant control sound, only in females with breeding-typical plasma E2 levels (reviewed by Maney & Pinaud, 2011). In untreated, non-breeding females, the genomic response to song is indistinguishable from the response to the control sound. Second, E2 treatment alone, independently of sound, induces IEG expression in auditory regions (Sanford, Lange, & Maney, 2010). E2 thus seems to induce plasticity in the auditory system, making it both more responsive and more selective for the sounds that are important in a breeding context.
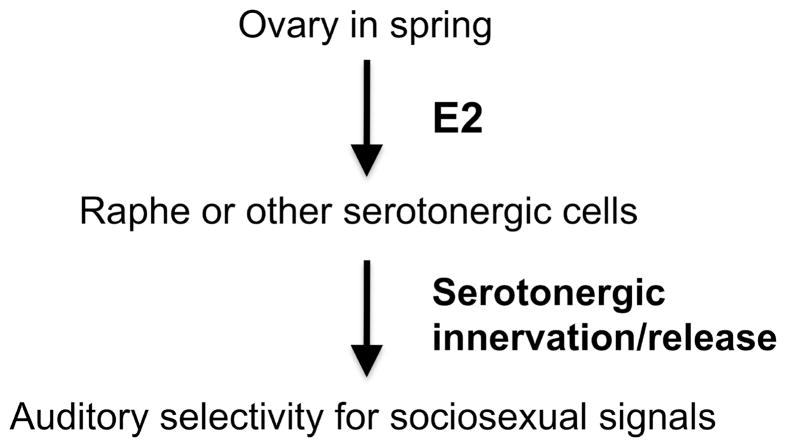
Several independent groups have shown that in finches and sparrows, E2 alters the physiology of central auditory circuits in both males and females by inducing the expression of genes involved in synaptic plasticity (reviewed by Maney & Pinaud, 2011). Application of exogenous E2 directly into the auditory forebrain enhances the responsiveness and coding efficiency of auditory neurons, demonstrating a possible direct effect. Local application does not, however, increase the selectivity of IEG responses in that region (Tremere, Jeong, & Pinaud, 2009). Furthermore, whereas the auditory forebrain expresses both ER-alpha and ER-beta (Bernard, Bentley, Balthazart, Turek, & Ball, 1999), estrogen receptors have not been described in the subpallial auditory structures of songbirds (Gahr, 2001; Gahr, Guttinger, & Kroodsma, 1993; cf. Martinez-Vargas, Stumpf, & Sar, 1976). In order to fully understand E2-dependent selectivity of genomic responses in the auditory system, we need to look for indirect effects--possibly via neuromodulatory systems. In the current study, we have explored a model whereby E2 modulates the processing of conspecific song by acting on the serotonin system (Fig. 1). We have focused on this system because it is widely understood to be estrogen-sensitive (reviewed by McEwen & Alves, 1999) and because it is hypothesized to convey information about both internal state and environmental context to the auditory system, directly affecting the selectivity of auditory responses (reviewed by Hurley, Devilbiss, & Waterhouse, 2004; Hurley & Hall, 2011). In mammals, serotonergic neurons in the raphe nuclei project throughout the auditory system, for example to the auditory midbrain, thalamus, and cortex (e.g., Hurley & Thompson, 2001; Klepper & Herbert, 1991; Steinbusch, 1981). The system is dynamic and highly plastic in that patterns of activity are modulated by behavioral arousal (Hall, Rebec, & Hurley, 2010) and social context (Hurley & Hall, 2011). Serotonin is released in auditory areas during sound presentation (Hall et al., 2010) and increases auditory selectivity for conspecific vocalizations (Hurley & Pollak, 2005). Clearly, this system is in an ideal position to mediate the effects of internal state, in this case endocrine state, on selectivity for sociosexual signals in seasonal breeders.
Fig. 1.
We hypothesize that ovarian steroids associated with breeding act via serotonergic systems in the brain to enhance the processing of auditory sociosexual signals. In Experiment 1 of this study, we tested whether manipulating plasma estradiol (E2) affected the density of serotonergic innervation and serotonin release in auditory areas (top arrow).
The avian serotonergic system has been mapped using a variety of techniques in non-songbirds such as chicken, quail, and pigeons (Challet et al., 1996; Cozzi, Viglietti-Panzica, Aste, & Panzica, 1991; Dube & Parent, 1981; Fuxe & Ljunggren, 1965; Ikeda, Inugai, & Gotoh, 1971; Kaiser & Covey, 1997; Metzger, Toledo, & Braun, 2002; Yamada, Takeuchi, & Sano, 1984). Its anatomical distribution is similar to that of mammals, with cell bodies organized into subdivisions comparable to mammalian cell groups B1-B9 (Dahlström & Fuxe, 1964; Ikeda, Inugai, & Gotoh, 1971; Yamada et al., 1984). The cell bodies are located primarily in the brainstem along the midline, with some groups stretching laterally (Cozzi et al., 1991; Parent, 1981; Yamada et al., 1984). Serotonergic fibers project widely throughout the entire brain, suggesting that like in mammals, this neuromodulator plays important roles in many physiological processes in birds (Cozzi et al., 1991). In the avian species studied so far, serotonergic fibers have been noted in auditory areas (Challet et al., 1996; Kaiser & Covey, 1997; Metzger, Toledo, & Braun, 2002; Zeng et al., 2007). In this study, we aimed to show evidence that in a seasonally breeding songbird, auditory areas are innervated by serotonergic fibers, that the density of this innervation is modulated by plasma E2, and that serotonin is released in the auditory system during the processing of conspecific vocalizations.
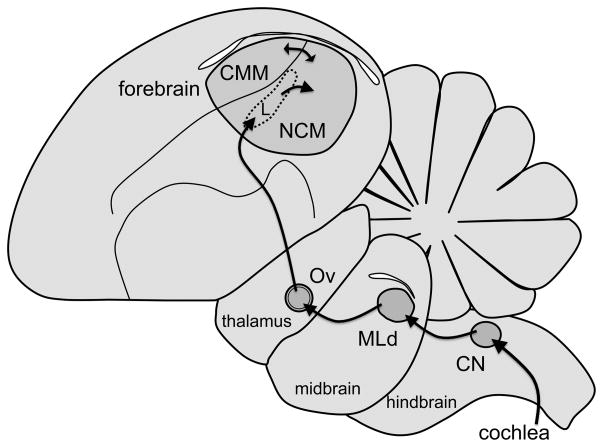
The anatomical and functional organization of central auditory pathways in songbirds largely resembles those found in other vertebrates, including mammals (Fig. 2; reviewed by Maney & Pinaud, 2011). Auditory input is transduced in the cochlea, ascends through brainstem areas analogous to mammalian cochlear nuclei, and arrives at the dorsal lateral mesencephalic nucleus (MLd), the avian homolog of the mammalian inferior colliculus (Karten, 1967). MLd neurons send direct projections to the thalamic nucleus Ovoidalis (Ov), the avian homolog of the ventral medial geniculate (Karten, 1967). Both structures participate in auditory discrimination and are tuned to behaviorally relevant signals (Amin, Gill, & Theunissen, 2010; Woolley, Fremouw, Hsu, & Theunissen, 2005). Ov projects to a pronounced lobe in the forebrain that contains auditory areas (Fig. 2). Inside this lobe, the caudomedial nidopallium (NCM) receives input from the thalamo-recipient Field L and is heavily interconnected with the caudomedial mesopallium (CMM). NCM and CMM are analogous to the supragranular layers of mammalian auditory cortex (Vates, Broome, Mello, & Nottebohm, 1996) or to mammalian auditory association cortex (Pinaud & Terleph, 2008; Tremere et al., 2009) and respond selectively to behaviorally relevant stimuli. For example, the factors that affect behavioral responses to song, such as attractiveness or familiarity, also affect the expression of the IEG ZENK (e.g., Gentner, Hulse, Duffy, & Ball, 2001; Maney, MacDougall-Shackleton, MacDougall-Shackleton, Ball, & Hahn, 2003; Terpstra, Bolhuis, Riebel, Van Der Burg, & Den Boer-Visser, 2006). Changes in the behavioral relevance of a signal are thus expected to be accompanied by changes in the ZENK response to that signal (Maney, Cho, & Goode, 2006).
Fig. 2.
Diagram showing a parasagittal view of the auditory pathway in songbirds. The auditory nerve enters the brainstem and arrives at the cochlear nucleus (CN, also called nucleus magnocellularis) which projects to the auditory midbrain (the dorsal portion of the lateral mesencephalic nucleus, or MLd). MLd projects to the core region of the auditory thalamus, also called nucleus ovoidalis (Ov). The core of Ov is relatively devoid of serotonergic fibers but is surrounded by a serotonin-rich shell region. The Ov core projects to the thalamorecipient region of the auditory forebrain, Field L, which then projects to the caudomedial nidopallium (NCM). NCM is reciprocally connected to the caudomedial mesopallium (CMM). NCM and CMM are thought to be analogous to the supragranular layers of mammalian auditory cortex (Vates et al., 1996) or to mammalian auditory association cortex (Pinaud & Terleph, 2008; Tremere et al. 2009).
We have shown such changes in seasonally breeding sparrows. When plasma E2 reaches breeding levels, which presumably happens when conspecific male song peaks in behavioral relevance (see Maney, 2010), ZENK responses in the auditory forebrain, thalamus, and midbrain become selective for that stimulus over a non-relevant sound (reviewed by Maney & Pinaud, 2011). In the current study, we tested whether this increase in selectivity is accompanied by an E2-mediated change in the serotonergic innervation of auditory areas (Fig. 3). We manipulated plasma E2 in female white-throated sparrows (Zonotrichia albicollis) and measured the subsequent effects on the density of serotonergic fibers and levels of the serotonin metabolite 5-hydroxyindoleacetic acid, 5-HIAA, in auditory areas. If E2 mediates seasonal changes in sensory tuning, then we expected these markers to be higher in females with breeding-typical E2 levels than in untreated, non-breeding females.
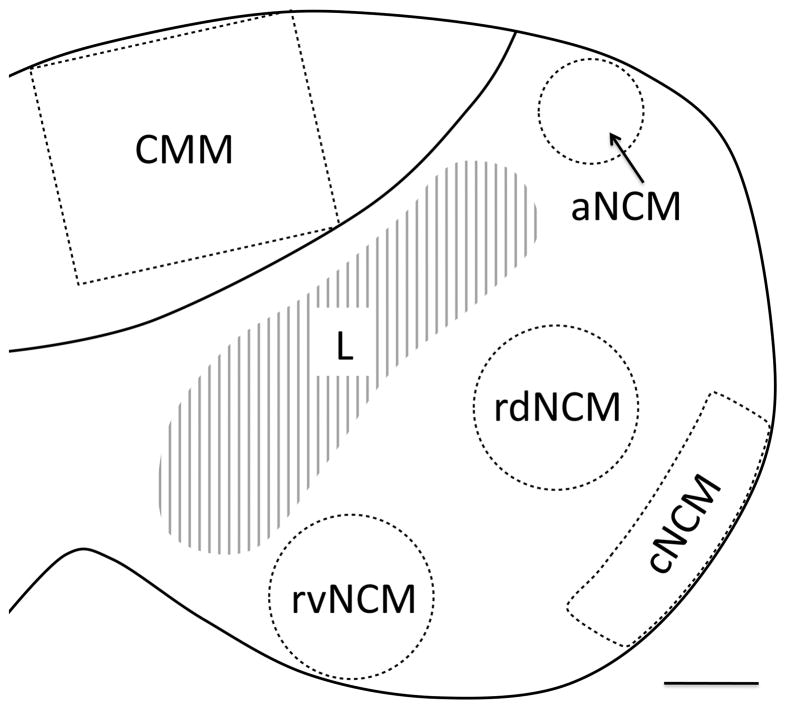
Fig. 3.
Diagram showing a parasagittal view of the auditory forebrain. Dashed lines delineate the areas we sampled when estimating the density of serotonergic innervation. We sampled from within four domains within the caudomedial nidopallium (NCM): apical NCM (aNCM), rostrodorsal NCM (rdNCM), rostroventral NCM (rvNCM), and caudal NCM (cNCM). Rostral is to the left. Scale bar = 300 μm.
In a second experiment, we addressed the function of E2-induced increases in serotonergic innervation of auditory areas. We reasoned that serotonergic input may regulate auditory responses via two independent mechanisms. First, E2 may increase the density of terminals that release serotonin in a nonsynaptic, or tonic fashion, altering the responsiveness or spontaneous firing activity in auditory areas (Beaudet & Descarries, 1978). Such changes, which may be sustained over prolonged periods, may enable auditory areas to respond differently to the same input depending on the season or social context. In this scenario, serotonergic neurons projecting to auditory regions need not themselves respond to song. Second, the serotonergic neurons may themselves fire and release transmitter in a phasic fashion during auditory stimulation (Cransac, Cottet-Emard, Hellstrom, & Peyrin, 1998; Hall et al., 2010), rapidly altering the responsiveness and selectivity of target cells. In order to test whether serotonin plays a priming role only or whether it is released rapidly during auditory stimulation, we performed a song playback experiment. We predicted that if serotonergic cells actively fire in response to conspecific song, exposure to song playback would increase local levels of serotonin metabolite rapidly in auditory areas.
METHODS
Animals
All procedures in this study adhered to NIH standards and were approved by the Emory University Institute for Animal Care and Use Committee. We collected female white-throated sparrows in mist nets in Atlanta, Georgia during fall migration. We determined their sex by polymerase chain reaction (PCR) analysis of DNA extracted from a blood sample (Griffiths, Double, Orr, & Dawson, 1998) and confirmed sex by necropsy at the end of the study. The birds were housed at the Emory University animal care facility in indoor walk-in flight cages and supplied with food and water ad libitum. We held day length constant at 10:14 h light-dark, which corresponds to the shortest day the birds would experience while wintering at the capture site. We kept the birds under these conditions for two months to ensure that they were not photorefractory prior to the start of the study (Shank, 1959; Wolfson, 1958).
Experiment 1: Effects of E2 treatment on serotonergic fiber density and 5-HIAA
Hormonal Manipulation
We transferred the birds in pairs to small, acoustically isolated rooms where they were housed one per cage (38 × 38 × 42 cm) in two adjacent cages. On the day of transfer, we implanted each bird with a subcutaneous silastic capsule (length 12 mm, ID 1.47 mm, OD 1.96 mm, Dow Corning, Midland, MI) sealed at both ends with A-100S Type A medical adhesive (Factor 2, Lakeside, AZ). One bird in each pair (n = 8) received an empty implant and the other (n = 8) received an implant containing 17β-estradiol (Steraloids, Newport, RI). This dose of E2 elevates plasma levels to those typical of the breeding season within seven days in this species (Maney et al., 2006, 2008) and likely does so within two days (Moore, 1983). After seven days of E2 treatment, which induces selectivity of sound-induced ZENK responses (Sanford et al., 2010) as well as enhanced catecholaminergic innervation of auditory areas (Matragrano, Sanford, Salvante, Sockman, & Maney, 2011), we rapidly decapitated the birds, quickly harvested the brains, and bisected each brain into two hemispheres. We fixed one hemisphere in 5% acrolein as previously described (Maney et al., 2003, 2005) and flash-froze the other in powdered dry ice for high performance liquid chromatography (HPLC) analysis. The hemisphere that was fixed (right or left) was balanced across treatments.
Immunohistochemistry
We cut the fixed hemispheres into 50 μm parasagittal sections using a freezing sliding microtome. We then used a standard immunohistochemistry (IHC) protocol (LeBlanc, Goode, MacDougall-Shackleton, & Maney, 2007; Maney et al., 2006; Matragrano et al., 2011) to immunolabel serotonin transporter (SERT). SERT is less liable to metabolism than serotonin; therefore immunolabeling with an anti-SERT antibody is a more stable marker of serotonergic fibers and has been shown to be a better indicator of serotonin axons than an anti-serotonin antibody (Nielsen, Brask, Knudsen, & Aznar, 2006). For this study, we used every third section; the first two series of sections were used for a separate study (Matragrano et al., 2011; see Discussion). Sections were incubated with an anti-SERT antibody (ImmunoStar; Hudson, WI; see below) diluted 1:5000 (Meyer, Grande, Johnson, & Ali, 2004), followed by a biotinylated antibody against anti-rabbit IgG (Vector, Burlingame, CA) diluted 1:250. We visualized the label via the ABC method (Vector) followed by diaminobenzidine. We processed all of the brain sections in a single run of IHC. Following IHC, we mounted all of the sections onto microscope slides, dehydrated them, and coverslipped in DPX (Sigma, St. Louis, MO). In order to better our understanding of the distribution of SERT-immunoreactive (ir) fibers, which has not been described in birds, we also processed coronal brain sections from several additional birds not otherwise used in this study.
The anti-SERT antibody was a rabbit polyclonal antibody generated against a synthetic peptide sequence corresponding to amino acids (602–622) of rat SERT coupled to keyhole limpet hemocyanin (ImmunoStar Cat#24330). We validated the specificity of the SERT antibody in brain sections of a white-throated sparrow not in the current study, following the procedure described by Saper and Sawchenko (2003). All labeling at antibody concentrations of 1:25,000 was eliminated by preadsorption of the antibody with 50 μg/ml of the SERT peptide (ImmunoStar Cat#24332). Omission of the primary or the secondary antibodies resulted in a complete loss of specific staining.
Regions of Interest
Our primary goals in this study were to test the effects of E2 on SERT-ir innervation of auditory areas and to determine the extent to which those effects overlap anatomically with previously described effects on the ZENK response (Sanford et al., 2010) and catecholaminergic innervation (Matragrano et al., 2011). In order to compare the anatomical distributions of the effects of E2 on SERT-ir innervation and the previously described effects, we needed to partition NCM into the same domains as in our previous studies. In the past, we have divided the region both rostro-caudally and dorso-ventrally into four primary domains (Sanford et al., 2010): rostro-dorsal (rdNCM), rostro-ventral (rvNCM), caudo-dorsal (cdNCM) and caudo-ventral (cvNCM). Because the dorsal and ventral domains of cNCM are similar hodologically and neurochemically, and because E2 does not affect ZENK expression in either domain (Sanford et al., 2010), we combined them into one caudal domain. As in our previous work, we also sampled from an apical domain (aNCM) located dorsal to Field L2 (Fig. 3). In the published literature, this region is usually considered part of NCM but may overlap the dorsal portion of Field L (Fortune & Margoliash, 1992).
We conducted all image acquisition and analyses while blind to treatment group. Regions of interest were identified with reference to Stokes et al. (1974) and Vates et al. (1996). To acquire the images we used the 10x objective on a Zeiss Axioskop microscope attached to a Leica DFC480 camera and Macintosh G5 computer. We captured rectangular images (approximately 46 MB in size) corresponding to the field of view of the camera (870 × 690 μm), holding the light level and exposure time constant for all photos. For each bird, we acquired images of NCM and CMM in four consecutive sections between ~350 and ~800 μm from the midline. Five separate images, each containing either CMM, aNCM, rdNCM, rvNCM, or cNCM, were acquired from each of the four sections. With the exception of CMM, for which the entire photo was used, all regions of interest were selected in the photos using ImageJ (version 1.41o, National Institutes of Health, Bethesda, MD) as previously described (Matragrano et al., 2011; Sanford et al., 2010; see Fig. 3).
To acquire images of CMM, the upper corners of the field of view of the camera were positioned along the dorsal boundary of CMM and one of the lower corners was positioned adjacent to the lamina mesopallium (Fig. 3). We defined cNCM as a strip of tissue approximately 275 μm from the caudal boundary of NCM (Sanford et al., 2010; Matragrano et al., 2011). The acquired images of cNCM captured the majority of that domain, spanning 870 μm from dorsal to ventral (Fig. 3). For aNCM, we placed a circle approximately 350 μm in diameter dorsal to Field L and just ventral to the ventricle. For rdNCM and rvNCM, we placed two circles, each approximately 550 μm in diameter, into the dorsal and ventral portions of this region (Matragrano et al., 2011; Sanford et al, 2010) so that they did not overlap with Field L or with the region we defined as cNCM. Because we did not conduct tract tracing or another method that would enable us to discern absolutely the boundary between the rostral domains of NCM and the adjacent subregions of Field L (Vates et al., 1996), it is possible that our samples of aNCM, rdNCM and rvNCM may have captured a bit of L1 and L3, respectively. We are confident in any case that the rostral regions we sampled correspond exactly to those exhibiting E2-dependent selectivity as described by Sanford et al. (2010). All five photos of each section of auditory forebrain were viewed at the same time to ensure that the regions of interest we selected in each section did not overlap.
In addition to the auditory forebrain, we estimated the density of SERT-ir fibers in the auditory midbrain (MLd) and thalamus (Ov). Five sections of MLd, spanning 600 μm, were chosen that encompassed the largest portion of this nucleus (Maney et al., 2006; Matragrano et al., 2011). We traced the outline of MLd in each acquired photograph using the ImageJ freehand tool. Ov was visible in at least three sections of each brain. We photographed the three consecutive sections in which Ov was the largest. Because the core region of Ov did not contain SERT-ir fibers (see also Belekhova et al., 2002; Kaiser & Covey, 1997); we included only the shell region in our analysis (Zeng, Li, Zhang, & Zuo, 2007). The Ov shell was traced with the ImageJ freehand tool. Finally, we estimated the density of fibers in a non-auditory region, the apical part of the hyperpallium (HA; see Stokes et al., 1974; Reiner et al., 2004), to test the specificity of the effects of E2 on auditory regions. We photographed HA in the same three brain sections we used for the Ov shell. Like CMM, the entire photo of HA was analyzed for fiber density.
Image acquisition and estimation of SERT-immunoreactive fiber density
We converted all the photos to 8-bit scale and selected SERT-ir fibers using the thresholding feature in ImageJ (Maney et al., 2005; Matragrano et al., 2011). Our method of selecting immunolabeled fibers has been fully validated and has high interrater reliability and low variability. Briefly, the thresholds selected by two independent observers were highly correlated (R = 0.98), not different from each other (P = 0.976; d = 0.0009), and were within one unit of each other in 30% of cases (Matragrano et al., 2011). In the present study, the same observer set the threshold for all images with the same lighting and computer monitor (LLM). We performed this procedure in 3–5 images per region (see above). For each region, we summed the total area covered by fibers and divided this sum by the total area measured to yield the area covered by the fibers in square microns per square mm of area measured.
Quantification of the serotonin metabolite and total protein
We measured the concentration of serotonin metabolite in the auditory pathway via HPLC. Because the number of brain regions we could sample was limited, we decided to sample regions in which we had previously shown E2-dependent selectivity of the ZENK response: NCM, CMM, and MLd (Maney et al., 2006). We collected NCM, CMM and MLd with micropunch tools (i.d.: 1.5, 1.0 and 1.0 mm, respectively) in sagittal sections (300 μm) from the frozen, non-fixed hemispheres. All of the sectioning and sampling was done in one day by the same person using the same punch tool for each region. The regions of interest were located by referring to Sockman, Gentner & Ball (2002) as well as two sets of Nissl-stained brain sections from white-throated sparrow. For NCM and CMM, we used Field L as a landmark. For MLd we used the tectal ventricle, which appears as a dark line in the fresh frozen tissue. We determined the concentration of 5-HIAA by reversed-phase HPLC with electrochemical detection (Kilts, Breese, & Mailman, 1981). This method has been precisely described elsewhere (see Sockman & Salvante, 2008). We also measured the protein content of each sample by dissolving the remaining sample pellet in 0.2 N NaOH (100 μl) and performing the Bradford protein-dye binding assay (Quick Start Bradford Protein Assay, Bio-Rad) with bovine serum albumin as a standard (Bio-Rad) on a μQuant microplate spectrophotometer (BioTek) (Bradford, 1976). Concentrations of 5-HIAA were normalized by dividing 5-HIAA levels by protein content of each sample.
Statistical Analysis
For each dependent variable, we plotted histograms and performed Shapiro-Wilk tests (SPSS) to determine whether the distribution of the data was normal. In the event of significant deviation (P < 0.05), we performed a square-root transformation to normalize the distribution. The transformation was necessary and sufficient to normalize the distributions for 3 of the 11 variables: 5-HIAA in NCM and MLd, and SERT-immunoreactivity in MLd. The other 8 variables did not require transformation. In all cases, visual inspection of the histogram confirmed the necessity of transformation and its effectiveness.
We looked for effects of treatment on 5-HIAA concentration and the estimated density of SERT-ir fibers using a mixed-effects linear model (Stata), which uses restricted maximum likelihood to estimate parameters. For each of the 11 dependent variables (concentration of 5-HIAA in CMM, NCM, and MLd and estimated density of SERT-immunoreactivity in CMM, aNCM, rdNCM, rvNCM, cNCM, Ov, MLd, and HA), hormone treatment was a predictor and bird was nested as both a random intercept and random coefficient on treatment (Schiezelth & Forstmeier, 2009). Effect sizes were estimated using Cohen’s d on untransformed data.
Experiment Two: Rapid effects of hearing song on serotonin and 5-HIAA in auditory regions
In order to determine whether the serotonin system is rapidly engaged during sound presentation, we conducted a playback study. We treated 12 female white-throated sparrows, collected and housed as for Experiment 1, with E2 as described above and presented recordings of male song seven days later. The sound stimuli and playback protocol have been published previously (Maney et al., 2006, 2007; 2009; Sanford et al., 2010). On the afternoon prior to sound presentation, we isolated each female in a sound attenuation booth equipped with a video camera, microphone and speaker. The following morning approximately 1 h after lights-on, we played male white-throated sparrow songs at 70 dB via the speaker. Each female heard either 30 min of song (n = 4), 15 min of song (n = 4), or no song (n = 4). The stimulus presentations contained songs from a variety of males (five males in the 15 min presentations and ten males in the 30 min presentations), with the identity of the singer changing to a new male every three minutes in order to prevent habituation to the stimulus (Maney et al., 2006). Each female heard the five or ten males in a unique order. We made audio and video recordings of all birds during the stimulus presentation (the birds hearing no song were recorded for 30 min).
We rapidly decapitated the birds and harvested the brains immediately following playback, with time of day balanced across stimulus treatments. As in Experiment 1, we carefully bisected each brain into hemispheres, flash froze them in powdered dry ice, and performed HPLC analysis using one hemisphere from each brain (left or right balanced across stimulus treatments). 5-HIAA was assayed in CMM, NCM, and MLd as described for Experiment 1. At the time of Experiment 2, we had revised our method and used punch tools of smaller diameter (0.5 mm in diameter for CMM and MLd, and 1.0 mm in diameter for NCM) in order to more accurately sample the regions of interest. We had also developed a method to detect serotonin in micropunches; we therefore assayed serotonin in these samples as well. We used the behavioral recordings to quantify the number of vocalizations during the period 30 min prior to sacrifice. We counted the number of trills, which are associated with copulation solicitation displays, as well as chip-ups, pinks, seets, and songs (see Maney et al., 2009 for descriptions of these vocalizations).
Statistical Analysis
In some cases, we had difficulty getting reliable protein measurements from the smaller punches. As a result, we had a complete set of protein measurements only for NCM; for CMM and MLd we were missing the protein measurement for at least one sample. We therefore normalized for protein content only for NCM. The data from CMM and MLd were not normalized. Since 5-HIAA concentrations were compared only between treatment groups and not regions of interest, this discrepancy did not pose a problem for the data analysis.
To analyze the effects of song duration on the concentrations 5-HIAA and serotonin, we used a separate general linear model (Stata) for each compound and each brain region. For each model, the response variable was the mean concentration from the two punches for that region (in pg/mg protein for NCM and pg for CMM and MLd; see Methods) and the predictor song duration was expanded into a dummy-variable set to model the contrast between 0 and 15 minutes and the independent contrast between 0 and 30 minutes.
In order to rule out the birds’ own vocalizations as a factor influencing 5-HIAA and serotonin concentrations in CMM, NCM, and MLd, we ran Spearman correlation tests to look for relationships between the number of vocalizations (total number as well as the number of each type) and the level of each compound for each region.
RESULTS
Experiment 1: Effects of E2 treatment on serotonergic fiber density and 5-HIAA
Distribution of SERT-ir Fibers
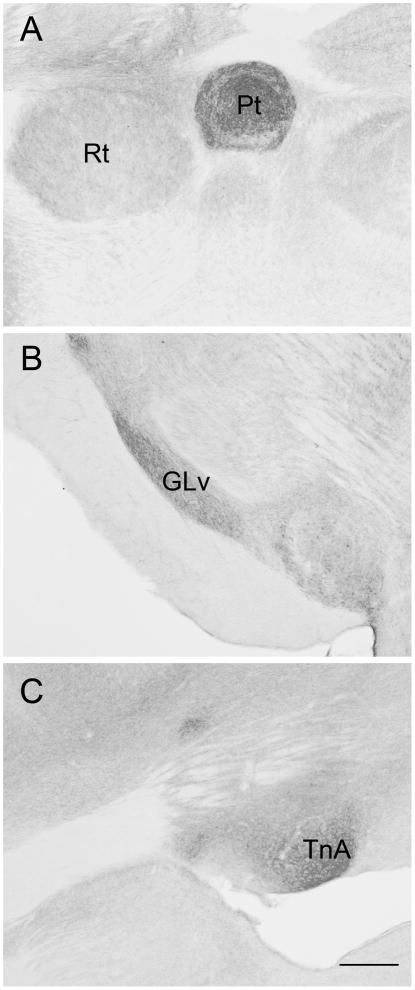
The distribution of serotonergic fibers was similar to what has been described in chickens, Japanese quail, and pigeons (Challet et al., 1996; Cozzi, Viglietti-Panzica, Aste, & Panzica, 1991; Kaiser & Covey, 1997; Metzger, Toledo, & Braun, 2002; Zeng et al., 2007). SERT-ir fibers were densely distributed throughout the entire brain. We noted the highest densities in the striatum, diencephalon, and midbrain. The most intensely labeled structures in the brain were the pretectal nucleus, the lateral geniculate nucleus, and the nucleus taeniae of the amygdala (Fig. 4). Basket-like immunoreactive structures appeared to surround neuronal somata in the ventral tegmental area, substantia nigra, and locus coeruleus. We noted high densities of fibers in the medial septum, lateral bed nucleus, hippocampus, ventromedial hypothalamus, habenula, and periaqueductal gray. The song control nucleus HVC (proper name) stood out as unlabeled compared to the surrounding tissue. Many SERT-ir fibers appeared to contact the intraventricular space, particularly in the lateral ventricles and the cerebral aqueduct. The median eminence also contained labeled fibers, suggesting secretion into the portal vasculature.
Fig. 4.
Immunoreactivity for serotonin transporter was seen throughout the brain, most notably in the pretectal nucleus (Pt; A), the lateral geniculate nucleus (GLv; B), and the nucleus taeniae of the amygdala (TnA; C). In general, the labeling agreed with the previously reported distribution of serotonin immunoreactivity in birds (see text). Rt, nucleus rotundus. Rostral is to the left. Scale bar = 300 μm.
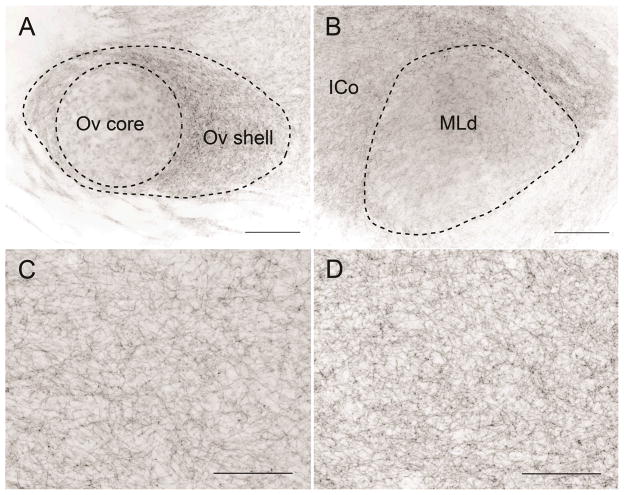
With the exception of Ov, within which only the shell region contained appreciable labeled fibers (see also Belekhova et al., 2002; Kaiser & Covey, 1997; Zeng et al., 2007), the auditory structures we focused on for this study were densely innervated (Fig. 5). The distribution of SERT-ir fibers was relatively homogenous across CMM and NCM, with clusters of fibers appearing to surround unlabeled cell bodies in some individuals. Whereas Zeng et al. (2007) reported that MLd in Bengalese finches is nearly devoid of serotonergic fibers, we found that although it was less densely labeled than the surrounding nucleus intercollicularis, it was clearly innervated by SERT-ir fibers (Fig. 5B). We did not detect convincing labeling in nucleus magnocellularis or nucleus laminaris, which are avian homologues of the cochlear nuclei and the medial superior olive, respectively, in mammals (Boord & Rasmussen, 1963).
Fig. 5.
Fibers immunoreactive for serotonin transporter in the auditory thalamus (Ov; A), the auditory midbrain (MLd; B), the rostrodorsal caudomedial nidopallium (rdNCM; C), and the apical part of the hyperpallium (HA; D), a visual area, in a female white-throated sparrow. All of the images are from the same female, which had a blank implant (no estradiol). Note that immunolabeling in Ov is limited to the shell region; the core region is relatively devoid of fibers (see also Kaiser & Covey, 1997; Zeng et al., 2007). ICo, nucleus intercollicularis. Rostral is to the left. Scale bar = 150 μm.
Effects of E2 on serotonergic fiber density and 5-HIAA
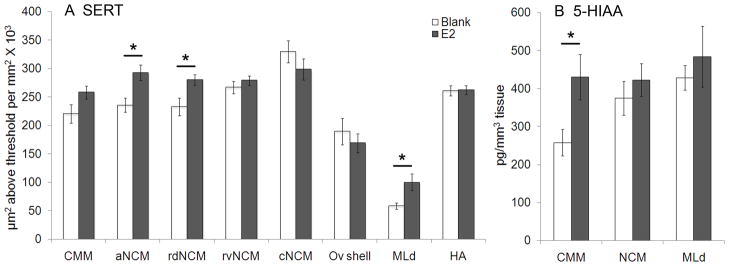
The effects of E2 treatment on SERT fiber density are shown in Fig. 6A. E2 significantly increased the density of SERT-ir fibers in aNCM (z = 2.95, P = 0.003, d = 1.546), rdNCM (z = 2.14, P = 0.032, d = 1.186), and MLd (z= 4.51, P < 0.001, d = 1.325). The effect of E2 treatment in CMM was not significant, but there was a compelling trend (z = 1.83, P = 0.067, d = 0.946). We did not detect an effect of E2 on the density of SERT-ir fibers in rvNCM (z = 1.02, P = 0.306, d = 0.449), cNCM (z = −1.43, P = 0.156, d = 0.583), the shell of Ov (z= − 0.97, P = 0.333, d = 0.351), or the non-auditory region HA (z = 0.07, P = 0.942, d = 0.038).
Fig. 6.
The effects of estradiol (E2) on the density of fibers immunoreactive for serotonin transporter (SERT; A) and the serotonin metabolite 5-hydroxyindoleacetic acid (5-HIAA; B). E2 treatment increased the density of fibers immunoreactive for SERT in two domains of the caudomedial nidopallium (NCM), aNCM and rdNCM, and the auditory midbrain (MLd; A) and increased the concentration of 5-HIAA in the caudomedial mesopallium (CMM; B). aNCM, apical NCM. cNCM, caudal NCM. rdNCM, rostrodorsal NCM. rvNCM, rostroventral NCM. Ov, n. Ovoidalis. HA, apical part of the hyperpallium. * P < 0.05 compared to blank (placebo) condition. See text for P values.
The effects of hormone treatment on the concentration of the serotonergic metabolite 5-HIAA in our regions of interest are shown in Fig. 6B. E2 treatment significantly increased concentrations of 5-HIAA in CMM (z = 2.86; P = 0.004; d = 1.241). We did not detect an effect of treatment in NCM (z = 1.07; P = 0.284; d = 0.383) or MLd (z = 0.43; P = 0.669; d = 0.321).
Experiment 2: Rapid effects of hearing song on serotonin and 5-HIAA in auditory regions
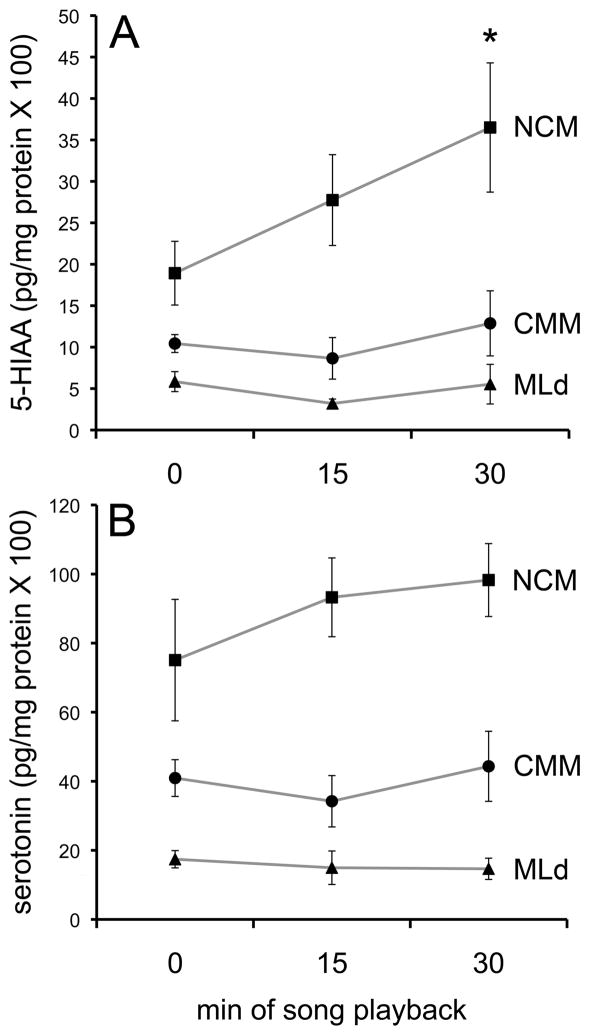
The serotonin metabolite 5-HIAA increased in NCM during song playback (Fig. 7). After 15 min of song presentation, 5-HIAA levels were not significantly higher than at 0 min, but after 30 min the metabolite had increased significantly (z = 2.25, P = 0.024). Hearing song did not increase 5-HIAA in CMM or MLd, nor did it affect serotonin concentrations in any of the regions of interest. We did not detect any significant relationships between serotonin or 5-HIAA and either the total number of vocalizations or the number of each type, indicating that the increase in 5-HIAA in NCM was unlikely to be driven by self-stimulation.
Fig. 7.
5-HIAA (A) and serotonin (B) concentrations in CMM, NCM, and the auditory midbrain, MLd after exposure to 15 or 30 min of conspecific song. Hearing 30 min of song significantly increased levels of 5-HIAA in NCM (A). * significantly different from levels at time 0, P = 0.024. Total protein content was available for all samples only for NCM (see Methods); for consistency in the graphs, plotted values for CMM and MLd have been normalized using the average protein values for those regions.
DISCUSSION
The serotonergic system is a prime candidate for mediating the effects of internal state and behavioral context on auditory processing in vertebrates (reviewed by Hurley & Hall, 2011). In this study, we have explored a model wherein E2 regulates both tonic and phasic release of serotonin in auditory areas. In Experiment 1, we showed evidence that E2, which varies in plasma according to reproductive state and alters IEG responses in auditory areas, also affects serotonin markers in the same areas independently of sound stimulation. First, E2 treatment of nonbreeding female white-throated sparrows enhanced the density of SERT-ir innervation of the auditory midbrain as well as the auditory forebrain (Fig. 6A). Because these fibers may release serotonin tonically, an increase in their density suggests a mechanism by which E2 might increase local levels of serotonin, thereby altering the firing properties of local auditory neurons. Second, we found an E2-induced increase in the concentration of the serotonin metabolite 5-HIAA (Fig. 6B), which indicates enhanced synthesis or release of serotonin (Commissiong, 1985; Houdouin, Cespuglio, Gharib, Sarda, & Jouvet, 1991). The females in Experiment 1 were isolated from males and male song during the course of hormone treatment, so any increase in serotonergic innervation or release occurred independently of sociosexual stimuli. In Experiment 2, we tested whether hearing song induces phasic serotonergic responses in auditory areas. Exposure to conspecific male song increased the concentration of 5-HIAA in NCM within minutes (Fig. 7) suggesting either an increase in intracellular serotonin turnover or actual release in response to hearing song. When considered together, the results of Experiments 1 and 2 are thus consistent with a model wherein tonic release of serotonin is enhanced by E2 and phasic release may be triggered by behaviorally relevant sound.
The effects of E2 on serotonergic activity may depend on the region. For example, we show here that E2 treatment enhanced serotonergic innervation of MLd and NCM but not CMM. In contrast, E2 treatment significantly increased levels of 5-HIAA in CMM but not the other regions. The hormone treatment may therefore have very different functional consequences depending on the region. Note however that the evidence of such differences is not particularly strong in this study. For example, although we did not find a significant effect of E2 on SERT-ir in CMM, there was a strong trend in that direction (P = 0.067). Similarly, the trends for 5-HIAA were in the same direction for all regions (Fig. 5B). Thus, although it is tempting to speculate that E2 may have site-specific effects on serotonergic activity in the auditory pathway, a more detailed analysis will be necessary to draw those contrasts with confidence.
A major goal of the current study was to map the effects of E2 on serotonergic innervation of the auditory system and to determine the extent to which those effects overlap anatomically with the effects of E2 on the ZENK response. In a previous series of studies, E2 induced ZENK expression and increased the selectivity of the ZENK response in the auditory midbrain (Maney et al., 2006) and the rostral domains of the auditory forebrain (CMM, aNCM, rdNCM, and rvNCM; Maney et al., 2006; Sanford et al., 2010). In contrast, we have consistently failed to detect an effect of E2 on the ZENK response in the caudomedial area of NCM (cNCM; Maney & Pinaud, 2011; Sanford et al., 2010). In the latter region, the ZENK response was selective for song over synthetic tones no matter the endocrine state of the listener. In the present study, the anatomical distribution of E2 effects on serotonergic innervation overlapped exactly with previously demonstrated effects on the ZENK response. E2 treatment enhanced serotonergic fiber density in the auditory midbrain and in the rostral but not the caudal areas of the auditory forebrain. The anatomical match between the effects of E2 on the ZENK response and serotonergic fiber density thus supports our hypothesis that E2-dependent plasticity in the ZENK response may be mediated by the serotonin system.
E2-dependent plasticity in the auditory system may involve not only serotonin, but also catecholamines. In a separately published study using material from the same animals, we reported that E2-treatment of nonbreeding female white-throated sparrows increased the density of fibers immunopositive for tyrosine hydroxylase or dopamine beta-hydroxylase in the auditory midbrain as well as part of the rostral but not the caudal auditory forebrain (Matragrano et al., 2011). Thus, the E2-induced changes in catecholaminergic innervation that we previously reported map closely onto the changes in serotonergic innervation that we report here. The effects of E2 on catecholaminergic and serotonergic innervation were not identical however, particularly with regard to their distribution within rostral NCM. Whereas E2 treatment affected the density of catecholaminergic fibers similarly in the dorsal compared with the ventral domain (rdNCM and rvNCM; Fig. 3), SERT-ir fibers increased in the dorsal domain only (Fig. 6A), suggesting the possibility of a functional distinction between the two domains.
The practice of dividing NCM into dorsal and ventral domains was introduced by Gentner et al. (2001) and the majority of published studies have since followed this convention (e.g., Avey, Kanyo, Irwin, & Sturdy, 2008; Eda-Fujiwara, Satoh, Bolhuis, & Kimura, 2003; Hernandez and MacDougall-Shackleton, 2004; Lynch and Ball, 2008; Maney et al., 2003; Sockman and Salvante, 2008; Sockman, Gentner, & Ball, 2005; Velho and Mello, 2008). Pinaud, Fortes, Lovell, & Mello (2006) argued that based on connectivity, electrophysiological responses, and neurochemical markers, NCM may be better divided into rostral and caudal domains. Sanford et al. (2010) demonstrated that whereas ZENK responses in the rostral domains are both induced and made more selective by E2 treatment, ZENK responses in the caudal domain are unaffected by E2. Matragrano et al. (2011), after finding similar effects of E2 treatment on catecholaminergic innervation of rdNCM and rvNCM, combined them into one domain (rNCM). In the current study, we detected a strong effect of E2 treatment in rdNCM but not rvNCM and, given that other researchers have reported that dorsal and ventral NCM differ in their sensitivity to and selectivity for a variety of experimental stimuli (Avey et al., 2008; Eda-Fujiwara et al., 2003; Gentner et al., 2001; Maney et al., 2003; Phillmore, Bloomfield, & Weisman, 2003; Sockman & Salvante, 2008), we could not justify combining them. Perhaps our current findings will ultimately contribute toward a more detailed understanding of the functional neuroanatomy of this large, heterogeneous region.
The literature on estrogen-dependent regulation of the mammalian serotonin system is vast and reflects a predominantly positive effect of E2 on serotonin markers in the dorsal and median raphe. E2 treatment of ovariectomized rats, for example, increased firing rate (Robichaud & Debonnel, 2005), serotonin content (Cone, Davis, & Goy, 1981), and the expression of SERT and tryptophan hydroxylase-2 mRNA (Bethea et al., 2000; Donner & Handa, 2009; Hiroi, McDevitt, & Neumaier, 2006). These effects may be direct, as many dorsal raphe neurons contain estrogen receptors (Gundlah, Lu, Mirkes, & Bethea, 2001; Lu, Ozawa, Nishi, & Kawata, 2001; Lu et al., 1999; Sheng et al., 2004). The reported effects of E2 treatment on the projections of these neurons, however, vary widely and appear to be highly region- and species-specific. For example, whereas ovariectomy decreased the concentration of serotonin in the prefrontal cortex of rats (Inagaki, Gautreaux, & Luine, 2010), it increased the density of serotonergic fibers in the same region of rhesus macaques (Kritzer & Kohama, 1999). In mountain spiny lizards, ovariectomy reduced and E2 treatment restored 5-HIAA concentrations in the lateral septum, but other regions were not affected (Woodley, Matt, & Moore, 2000). In ovariectomized rats, E2 treatment decreased serotonergic fiber density, serotonin, and 5-HIAA in the ventromedial hypothalamus and medial preoptic area but not the lateral septum (Lu, Yuri, Ito, Yoshimoto, & Kawata, 1998) and increased serotonin turnover in the hippocampus and nucleus accumbens whereas many other regions were not affected (Pandaranandaka, Poonyachoti, & Kalandakanond-Thongsong, 2009). The expression of SERT, as measured by the binding of [3H]-paroxetine, increased in the lateral septum, basolateral amygdala, and ventromedial hypothalamus but decreased in the periaqueductal gray (McQueen, Wilson, & Fink, 1997; see also Bertrand et al., 2005). In ovariectomized rhesus macaques, E2 treatment increased the density of SERT-ir fibers in the zona incerta but not the lateral tuberal nuclei (Lu, Eshleman, Janowsky, & Bethea, 2003). McQueen et al. noted that in rats, each of the regions in which SERT is upregulated by E2 contains high levels of estrogen receptor, and suggested that local effects on posttranslational processing, rather than effects in the dorsal raphe, may contribute toward E2-evoked changes in reuptake. Clearly, the effects of E2 manipulation on serotonergic innervation cannot be predicted by a simple rule for all brain areas in all species. Just as the receptors are remarkably diverse, serotonin release and reuptake appear to be regulated by heterogeneous mechanisms that depend on the local environment.
We interpret the increases in the density of SERT-ir fibers as an increase in the density of serotonergic axons. In other words, we are interested primarily in a hormone-dependent structural change. The well-known trophic effects of E2, for example on axonal outgrowth in culture and in vivo (reviewed by de Lacalle, 2006) are observable without IHC by using tract tracing or Golgi impregnation (e.g. Vanderhorst, Terasawa, & Ralston, 2002). In this study, however, because we labeled a marker that can be regulated independently of structural plasticity, we must consider alternative explanations. It is possible that E2 treatment altered the abundance of SERT protein, affecting our ability to detect immunolabeled fibers. Regardless of whether the increase in SERT-ir fiber density reflects structural plasticity, it likely indicates an increase in serotonin reuptake activity. Studies using in vitro and cell culture preparations have demonstrated that E2 inhibits SERT activity (Chang & Chang, 1999; Koldzic-Zivanovic, Seitz, Watson, Cunningham, & Thomas, 2004), which would lead to serotonin accumulation in the synapse and compensatory upregulation of SERT (see also Charoenphandhu, Teerapornpuntakit, Nuntapornsak, Krishnamra, & Charoenphandhu, 2011). As noted above, E2 increases serotonin synthesis (Bethea et al., 2000; Donner & Handa, 2009; Hiroi et al., 2006), which could also result in compensatory upregulation of SERT (see also Attali, Weizman, Gil-Ad, & Rehavi, 1997). Thus, the increase in SERT-immunoreactivity that we observed in auditory structures could have multiple explanations and implications. We hope eventually to confirm and extend our findings using additional markers such as immunoreactivity for serotonin itself.
In Experiment 2, we showed that hearing conspecific male song increases the concentration of the serotonin metabolite 5-HIAA in NCM, suggesting release of serotonin (Commissiong, 1985; Houdouin et al., 1991). Sound-induced release has been demonstrated in several species of rodents (Cransac et al., 1998; Hall et al., 2010; Stark & Scheich, 1997); presentation of broadband noise increases serotonin concentrations in the inferior colliculus within 5 min (Hall et al., 2010) and 5-HIAA concentrations in the cochlear nuclei and the auditory cortex within 45 min (Cransac et al., 1998). Sound-induced serotonin transmission in auditory areas may modulate auditory responses by conveying information about internal state of the animal, in that it is modulated by stress (Stark & Scheich, 1997) and possibly by other internal and external factors (Hall et al., 2010; Hurley & Hall, 2011). Because the serotonin system is already widely understood to be affected by endocrine state, we hypothesize that seasonal changes in plasma E2 may underlie plasticity in auditory responses to the conspecific signals that are important for breeding. In this study, since all of the animals in Experiment 2 were treated with E2, we do not know whether the sound-induced increase in 5-HIAA we observed in NCM depends on breeding-typical plasma levels. Because we played only song, neither do we know whether sound-induced serotonin release in NCM depends on the type or behavioral relevance of the stimulus. Future experiments will explore the selectivity of sound-induced serotonin release and how it may be modulated by endocrine state.
In this study, we have shown that an increase from nonbreeding to breeding-typical levels of plasma E2 enhances serotonergic innervation of auditory centers. Our results thus support a model wherein seasonal increases in plasma E2 may induce changes in auditory tuning such that selectivity for courtship signals is increased (Fig. 1). This study has addressed only the first step in this model, however, since we do not know the physiological effects of serotonin on auditory responses in songbirds. When iontophoretically applied to areas of the auditory pathway in mammals, serotonin inhibits or enhances sound-evoked activity in a pattern probably related to the distribution of receptor subtypes (e.g. Ebert & Ostwald, 1992; Hurley & Pollak, 1999; Ji & Suga, 2007). Serotonin-induced changes in the responses to species-specific vocalizations have been demonstrated in the inferior colliculus of bats (e.g., Hurley & Pollak, 2005) but not other vertebrates. Future studies should investigate whether increased serotonergic activity in the auditory pathway alters auditory tuning in songbirds.
Acknowledgments
We thank Allison Reid, Sarah Green, Brent Horton, David Lee, Danielle Racke, and Said Saab for technical assistance and Dr. Richard B. Mailman and Stan B. Southerland for providing and helping with the HPLC system. This research was supported by NINDS R01 NS055125 to KWS, NSF IBN-0346984 to DLM, and the Center for Behavioral Neuroscience IBN-9876754.
Footnotes
Publisher's Disclaimer: The following manuscript is the final accepted manuscript. It has not been subjected to the final copyediting, fact-checking, and proofreading required for formal publication. It is not the definitive, publisher-authenticated version. The American Psychological Association and its Council of Editors disclaim any responsibility or liabilities for errors or omissions of this manuscript version, any version derived from this manuscript by NIH, or other third parties. The published version is available at www.apa.org/pubs/journals/bne
LITERATURE CITED
- Amin N, Gill P, Theunissen FE. Role of the zebra finch auditory thalamus in generating complex representations for natural sounds. Journal of Neurophysiology. 2010;104:784–798. doi: 10.1152/jn.00128.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Attali G, Weizman A, Gil-Ad I, Rehavi M. Opposite modulatory effects of ovarian hormones on rat brain dopamine and serotonin transporters. Brain Research. 1997;756:153–159. doi: 10.1016/s0006-8993(97)00136-4. [DOI] [PubMed] [Google Scholar]
- Avey MT, Kanyo RA, Irwin EL, Sturdy CB. Differential effects of vocalization type, singer and listener on ZENK immediate early gene response in black-capped chickadees (Poecile atricapillus) Behavioral Brain Research. 2008;188:201–208. doi: 10.1016/j.bbr.2007.10.034. [DOI] [PubMed] [Google Scholar]
- Barni T, Maggi M, Fantoni G, Granchi S, Mancina R, Gulisano M, Marra F, Macorsini E, Luconi M, Rotella C, Serio M, Balboni GC, Vannelli GB. Sex steroids and odorants modulate gonadotropin-releasing hormone secretion in primary cultures of human olfactory cells. Journal of Clinical Endocrinology & Metabolism. 1999;84:4266–4273. doi: 10.1210/jcem.84.11.6150. [DOI] [PubMed] [Google Scholar]
- Beach FA. Hormones and Behavior: A Survey of Interrelationships Between Endocrine Secretions and Patterns of Overt Response. NY: Paul B. Hoeber Inc; 1948. [Google Scholar]
- Beaudet A, Descarries L. The monoamine innervation of rat cerebral cortex: synaptic and nonsynaptic axon terminals. Neuroscience. 1978;3:851–860. doi: 10.1016/0306-4522(78)90115-x. [DOI] [PubMed] [Google Scholar]
- Begay V, Valotaire Y, Ravault JP, Collin JP, Falcon J. Detection of estrogen receptor mRNA in trout pineal and retina: estradiol-17 beta modulates melatonin production by cultured pineal photoreceptor cells. General and Comparative Endocrinology. 1994;93:61–69. doi: 10.1006/gcen.1994.1008. [DOI] [PubMed] [Google Scholar]
- Belekhova MG, Kenigfest-Rio NB, Vesselkin NP, Rio JP, Repérant J, Ward R. Evolutionary significance of different neurochemical organization of the internal and external regions of auditory centres in the reptilian brain: an immunocytochemical and reduced NADPH-diaphorase histochemical study in turtles. Brain Research. 2002;925:100–106. doi: 10.1016/s0006-8993(01)03255-3. [DOI] [PubMed] [Google Scholar]
- Bernard DJ, Bentley GE, Balthazart J, Turek FW, Ball GF. Androgen receptor, estrogen receptor alpha, and estrogen receptor beta show distinct patterns of expression in forebrain song control nuclei of European starlings. Endocrinology. 1999;140:4633–4643. doi: 10.1210/endo.140.10.7024. [DOI] [PubMed] [Google Scholar]
- Bertrand PP, Paranavitane UT, Chavez C, Gogos A, Jones M, van den Buuse M. The effect of low estrogen state on serotonin transporter function in mouse hippocampus: A behavioral and electrochemical study. Brain Research. 2005;1064:10–20. doi: 10.1016/j.brainres.2005.10.018. [DOI] [PubMed] [Google Scholar]
- Bethea CL, Mirkes SJ, Shively CA, Adams MR. Steroid regulation of tryptophan hydroxylase protein in the dorsal raphe of macaques. Biological Psychiatry. 2000;47:562–576. doi: 10.1016/s0006-3223(99)00156-0. [DOI] [PubMed] [Google Scholar]
- Boord RL, Rasmussen GL. Projection of the cochlear and lagenar nerves on the cochlear nuclei of the pigeon. Journal of Comparative Neurology. 1963;120:463–475. doi: 10.1002/cne.901200305. [DOI] [PubMed] [Google Scholar]
- Bradford MM. A rapid and sensitive method for quantification of microgram quantities of protein utilizing the principle of protein dye binding. Analytical Biochemistry. 1976;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- Caruso S, Grillo C, Agnello C, Maiolino L, Intelisano G, Serra A. A prospective study evidencing rhinomanometric and olfactometric outcomes in women taking oral contraceptives. Human Reproduction. 2001;16:2288–2294. doi: 10.1093/humrep/16.11.2288. [DOI] [PubMed] [Google Scholar]
- Challet E, Miceli D, Pierre J, Repérant J, Masicotte G, Herbin M, Vesselkin NP. Distribution of serotonin-immunoreactivity in the brain of the pigeon (Columba livia) Anatomy and Embryology. 1996;193:209–227. doi: 10.1007/BF00198325. [DOI] [PubMed] [Google Scholar]
- Chang AS, Chang SM. Nongenomic steroidal modulation of high-affinity serotonin transport. Biochimica et Biophysica Acta. 1999;1417:157–166. doi: 10.1016/s0005-2736(98)00255-7. [DOI] [PubMed] [Google Scholar]
- Charoenphandhu J, Teerapornpuntakit J, Nuntapornsak A, Krishnamra N, Charoenphandhu N. Anxiety-like behaviors and expression of SERT and TPH in the dorsal raphe of estrogen- and fluoxetine-treated ovariectomized rats. Pharmacology, Biochemistry, and Behavior. 2011;98:503–510. doi: 10.1016/j.pbb.2011.02.023. [DOI] [PubMed] [Google Scholar]
- Commissiong JW. Monoamine metabolites: their relationship and lack of relationship to monoaminergic neuronal activity. Biochemical Pharmacology. 1985;34:1127–1131. doi: 10.1016/0006-2952(85)90484-8. [DOI] [PubMed] [Google Scholar]
- Cone RI, Davis GA, Goy RW. Effects of ovarian steroids on serotonin metabolism within grossly dissected and microdissected brain regions of the ovariectomized rat. Brain Research Bulletin. 1981;7:639–644. doi: 10.1016/0361-9230(81)90111-8. [DOI] [PubMed] [Google Scholar]
- Cozzi B, Viglietti-Panzica C, Aste N, Panzica GC. The serotoninergic system in the brain of the Japanese quail: An immunohistochemical study. Cell and Tissue Research. 1991;263:271–284. doi: 10.1007/BF00318769. [DOI] [PubMed] [Google Scholar]
- Cransac H, Cottet-Emard JM, Hellstrom S, Peyrin L. Specific sound-induced noradrenergic and serotonergic activation in central auditory structures. Hearing Research. 1998;118:151–156. doi: 10.1016/s0378-5955(98)00031-8. [DOI] [PubMed] [Google Scholar]
- Dahlström A, Fuxe K. Localization of monoamines in the lower brain stem. Experientia. 1964;20:398–399. doi: 10.1007/BF02147990. [DOI] [PubMed] [Google Scholar]
- de Lacalle S. Estrogen effects on neuronal morphology. Endocrine. 2006;29:185–190. doi: 10.1385/ENDO:29:2:185. [DOI] [PubMed] [Google Scholar]
- Donner N, Handa RJ. Estrogen receptor beta regulates the expression of tryptophan-hydroxylase 2 mRNA within serotonergic neurons of the rat dorsal raphe nuclei. Neuroscience. 2009;163:705–718. doi: 10.1016/j.neuroscience.2009.06.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dube L, Parent A. The monoamine-containing neurons in avian brain: I. A study of the brain stem of the chicken (Gallus domesticus) by means of fluorescence and acetylcholinesterase histochemistry. Journal of Comparative Neurology. 1981;196:695–708. doi: 10.1002/cne.901960413. [DOI] [PubMed] [Google Scholar]
- Ebert U, Ostwald J. Serotonin modulates auditory information processing in the cochlear nucleus of the rat. Neuroscience Letters. 1992;145:51–54. doi: 10.1016/0304-3940(92)90201-h. [DOI] [PubMed] [Google Scholar]
- Eda-Fujiwara H, Satoh R, Bolhuis JJ, Kimura T. Neuronal activation in female budgerigars is localized and related to male song complexity. European Journal of Neuroscience. 2003;17:149–154. doi: 10.1046/j.1460-9568.2003.02414.x. [DOI] [PubMed] [Google Scholar]
- Fortune ES, Margoliash D. Cytoarchitectonic organization and morphology of cells of the field L complex in zebra finches (Taeniopygia guttata) Journal of Comparative Neurology. 1992;325:388–404. doi: 10.1002/cne.903250306. [DOI] [PubMed] [Google Scholar]
- Fuxe K, Ljunggren L. Cellular localization of monoamines in the upper brain stem of the pigeon. Journal of Comparative Neurology. 1965;125:355–382. doi: 10.1002/cne.901250306. [DOI] [PubMed] [Google Scholar]
- Gahr M. Distribution of sex steroid hormone receptors in the avian brain: functional implications for neural sex differences and sexual behaviors. Microscopy Research and Technique. 2001;55:1–11. doi: 10.1002/jemt.1151. [DOI] [PubMed] [Google Scholar]
- Gahr M, Guttinger HR, Kroodsma DE. Estrogen receptors in the avian brain: survey reveals general distribution and forebrain areas unique to songbirds. Journal of Comparative Neurology. 1993;327:112–122. doi: 10.1002/cne.903270109. [DOI] [PubMed] [Google Scholar]
- Gentner TQ, Hulse SH, Duffy D, Ball GF. Response biases in auditory forebrain regions of female songbirds following exposure to sexually relevant variation in male song. Journal of Neurobiology. 2001;46:48–58. doi: 10.1002/1097-4695(200101)46:1<48::aid-neu5>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- Griffiths R, Double MC, Orr K, Dawson RJG. A DNA test to sex most birds. Molecular Ecology. 1998;7:1071–1075. doi: 10.1046/j.1365-294x.1998.00389.x. [DOI] [PubMed] [Google Scholar]
- Gundlah C, Lu NZ, Mirkes SJ, Bethea CL. Estrogen receptor beta (ER beta) mRNA and protein in serotonin neurons of macaques. Molecular Brain Research. 2001;91:14–22. doi: 10.1016/s0169-328x(01)00108-5. [DOI] [PubMed] [Google Scholar]
- Hall IC, Rebec GV, Hurley LM. Serotonin in the inferior colliculus fluctuates with behavioral state and environmental stimuli. Journal of Experimental Biology. 2010;213:1009–1017. doi: 10.1242/jeb.035956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernandez AM, MacDougall-Shackleton SA. Effects of early song experience on song preferences and song control and auditory brain regions in female house finches (Carpodacus mexicanus) Journal of Neurobiology. 2004;59:247–258. doi: 10.1002/neu.10312. [DOI] [PubMed] [Google Scholar]
- Hiroi R, McDevitt RA, Neumaier JF. Estrogen selectively increases tryptophan hydroxylase-2 mRNA expression in distinct subregions of rat midbrain raphe nucleus: association between gene expression and anxiety behavior in the open field. Biological Psychiatry. 2006;60:288–295. doi: 10.1016/j.biopsych.2005.10.019. [DOI] [PubMed] [Google Scholar]
- Houdouin F, Cespuglio R, Gharib A, Sarda N, Jouvet M. Detection of the release of 5-hydroxyindole compounds in the hypothalamus and the n. raphe dorsalis throughout the sleep-waking cycle and during stressful situations in the rat: a polygraphic and voltammetric approach. Experimental Brain Research. 1991;85:153–162. doi: 10.1007/BF00229997. [DOI] [PubMed] [Google Scholar]
- Hurley LM, Devilbiss DM, Waterhouse BD. A matter of focus: Monoaminergic modulation of stimulus coding mammalian sensory networks. Current Opinion in Neurobiology. 2004;14:488–495. doi: 10.1016/j.conb.2004.06.007. [DOI] [PubMed] [Google Scholar]
- Hurley LM, Hall IC. Context-dependent modulation of auditory processing by serotonin. Hearing Research. 2011;279:74–84. doi: 10.1016/j.heares.2010.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurley LM, Pollak GD. Serotonin differentially modulates responses to tones and frequency-modulated sweeps in the inferior colliculus. Journal of Neuroscience. 1999;19:8071–8082. doi: 10.1523/JNEUROSCI.19-18-08071.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurley LM, Pollak GD. Serotonin modulates responses to species-specific vocalizations in the inferior colliculus. Journal of Comparative Physiology A. 2005;191:535–546. doi: 10.1007/s00359-005-0623-y. [DOI] [PubMed] [Google Scholar]
- Hurley LM, Thompson AM. Serotonergic innervation of the auditory brainstem of the Mexican free-tailed bat, Tadarida brasiliensis. Journal of Comparative Neurology. 2001;435:78–88. doi: 10.1002/cne.1194. [DOI] [PubMed] [Google Scholar]
- Ikeda H, Inugai H, Gotoh J. Localization of monoamine-containing fibers and cells in the alimentary canal of chickens. Japanese Journal of Veterinary Science. 1971;33:187–193. doi: 10.1292/jvms1939.33.187. [DOI] [PubMed] [Google Scholar]
- Inagaki T, Gautreaux C, Luine V. Acute estrogen treatment facilitates recognition memory consolidation and alters monoamine levels in memory-related brain areas. Hormones and Behavior. 2010;58:415–426. doi: 10.1016/j.yhbeh.2010.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji W, Suga N. Serotonergic modulation of plasticity of the auditory cortex elicited by fear conditioning. Journal of Neuroscience. 2007;27:4910–4918. doi: 10.1523/JNEUROSCI.5528-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaiser A, Covey E. 5-HT innervation of the auditory pathway in birds and bats. In: Syka J, editor. Acoustical Signal Processing in the Central Auditory System. New York, NY: Plenum; 1997. pp. 71–78. [Google Scholar]
- Karten HJ. The organization of the ascending auditory pathway in the pigeon (Columba livia). I. Diencephalic projections of the inferior colliculus (nucleus mesencephalicus lateralis, pars dorsalis) Brain Research. 1967;6:409–427. doi: 10.1016/0006-8993(67)90055-8. [DOI] [PubMed] [Google Scholar]
- Kilts CD, Breese GR, Mailman RB. Simultaneous quantification of dopamine, 5-hydroxytryptamine and four metabolically related compounds by means of reversed-phase high-performance liquid chromatography with electrochemical detection. Journal of Chromatography B: Biomedical Sciences. 1981;225:347–357. doi: 10.1016/s0378-4347(00)80283-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klepper A, Herbert H. Distribution and origin of noradrenergic and serotonergic fibers in the cochlear nucleus and inferior colliculus of the rat. Brain Research. 1991;557:190–201. doi: 10.1016/0006-8993(91)90134-h. [DOI] [PubMed] [Google Scholar]
- Koldzic-Zivanovic N, Seitz PK, Watson CS, Cunningham KA, Thomas ML. Intracellular signaling involved in estrogen regulation of serotonin reuptake. Molecular and Cellular Endocrinology. 2004;29:33–42. doi: 10.1016/j.mce.2004.07.017. [DOI] [PubMed] [Google Scholar]
- Kritzer MF, Kohama SG. Ovarian hormones differentially influence immunoreactivity for dopamine beta-hydroxylase, choline acetyltransferase, and serotonin in the dorsolateral prefrontal cortex of adult rhesus monkeys. Journal of Comparative Neurology. 1999;409:438–451. doi: 10.1002/(sici)1096-9861(19990705)409:3<438::aid-cne8>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- LeBlanc MM, Goode CT, MacDougall-Shackleton EA, Maney DL. Estradiol modulates brainstem catecholaminergic cell groups and projections to the auditory forebrain in a female songbird. Brain Research. 2007;1171:93–103. doi: 10.1016/j.brainres.2007.06.086. [DOI] [PubMed] [Google Scholar]
- Lu H, Ozawa H, Nishi M, Kawata M. Serotonergic neurons in the dorsal raphe nucleus that project into the medial preoptic area contain oestrogen receptor β. Journal of Neuroendocrinology. 2001;13:839–845. doi: 10.1046/j.1365-2826.2001.00695.x. [DOI] [PubMed] [Google Scholar]
- Lu H, Yuri K, Ito T, Yoshimoto K, Kawata M. The effects of oestrogen and progesterone on serotonin and its metabolite in the lateral septum, medial preoptic area and ventromedial hypothalamic nucleus of female rats. Journal of Neuroendocrinology. 1998;10:919–926. doi: 10.1046/j.1365-2826.1998.00280.x. [DOI] [PubMed] [Google Scholar]
- Lu NZ, Eshleman AJ, Janowsky A, Bethea CL. Ovarian steroid regulation of serotonin reuptake transporter (SERT) binding, distribution, and function in female macaques. Molecular Psychiatry. 2003;8:353–360. doi: 10.1038/sj.mp.4001243. [DOI] [PubMed] [Google Scholar]
- Lu NZ, Shlaes TA, Gundlah C, Dziennis SE, Lyle RE, Bethea CL. Ovarian steroid action on tryptophan hydroxylase protein and serotonin compared to localization of ovarian steroid receptors in midbrain of guinea pigs. Endocrine. 1999;11:257–267. doi: 10.1385/ENDO:11:3:257. [DOI] [PubMed] [Google Scholar]
- Lynch KS, Ball GF. Noradrenergic deficits alter processing of communication signals in female songbirds. Brain, Behavior & Evolution. 2008;72:207–214. doi: 10.1159/000157357. [DOI] [PubMed] [Google Scholar]
- Maney DL. Hormonal control of sexual behavior in female nonmammalian vertebrates. In: Breed MD, Moore J, editors. Encyclopedia of Animal Behavior. Vol. 1. Oxford: Elsevier; 2010. pp. 697–703. [Google Scholar]
- Maney DL, Cho E, Goode CT. Estrogen-dependent selectivity of genomic responses to birdsong. European Journal of Neuroscience. 2006;23:1523–1529. doi: 10.1111/j.1460-9568.2006.04673.x. [DOI] [PubMed] [Google Scholar]
- Maney DL, Erwin KL, Goode CT. Neuroendocrine correlates of behavioral polymorphism in white-throated sparrows. Hormones and Behavior. 2005;48:196–206. doi: 10.1016/j.yhbeh.2005.03.004. [DOI] [PubMed] [Google Scholar]
- Maney DL, Goode CT, Lange HS, Sanford SE, Solomon BL. Estradiol modulates neural responses to song in a seasonal songbird. Journal of Comparative Neurology. 2008;511:173–186. doi: 10.1002/cne.21830. [DOI] [PubMed] [Google Scholar]
- Maney DL, Lange HS, Raees MQ, Reid AE, Sanford SE. Behavioral phenotypes persist after gonadal steroid manipulation in white-throated sparrows. Hormones and Behavior. 2009;55:113–120. doi: 10.1016/j.yhbeh.2008.09.002. [DOI] [PubMed] [Google Scholar]
- Maney DL, MacDougall-Shackleton EA, MacDougall-Shackleton SA, Ball GF, Hahn TP. Immediate early gene response to hearing song correlates with receptive behavior and depends on dialect in a female songbird. Journal of Comparative Physiology A. 2003;189:667–674. doi: 10.1007/s00359-003-0441-z. [DOI] [PubMed] [Google Scholar]
- Maney DL, Pinaud R. Estradiol-dependent modulation of auditory processing and selectivity in songbirds. Frontiers in Neuroendocrinology. 2011;32:287–302. doi: 10.1016/j.yfrne.2010.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez-Vargas MC, Stumpf WE, Sar M. Anatomical distribution of estrogen target cells in the avian CNS: a comparison with the mammalian CNS. Journal of Comparative Neurology. 1976;167:83–103. doi: 10.1002/cne.901670106. [DOI] [PubMed] [Google Scholar]
- Maruska KP, Fernald RD. Steroid receptor expression in the fish inner ear varies with sex, social status, and reproductive state. BMC Neuroscience. 2010;11:58. doi: 10.1186/1471-2202-11-58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matragrano LL, Sanford SE, Salvante KG, Sockman KW, Maney DL. Estradiol-dependent catecholaminergic innervation of auditory areas in a seasonally breeding songbird. European Journal of Neuroscience. 2011;34:416–425. doi: 10.1111/j.1460-9568.2011.07751.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEwen BS, Alves SE. Estrogen actions in the central nervous system. Endocrine Reviews. 1999;20:279–307. doi: 10.1210/edrv.20.3.0365. [DOI] [PubMed] [Google Scholar]
- McQueen JK, Wilson H, Fink G. Estradiol-17β increases serotonin transporter (SERT) mRNA levels and the density of SERT-binding sites in female rat brain. Molecular Brain Research. 1997;45:13–23. doi: 10.1016/s0169-328x(96)00233-1. [DOI] [PubMed] [Google Scholar]
- Metzger M, Toledo C, Braun K. Serotonergic innervation of the telencephalon in the domestic chick. Brain Research Bulletin. 2002;57:547–551. doi: 10.1016/s0361-9230(01)00688-8. [DOI] [PubMed] [Google Scholar]
- Meyer JS, Grande M, Johnson K, Ali SF. Neurotoxic effects of MDMA (“ecstasy”) administration to neonatal rats. International Journal of Developmental Neuroscience. 2004;22:261–271. doi: 10.1016/j.ijdevneu.2004.04.007. [DOI] [PubMed] [Google Scholar]
- Moore MC. Effect of female sexual displays on the endocrine physiology and behavior of male white-crowned sparrows, Zonotrichia leucophrys. Journal of Zoology. 1983;199:137–148. [Google Scholar]
- Nielsen K, Brask D, Knudsen GM, Aznar S. Immunodetection of the serotonin transporter protein is a more valid marker for serotonergic fibers than serotonin. Synapse. 2006;59:270–276. doi: 10.1002/syn.20240. [DOI] [PubMed] [Google Scholar]
- Noirot IC, Adler HJ, Cornil CA, Harada N, Dooling RJ, Balthazart J, Ball GF. Presence of aromatase and estrogen receptor alpha in the inner ear of zebra finches. Hearing Research. 2009;252:49–55. doi: 10.1016/j.heares.2009.04.012. [DOI] [PubMed] [Google Scholar]
- Pandaranandaka J, Poonyachoti S, Kalandakanond-Thongsong S. Differential effects of exogenous and endogenous estrogen on anxiety as measured by elevated T-maze in relation to the serotonergic system. Behavioural Brain Research. 2009;198:142–148. doi: 10.1016/j.bbr.2008.10.043. [DOI] [PubMed] [Google Scholar]
- Parent A. Comparative anatomy of the serotoninergic systems. Journal of Physiology. 1981;77:147–156. [PubMed] [Google Scholar]
- Phillmore LS, Bloomfield LL, Weisman RG. Effects of songs and calls on ZENK expression in the auditory telencephalon of field- and isolate-reared black capped chickadees. Behavioural Brain Research. 2003;147:125–134. doi: 10.1016/s0166-4328(03)00155-4. [DOI] [PubMed] [Google Scholar]
- Pietras RJ, Moulton DG. Hormonal influences on odor detection in rats: changes associated with the estrous cycle, pseudopregnancy, ovariectomy, and administration of testosterone propionate. Physiology & Behavior. 1974;12:475–491. doi: 10.1016/0031-9384(74)90125-5. [DOI] [PubMed] [Google Scholar]
- Pinaud R, Fortes AF, Lovell P, Mello CV. Calbindin-positive neurons reveal a sexual dimorphism within the songbird analogue of the mammalian auditory cortex. Journal of Neurobiology. 2006;66:182–195. doi: 10.1002/neu.20211. [DOI] [PubMed] [Google Scholar]
- Pinaud R, Terleph TA. A songbird forebrain area potentially involved in auditory discrimination and memory formation. Journal of Bioscience. 2008;33:145–155. doi: 10.1007/s12038-008-0030-y. [DOI] [PubMed] [Google Scholar]
- Reiner A, Perkel DJ, Bruce LL, Butler AB, Csillag A, Kuenzel W, Striedter G. Revised nomenclature for avian telencephalon and some related brainstem nuclei. Journal of Comparative Neurology. 2004;473:377–414. doi: 10.1002/cne.20118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robichaud M, Debonnel G. Oestrogen and testosterone modulate the firing activity of dorsal raphe nucleus serotonergic neurons in both male and female rats. Journal of Neuroendocrinology. 2005;17:179–185. doi: 10.1111/j.1365-2826.2005.01292.x. [DOI] [PubMed] [Google Scholar]
- Sanford SE, Lange HS, Maney DL. Topography of estradiol-modulated genomic responses in the songbird auditory forebrain. Developmental Neurobiology. 2010;70:73–86. doi: 10.1002/dneu.20757. [DOI] [PubMed] [Google Scholar]
- Saper CB, Sawchenko PE. Magic peptides, magic antibodies: Guidelines for appropriate controls for immunohistochemistry. Journal of Comparative Neurology. 2003;465:161–163. doi: 10.1002/cne.10858. [DOI] [PubMed] [Google Scholar]
- Schielzeth H, Forstmeier W. Conclusions beyond support: overconfident estimates in mixed models. Behavioral Ecology. 2009;20:416–420. doi: 10.1093/beheco/arn145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shank MC. The natural termination of the refractory period in the slate-colored junco and in the white-throated sparrow. Auk. 1959;76:44–54. [Google Scholar]
- Sheng Z, Kawano J, Yanai A, Fujinaga R, Tanaka M, Watanabe Y, Shinoda K. Expression of estrogen receptors (α, β) and androgen receptor in serotonin neurons of the rat and mouse dorsal raphe nuclei; sex and species differences. Neuroscience Research. 2004;49:185–196. doi: 10.1016/j.neures.2004.02.011. [DOI] [PubMed] [Google Scholar]
- Sisneros JA, Forlano PM, Deitcher DL, Bass AH. Steroid-dependent auditory plasticity leads to adaptive coupling of sender and receiver. Science. 2004;305:404–407. doi: 10.1126/science.1097218. [DOI] [PubMed] [Google Scholar]
- Sockman KW, Salvante KG. The integration of song environment by catecholaminergic systems innervating the auditory telencephalon of adult female European starlings. Developmental Neurobiology. 2008;68:656–668. doi: 10.1002/dneu.20611. [DOI] [PubMed] [Google Scholar]
- Sockman KW, Gentner TQ, Ball GF. Recent experience modulates forebrain gene-expression in response to mate-choice cues in European starlings. Proceedings of the Royal Society of London Series B. 2002;269:2479–2485. doi: 10.1098/rspb.2002.2180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sockman KW, Gentner TQ, Ball GF. Complementary neural systems for the experience-dependent integration of mate-choice cues in European starlings. Journal of Neurobiology. 2005;62:72–81. doi: 10.1002/neu.20068. [DOI] [PubMed] [Google Scholar]
- Stark H, Scheich H. Dopaminergic and serotonergic neurotransmission systems are differentially involved in auditory cortex learning: a long-term microdialysis study of metabolites. Journal of Neurochemistry. 1997;68:691–697. doi: 10.1046/j.1471-4159.1997.68020691.x. [DOI] [PubMed] [Google Scholar]
- Steinbusch HW. Distribution of serotonin-immunoreactivity in the central nervous system of the rat-cell bodies and terminals. Neuroscience. 1981;6:557–618. doi: 10.1016/0306-4522(81)90146-9. [DOI] [PubMed] [Google Scholar]
- Stokes TM, Leonard CM, Nottebohm F. Telencephalon, diencephalon, and mesencephalon of canary, Serinus canaria, in stereotaxic coordinates. Journal of Comparative Neurology. 1974;156:337–374. doi: 10.1002/cne.901560305. [DOI] [PubMed] [Google Scholar]
- Terpstra NJ, Bolhuis JJ, Riebel K, Van Der Burg JMM, Den Boer-Visser AM. Localized brain activation specific to auditory memory in a female songbird. Journal of Comparative Neurology. 2006;494:784–791. doi: 10.1002/cne.20831. [DOI] [PubMed] [Google Scholar]
- Tremere LA, Jeong JK, Pinaud R. Estradiol shapes auditory processing in the adult brain by regulating inhibitory transmission and plasticity-associated gene expression. Journal of Neuroscience. 2009;29:5949–5963. doi: 10.1523/JNEUROSCI.0774-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolfson A. Regulation of refractory period in the photoperiodic responses of the white-throated sparrow. Journal of Experimental Zoology. 1958;139:349–379. doi: 10.1002/jez.1401390207. [DOI] [PubMed] [Google Scholar]
- Woodley SK, Matt KS, Moore MC. Estradiol modulation of central monoamine activity in female mountain spiny lizards. Brain, Behavior, and Evolution. 2000;56:175–183. doi: 10.1159/000047202. [DOI] [PubMed] [Google Scholar]
- Woolley SM, Fremouw TE, Hsu A, Theunissen FE. Tuning for spectro-temporal modulations as a mechanism for auditory discrimination of natural sounds. Nature Neuroscience. 2005;8:1371–1379. doi: 10.1038/nn1536. [DOI] [PubMed] [Google Scholar]
- Vanderhorst VGJM, Terasawa E, Ralston HJ. Axonal sprouting of a brainstem-spinal pathway after estrogen administration in the adult female rhesus monkey. The Journal of Comparative Neurology. 2002;454:82–103. doi: 10.1002/cne.10446. [DOI] [PubMed] [Google Scholar]
- Vates GE, Broome BM, Mello CV, Nottebohm F. Auditory pathways of caudal telencephalon and their relation to the song system of adult male zebra finches (Taeniopygia guttata) Journal of Comparative Neurology. 1996;366:613–642. doi: 10.1002/(SICI)1096-9861(19960318)366:4<613::AID-CNE5>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- Velho TAF, Mello CV. Synapsins are late activity-induced genes regulated by birdsong. Journal of Neuroscience. 2008;28:11871–11882. doi: 10.1523/JNEUROSCI.2307-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada H, Takeuchi Y, Sano Y. Immunohistochemical studies on the serotonin neuron system in the brain of the chicken (Gallus domesticus). I. The distribution of the neuronal somata. Biogenic Amines. 1984;1:83–94. [Google Scholar]
- Zeng S, Li J, Zhang X, Zuo M. Distinction of neurochemistry between the cores and their shells of auditory nuclei in tetrapod species. Brain, Behavior and Evolution. 2007;70:1–20. doi: 10.1159/000101066. [DOI] [PubMed] [Google Scholar]