Abstract
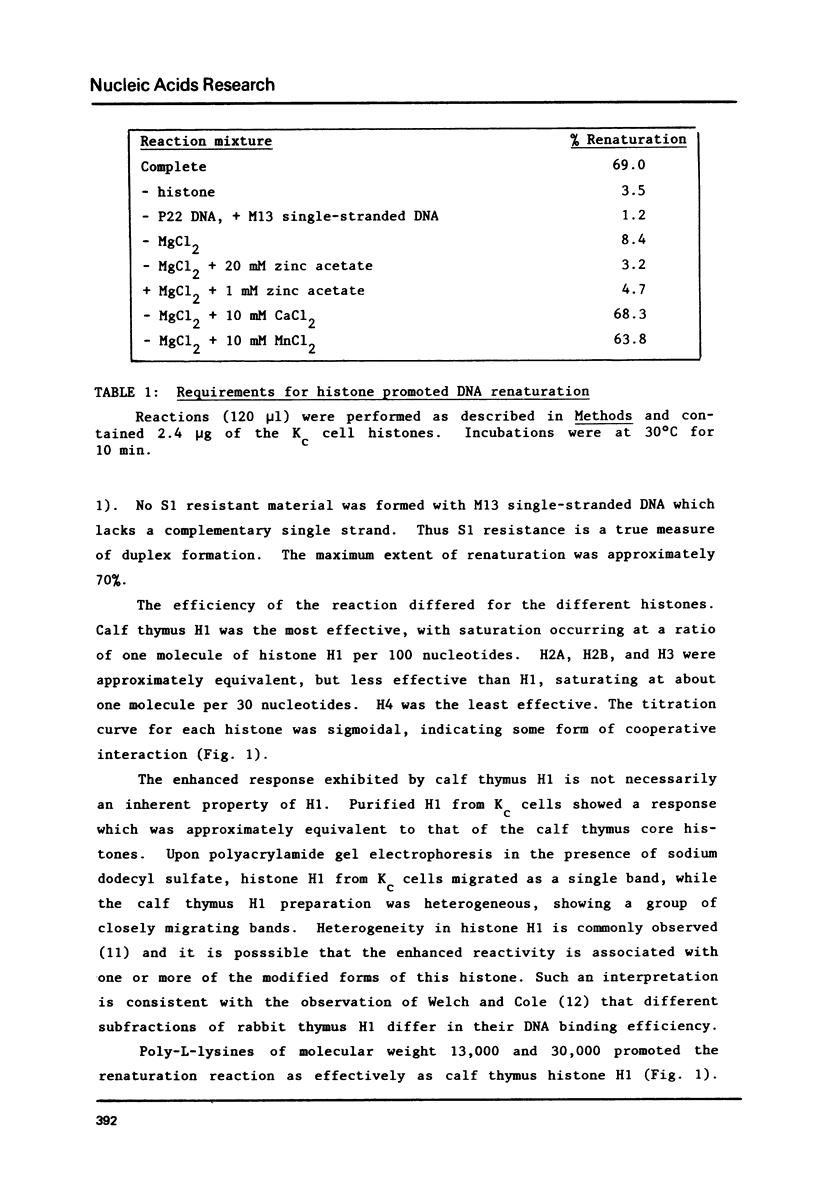
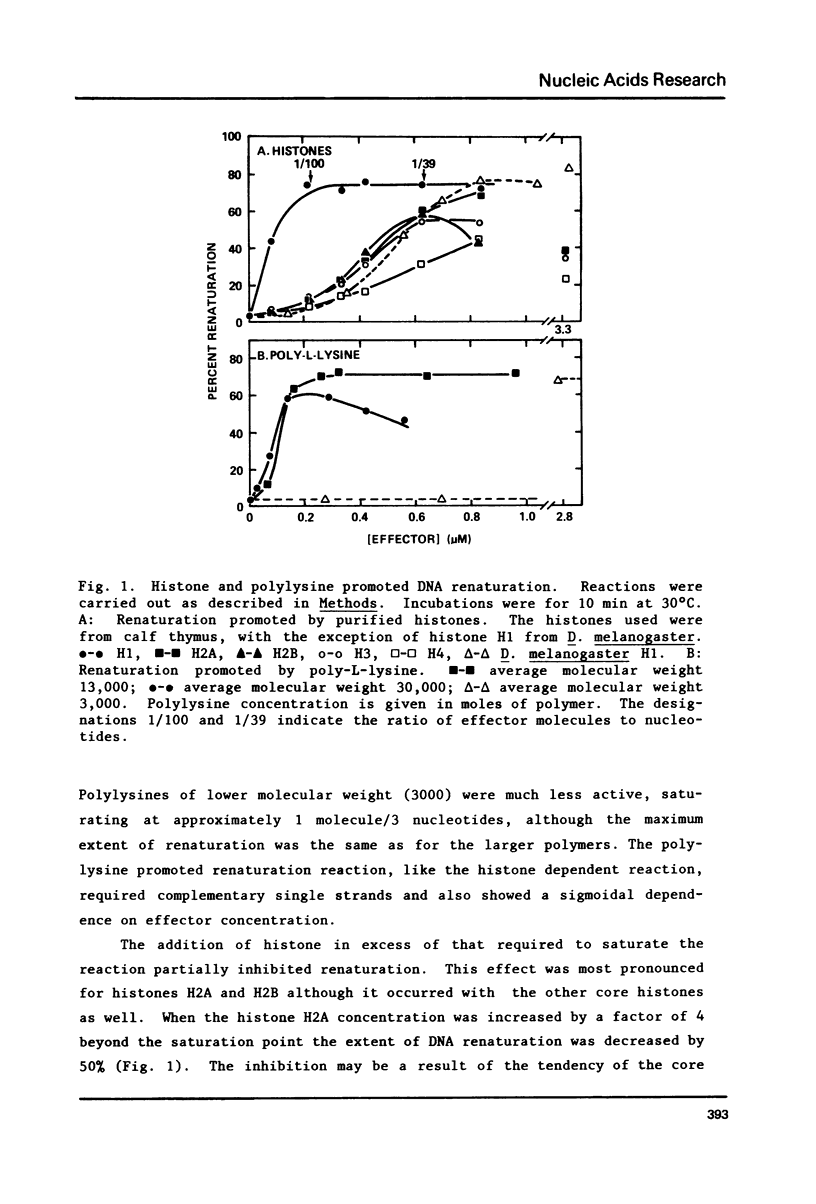
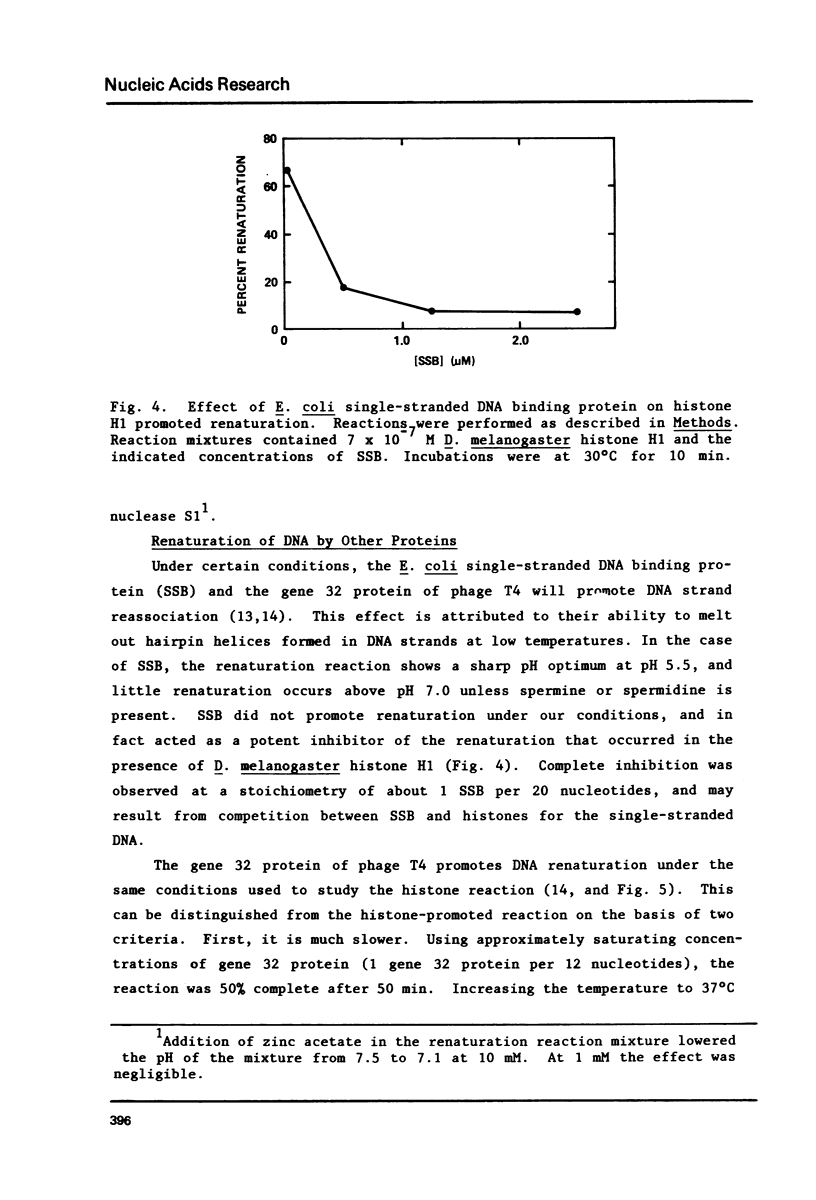
Histones isolated from several sources, either singly or in combination promote the renaturation of complementary single strands of DNA, as measured by the acquisition of resistance to S1 nuclease. The reaction is rapid (T1/2 less than 1 min), and is stoichiometric rather than catalytic. Renaturation is stimulated by Mg2+, Mn2+, and Ca2+, but is strongly inhibited by Zn2+. Crude extracts of early embryos of Drosophila melanogaster possess renaturation activity which is protease sensitive, heat-stable, and acid-soluble, suggesting that most or all of it can be attributed to histones. This observation thus provides a functional assay for histones that should prove useful in studies of chromatin and histone-DNA interactions, as well as for the identification and isolation of histones and histone-like proteins in crude extracts.
Full text
PDF








Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alberts B. M., Frey L. T4 bacteriophage gene 32: a structural protein in the replication and recombination of DNA. Nature. 1970 Sep 26;227(5265):1313–1318. doi: 10.1038/2271313a0. [DOI] [PubMed] [Google Scholar]
- Botstein D. Synthesis and maturation of phage P22 DNA. I. Identification of intermediates. J Mol Biol. 1968 Jun 28;34(3):621–641. doi: 10.1016/0022-2836(68)90185-x. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Christiansen C., Baldwin R. L. Catalysis of DNA reassociation by the Escherichia coli DNA binding protein: A polyamine-dependent reaction. J Mol Biol. 1977 Sep 25;115(3):441–454. doi: 10.1016/0022-2836(77)90164-4. [DOI] [PubMed] [Google Scholar]
- Dolfini S. Karyotype polymorphism in a cell population of Drosophila melanogaster cultured in vitro. Chromosoma. 1971;33(2):196–208. doi: 10.1007/BF00285633. [DOI] [PubMed] [Google Scholar]
- Echalier G., Ohanessian A. In vitro culture of Drosophila melanogaster embryonic cells. In Vitro. 1970 Nov-Dec;6(3):162–172. doi: 10.1007/BF02617759. [DOI] [PubMed] [Google Scholar]
- Germond J. E., Bellard M., Oudet P., Chambon P. Stability of nucleosomes in native and reconstituted chromatins. Nucleic Acids Res. 1976 Nov;3(11):3173–3192. doi: 10.1093/nar/3.11.3173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hancock R., Faber A. J., Fakan S. Isolation of interphase chromatin structures from cultured cells. Methods Cell Biol. 1977;15:127–147. doi: 10.1016/s0091-679x(08)60213-7. [DOI] [PubMed] [Google Scholar]
- Huberman J. A. Structure of chromosome fibers and chromosomes. Annu Rev Biochem. 1973;42:355–378. doi: 10.1146/annurev.bi.42.070173.002035. [DOI] [PubMed] [Google Scholar]
- Isenberg I. Histones. Annu Rev Biochem. 1979;48:159–191. doi: 10.1146/annurev.bi.48.070179.001111. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K., Favre M. Maturation of the head of bacteriophage T4. I. DNA packaging events. J Mol Biol. 1973 Nov 15;80(4):575–599. doi: 10.1016/0022-2836(73)90198-8. [DOI] [PubMed] [Google Scholar]
- Palter K. B., Alberts B. M. The use of DNA-cellulose for analyzing histone-DNA interactions. Discovery of nucleosome-like histone binding to single-stranded DNA. J Biol Chem. 1979 Nov 10;254(21):11160–11169. [PubMed] [Google Scholar]
- Rubin G. M., Hogness D. S. Effect of heat shock on the synthesis of low molecular weight RNAs in drosophilia: accumulation of a novel form of 5S RNA. Cell. 1975 Oct;6(2):207–213. doi: 10.1016/0092-8674(75)90011-2. [DOI] [PubMed] [Google Scholar]
- Shin Y. A., Eichhorn G. L. Interactions of metal ions with polynucleotides and related compounds. XI. The reversible unwinding and rewinding of deoxyribonucleic acid by zinc (II) Ions through temperature manipulation. Biochemistry. 1968 Mar;7(3):1026–1032. doi: 10.1021/bi00843a022. [DOI] [PubMed] [Google Scholar]
- Sperling R., Amos L. A. Arrangement of subunits in assembled histone H4 fibers. Proc Natl Acad Sci U S A. 1977 Sep;74(9):3772–3776. doi: 10.1073/pnas.74.9.3772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinstock G. M., McEntee K., Lehman I. R. ATP-dependent renaturation of DNA catalyzed by the recA protein of Escherichia coli. Proc Natl Acad Sci U S A. 1979 Jan;76(1):126–130. doi: 10.1073/pnas.76.1.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welch S. L., Cole R. D. Differences among subfractions of H1 histone in retention of linear and superhelical DNA on filters. J Biol Chem. 1980 May 25;255(10):4516–4518. [PubMed] [Google Scholar]



