Abstract
Cognitive decline is a common problem of aging. Whereas multiple neural and glial mechanisms may account for these declines, microglial sensitization and/or dystrophy has emerged as a leading culprit in brain aging and dysfunction. However, glial activation is consistently observed in normal brain aging as well, independent of frank neuroinflammation or functional impairment. Such variability suggests the existence of additional vulnerability factors that can impact neuronal-glial interactions and thus overall brain and cognitive health. The goal of this review is to elucidate our working hypothesis that an individual‘s risk or resilience to neuroinflammatory disorders and poor cognitive aging may critically depend on their early life experience, which can change immune reactivity within the brain for the remainder of the lifespan. For instance, early-life infection in rats can profoundly disrupt memory function in young adulthood, as well as accelerate age-related cognitive decline, both of which are linked to enduring changes in glial function that occur in response to the initial infection. We discuss these findings within the context of the growing literature on the role of immune molecules and neuroimmune crosstalk in normal brain development. We highlight the intrinsic factors (e.g., chemokines, hormones) that regulate microglial development and their colonization of the embryonic and postnatal brain, and the capacity for disruption or “re-programming” of this crucial process by external events (e.g, stress, infection). An impact on glia, which in turn alters neural development, has the capacity to profoundly impact cognitive and mental health function at all stages of life.
Introduction
Cognitive decline is one of the primary disabilities of aging. However, some individuals age more successfully than others; e.g., only a subset of the population develops dementias such as Alzheimer‘s disease (AD), and the risk factors are poorly defined. Neuroinflammation is strongly associated with AD, and is suspected in conditions as diverse as Parkinson‘s disease, schizophrenia, and depression — notably, all conditions that have not historically been considered “inflammatory” (Streit et al., 2004). Inflammation in the periphery typically occurs as an acute response to injury, with a critical repair role and a clear resolution, whereas neuroinflammation is largely synonymous in the literature with chronic glial activation (microglia and astrocytes) and exaggerated expression of proinflammatory mediators within the CNS (e.g., cytokines, reactive oxygen species) (Streit, 2010). However, whether neuroinflammation is a cause or a consequence of neural dysfunction remains unknown in the midst of conditions that may involve repeated cycles of injury and response extending well beyond their initial origin. Similarly, whether inflammatory processes (and microglial activation in particular) are ultimately helpful or harmful remains subject to intense debate; this is especially the case for AD (see (Streit, 2010).
Notably, changes in glial morphology, surface marker expression, and function are consistently observed in the normal aging brain in rodent models, including upregulation of major histocompatibility (MHC) II and CD11b on microglia, astrogliosis, and increased expression of central pro-inflammatory cytokines following immune challenge compared to young brains (Barrientos et al., 2006; Frank et al., 2006; Godbout and Johnson, 2009; Bilbo, 2010; Lynch, 2010). Thus, the factors that create a transition from normal age-related glial alterations to frank pathology (e.g., in disorders like AD) remain unclear.
The goal of this review is to elucidate our working hypothesis that an individual‘s risk or resilience to neuroinflammatory disorders, cognitive dysfunction, and poor cognitive aging may critically depend on their early life experience, which can change immune reactivity within the brain for the remainder of the lifespan. Diverse early-life events, such as infection, stress, nutrition, or maternal care, can impact the normal course of immune and brain development, and thereby permanently alter adult cognition and mood. Research on “developmental programming” has thus begun to provide valuable insight into the origins of neuropsychiatric diseases that exhibit a broad spectrum of prevalence and severity (Bennet and Gunn, 2006). For instance, we have demonstrated that early-life infection in rats can profoundly disrupt memory function later in life. However, memory deficits remain latent throughout young adulthood, and only emerge if ‘unmasked’ by a subsequent inflammatory challenge (a “second hit”) around the time of learning, implying a long-term change within the immune system that acutely impacts the neural processes underlying memory (Bilbo et al., 2005a; Bilbo et al., 2005b; Bilbo et al., 2006; Bilbo et al., 2008; Bilbo and Schwarz, 2009). Moreover, these same animals exhibit accelerated cognitive decline with age, independent of acute immune challenge, a change that is linked to exaggerated aging-related glial activation (Bilbo, 2010). Thus, aging itself, and its impact on glial reactivity, acts as a “second hit” only in rats made vulnerable by infection early in life.
We discuss these findings within the context of the growing literature on the role of neuroimmune communication in both normal and pathological brain development, the role of cytokines in cognition and plasticity, including critical stages of development juxtaposed with aging, and the capacity for early-life events to program mental health throughout the lifespan. We focus primarily on the role of microglia as the major immune cells of the brain.
The Immune System is a Regulator of Neural and Cognitive Function
Immune-CNS Communication
Beyond its traditional role in host defense and tissue repair, the immune system is now considered a diffuse sensory organ that works in concert with the endocrine and nervous systems to achieve and maintain homeostasis throughout the body (Husband, 1995; Vitkovic et al., 2000). Immunocompetent cells are located throughout every organ of the body, including the brain, and regular communication occurs between the CNS and immune tissues during both health and disease processes, via several pathways: 1) the hormones of the hypothalamic-pituitary-adrenal (HPA) axis (e.g., glucocorticoids), for which immune cells have receptors, 2) the autonomic nervous system which innervates lymphoid tissues (e.g., vagus nerve), 3) circulating peripheral immune cells (e.g., CD4+ T cells) that can enter the CNS and interact with resident immune cells (microglia), and 4) the release of cytokines and chemokines by both peripheral and CNS immune cells, as well as other “non-immune” tissues (e.g., liver, adipose), which can have autocrine, paracrine, and endocrine functions. Many excellent reviews have been written on these topics (Maier and Watkins, 1998; Mignini et al., 2003; Wrona, 2006; Dantzer and Kelley, 2007). Importantly, bidirectional communication between the brain and immune system has significant consequences for plasticity mechanisms within the brain, including cognition, which is the focus of this review.
Cytokines and Cognition
Cytokines are increasingly implicated in synaptic plasticity mechanisms important for cognition (McAfoose and Baune, 2009). Tumor necrosis factor (TNF)-α is important for activity-dependent synaptic scaling within the hippocampus (Beattie et al., 2002; Stellwagen and Malenka, 2006). Moreover, TNFα, as well as multiple interleukins [IL] (e.g., IL-6, IL-1, IL-10) and prostaglandins can markedly impact cognitive function, primarily memory (reviewed in (Yirmiya and Goshen, 2011). One of the more surprising developments in this field within the last several years is compelling evidence that CD4+ T cells that specifically recognize CNS self-antigens are important for healthy brain plasticity, including neurogenesis and learning & memory, via interactions with meningeal myeloid cells and microglia and the production of IL-4 (Ziv et al., 2006; Derecki et al., 2010; Schwartz and Kipnis, 2010). Notably, in each of these literatures there exists a role for cytokines in the mechanisms underlying not only impairment in the midst of pathology, but also normal, healthy cognition. Perhaps the largest amount of research in this area has been done on IL-1β. For instance, IL-1β mRNA is induced within the hippocampus (HP) in response to learning (Abraham and Williams, 2003; Goshen et al., 2007), and is required for the maintenance of HP long-term potentiation (LTP) (Ross et al., 2003; Spulber et al., 2009). Moreover, mice lacking IL-1β or its type 1 receptor, and those with overexpression of the endogenous IL-1 receptor antagonist (IL-1ra), exhibit markedly impaired HP-dependent learning and memory (Goshen et al., 2007; Spulber et al., 2009). In contrast to IL-1β in the healthy brain, patients with AD, AIDS-related dementia, or chronic inflammatory diseases often exhibit exaggerated levels of IL-1β within the CNS along with cognitive impairment (Gallo et al., 1989; Griffin et al., 1989; Stanley et al., 1994; Meyers, 2000). Similarly, peripheral high dose lipopolysaccharide (LPS) injection in rodents interferes with HP LTP in vivo, and this effect depends upon increased IL-1β (Vereker et al., 2000). Finally, rats injected with high dose IL-1β directly into the dorsal HP also display memory impairments (Pugh et al., 1999; Barrientos et al., 2002). Thus, increasing evidence suggests an inverted “U” function for IL-1β and cognition, in which physiological, mid-range levels are necessary for normal learning & memory, whereas concentrations that are either too low or too high impair memory (Goshen et al., 2007). We believe this point is key - it is because cytokines are important for normal brain function that their dysregulation is pivotal in dysfunction.
Adult Neurogenesis
Immune factors influence several aspects of adult neurogenesis, including progenitor cell proliferation, differentiation, and survival (reviewed in (Carpentier and Palmer, 2009; McAfoose and Baune, 2009), again during homeostasis as well as pathology, though the latter has been much better defined. The dentate gyrus (DG) of the HP is one of only two brain regions in which adult neurogenesis is known to consistently occur in mammals, including humans. Adult DG neurogenesis may be important for learning and memory, and deficits are linked to a number of mental illnesses (Zhao et al., 2006). The majority of newborn neurons within the adult DG undergo programmed cell death within the first 4 days, and microglia play a critical role in this homeostatic process via phagocytosis of dying cells (Sierra et al., 2010). Inflammation can have multiple impacts on neurogenesis; in general, low levels of inflammation induce growth factors and cell survival, whereas moderate-to-high levels are either profoundly suppressive or aberrantly proliferative (reviewed in (Whitney et al., 2009). There is also evidence that inflammation can influence the function of newborn cells, which may be more important than a change in the number of cells. For instance, intrahippocampal LPS injection in adult rats does not influence the number or survival of new neurons, but does alter the likelihood that the new cells respond to excitatory input (Jakubs et al., 2008).
There is a growing literature on the role of chemokines (chemotactic cytokines) in stem cell migration and maturation within the adult brain as well (reviewed in (Miller et al., 2008). Chemokines are a family of small cytokines involved in cellular migration as well as intercellular communication more generally. In fact, these molecules have recently been suggested as the third major medium for communication within the brain, alongside neurotransmitters and neuropeptides (Adler et al., 2005). Although their role in development is much better known, chemokines such as monocyte chemoattractant protein (MCP)-1 mediate neural stem cell migration to areas of adult brain injury (Widera et al., 2004), and neural stem cells continue to express receptors for MCP-1 throughout life (Tran and Miller, 2003), suggesting a physiological role in non-injury neurogenesis as well.
Interestingly, neural stem cells in the adult mouse DG also express toll-like-receptors (TLRs), innate pattern recognition receptors present on most immune cells including microglia (Akira and Takeda, 2004). In vitro studies suggest TLR2 and 4 are important for stem cell fate determination, and mice lacking TLR2 exhibit impaired neurogenesis, whereas mice lacking TLR4 exhibit enhanced neurogenesis (Rolls et al., 2007). These data are especially intriguing given that the Toll family of receptors were initially characterized in Drosophila for their role in embryonic neural patterning, but are inseparable from the immune system in mammals (Anderson et al., 1985).
Glia and Plasticity
Microglia are the primary immunocompetent cells of the brain. However, astrocytes are the largest glial cell population within the brain, and their role in synaptic plasticity mechanisms is now well accepted (reviewed in (Eroglu and Barres, 2010)). In the adult brain, astrocytes are physically and functionally appositioned with most synapses, known as the “tripartite synapse” (Araque et al., 1999). Astrocytes contain most neurotransmitter receptors, allowing them to perceive and respond to synaptic activity (Haydon and Carmignoto, 2006). Astrocytes also produce the TNFα that mediates synaptic scaling following prolonged periods of inactivity within the HP (Beattie et al., 2002; Stellwagen and Malenka, 2006). D-serine release from astrocytes is necessary for HP LTP (Yang et al., 2003; Henneberger et al., 2010). Importantly, a recent report showed that IL-1 type 1 receptor expression in astrocytes is also necessary for HP-dependent LTP and long-term memory (Ben Menachem-Zidon et al., 2011).
Increasing evidence suggests a role for microglia in normal synaptic plasticity mechanisms within the adult brain as well, including interactions with extracellular matrix composition and geometry, and dendritic spine remodeling and elimination (Tremblay and Majewska, 2011). These cells are very dynamic, even when resting (Nimmerjahn et al., 2005), and continually survey their microenvironments by extending and contracting processes into nearby synapses, with a frequency that is activity-dependent (Wake et al., 2009; Tremblay et al., 2010). For instance, they sample individual synapses more frequently following visual stimulation, or in response to injury, and are likely responsible for synapse removal via phagocytosis (Tremblay et al., 2010). Microglia have receptors for multiple neurotransmitters and neuromodulators, including those important for learning and memory (e.g., ATP, norepinephrine, glutamate) (Pocock and Kettenmann, 2007), suggesting a rapid and direct role for these cells in normal cognition, similar to astrocytes, though this remains to be demonstrated.
The Immune System is a Regulator of Brain Development
The immune system is critical for normal brain development. A novel role for major histocompatibility class (MHC) I proteins in activity-dependent synapse formation within the visual cortex was identified over a decade ago (Corriveau et al., 1998), and a role for complement proteins in developmental synapse elimination was described several years later (Stevens et al., 2007), two pivotal findings that fundamentally changed the concept of “immune privilege” within the healthy brain (reviewed in (Garay and McAllister, 2010).
Cytokines and Neurodevelopment
A large number of chemokines and cytokines have now been characterized for their importance in neural induction, neuronal and glial cell migration, proliferation, differentiation, and synaptic maturation and pruning (reviewed in (Deverman and Patterson, 2009). These include members of the gp130, bone morphogenetic protein (BMP), and transforming growth factor beta (TGFβ) superfamilies, as well as many traditionally defined “pro-inflammatory” cytokines (e.g., IL-1β, TNFα) (Merrill, 1992). Notably, discrete time dependence and regional specificity has been demonstrated for different cytokines during brain development, suggesting physiological roles for these cytokines in the growth and organization of specific brain circuits. For instance, IL-1β is mitogenic for astrocytes and is expressed at high levels throughout the late prenatal/early postnatal HP and cortex, but at very low (constitutive) levels in the adult rodent (Giulian et al., 1988; Schmitz and Chew, 2008). Both IL-1β and TNFα are present early in the developing sheep brain, declining by birth and peaking again around the time of synaptogenesis (Dziegielewska et al., 2000). IL-6 is important for numerous developmental processes, including fetal growth at the maternal-placental-fetal interface (Hsiao and Patterson, 2011), and prenatal CNS vascular development (Fee et al., 2000), and increases markedly in striatum throughout development, suggesting a neurotrophic role within this region (Gadient and Otten, 1994).
In contrast to physiological levels, elevated proinflammatory cytokines in the amniotic fluid and/or neonatal blood stream are cited as a primary predictor of adverse long-term outcomes in children (Cai et al., 2000; Urakubo et al., 2001; Pang et al., 2003; Richardson-Burns and Tyler, 2004; Yu et al., 2004; Meyer et al., 2006). Concentrations of IL-1β, IL-6, and TNF are elevated in infants with severe perinatal complications (Miller et al., 1990), and children with bacterial meningitis have elevated levels of IL-1β that strongly correlate with the occurrence of neurological disorders (Miller et al., 1990). Increased levels of IL-6 in amniotic fluid have also been a clinically useful marker of increased risk for neurological disorders and morbidity (Yoon et al., 1995). Finally, a critical role for IL-6 in altering fetal-placental growth and in long-term changes in offspring brain development and behavior has also been demonstrated in a mouse model of maternal infection (Smith et al., 2007; Hsiao and Patterson, 2011).
Role of Glia
Glial cells direct normal brain development. Oligodendrocytes myelinate axons primarily during the postnatal period, and disruption of their function can be profoundly debilitating as in the case of periventricular leukomalacia leading to cerebral palsy (Bell and Hallenbeck, 2002). Astrocytes mediate synapse formation within the developing brain (Ullian et al., 2001), in part via the secretion of extracellular matrix proteins called thrombospondins (TSPs) (Christopherson et al., 2005; Eroglu et al., 2009). Alterations in spine density via a putative TSP mechanism are implicated in neurodevelopmental disorders such as Down‘s and Rett Syndrome (Garcia et al., 2010). Notably, astrocyte maturation marks the end of the perinatal synaptogenic period when the brain is most plastic (Muller and Best, 1989).
Important for this review is that the expression of many cytokines within the developing brain, including IL-1β and TGFβ, depends on the presence of amoeboid microglia (Giulian et al., 1988). Early in development, microglia are highly mobile and primarily amoeboid, consistent with their role in the phagocytosis of apoptotic cells (Rezaie and Male, 2002). They gradually transform into a highly branched, ramified morphology by adulthood in most brain regions. This morphological transition occurs in parallel with neural cell genesis and migration, synaptogenesis, and synaptic pruning, suggesting functions for microglia in each of these processes (Figure 1), though these are just beginning to be explored (Rakic and Zecevic, 2000; Streit, 2001;Garden and Moller, 2006).
Figure 1. Developing microglia participate in many processes of neural development.
Timeline representation of microglial development (upper panel of timeline) juxtaposed with known processes of neurodevelopment (lower panel). Microglia are derived from primitive macrophages that originate within the yolk sac, enter the neuroectoderm during embryogenesis, and begin to colonize the parenchyma around embryonic day (E)12–14 (Ginhoux et al., 2010). During the early postnatal period (P4) several chemokines, cytokines, and receptors are several-fold higher than at P60 (e.g., Ccl12, Ccl3, Cxcl6); these may be involved in the ongoing recruitment of microglia into the neural tissue, among other functions (Schwartz and Bilbo, unpublished). In the adult (P60), the pattern of chemokine expression changes, with significant upregulation of different factors (e.g., C3, Cx3cl1) (Schwartz and Bilbo, unpublished). Cx3cl1 (fractalkine) is expressed by neurons and tonically inhibits microglia (Harrison et al., 1998); thus, its upregulation over the course of development may be important for shifting the morphology and function of microglia into their mature, ramified state in adulthood. In parallel with the colonization of microglia, progenitor cells in situ undergo proliferation and differentiate into neurons and glia, a process dependent upon local factors or intrinsic signals. Bone morphogenic protein (BMP)/TGFβ and interleukin (IL)-1, both released from developing microglia (Deverman and Patterson, 2009), are known mediators of these processes. As microglia continue to develop, they produce cytokines (including IL-1β, Tumor Necrosis Factor (TNF) α, and IL-11) that are important for ongoing neurogenesis within the developing brain; they also produce chemokines (Cxcl12 and its receptor Cxcr4) that guide the axons of new neurons via chemoattraction toward their new synaptic targets (Lu et al., 2002; Lieberam et al., 2005). At the level of the synapse, major histocompatability complex (MHC) I and TNFα are important for strengthening new synaptic connections within the brain (Corriveau et al., 1998; Santello et al., 2011). Finally, in the later stages of neurodevelopment, abundant or inappropriate synaptic connections are eliminated (synaptic pruning) and phagocytosed by microglia. Immune proteins such as complement component (C)1q and C3 tag synapses for elimination during this process (Stevens et al., 2007). These timelines are broadly representative of the pattern in both males and females, though there are sex differences in relative glial density at distinct stages (see Figure 3).
Developmental Origins of Health and Disease
The role of early-life events that specifically impact immune factors in long-term synaptic plasticity, and in neural and glial cell genesis, connectivity, and function within the adult and aging brain has been relatively unexplored. However, developmental or “fetal programming” is an area of significant interest, based on evidence that experiences during critical or sensitive periods of perinatal life may modulate or “program” the normal course of development, with the result that adult outcomes, including cognition, are significantly and often permanently altered (Bennet and Gunn, 2006). Notably, there is strong evidence that early-life infection can permanently alter stress reactivity, disease susceptibility, and notably, vulnerability to cognitive and neuropsychiatric disorders, including AD, Parkinson‘s disease, schizophrenia, and autism (Rantakallio et al., 1997; Hornig et al., 1999; Nelson and Willoughby, 2000; Shi et al., 2003). We have recently reviewed this literature (Bilbo and Schwarz, 2009).
Given the many ways in which the immune system is important for normal brain development, the capacity for immune-inducing events to influence the long-term trajectory and function of these processes is likely profound, perhaps more so than at any other stage of life (Rice and Barone, 2000). The long-term impact of any early-life immune-activating stimulus on long-term function likely depends on several factors. These factors include timing relative to neural development, whether the insult occurs pre- vs. postnatally (the former of which may incorporate maternal and placental immune factors), the specific nature of the insult and pattern of cytokine expression (including activation of distinct TLRs and their downstream signaling pathways), and notably, the capacity to alter glial colonization or function long-term, which we highlight in the following sections.
Microglial Colonization of the Developing Brain
Microglia derive from primitive yolk sac macrophage precursors, which are of mesodermal origin and enter the neuroectoderm during embryogenesis, a founding colony of which proliferates to populate the entire developing parenchyma (Ginhoux et al., 2010). Little is known about the mechanisms underlying their initial recruitment into and throughout the white and gray matter, though MCP-1 and intercellular cell adhesion molecule (ICAM)-2 may play a role, in addition to signals released as a consequence of synapse formation and programmed cell death (Rezaie and Male, 1999). Moreover, a recent report shows that mice lacking the receptor for colony-stimulating factor (CSF)-1 do not develop microglia (Ginhoux et al., 2010). This same report demonstrates that adult microglia in normal mice arise exclusively from the founding population of primitive macrophages, with little or no contribution from peripheral bone marrow-derived myeloid precursors after initial colonization and proliferation during development (in the absence of trauma) (Ginhoux et al., 2010). These data address a critical issue, as they illustrate that resident microglia are maintained potentially throughout life, in stark contrast to peripheral macrophages that have a high rate of turn over.
Regional Differences
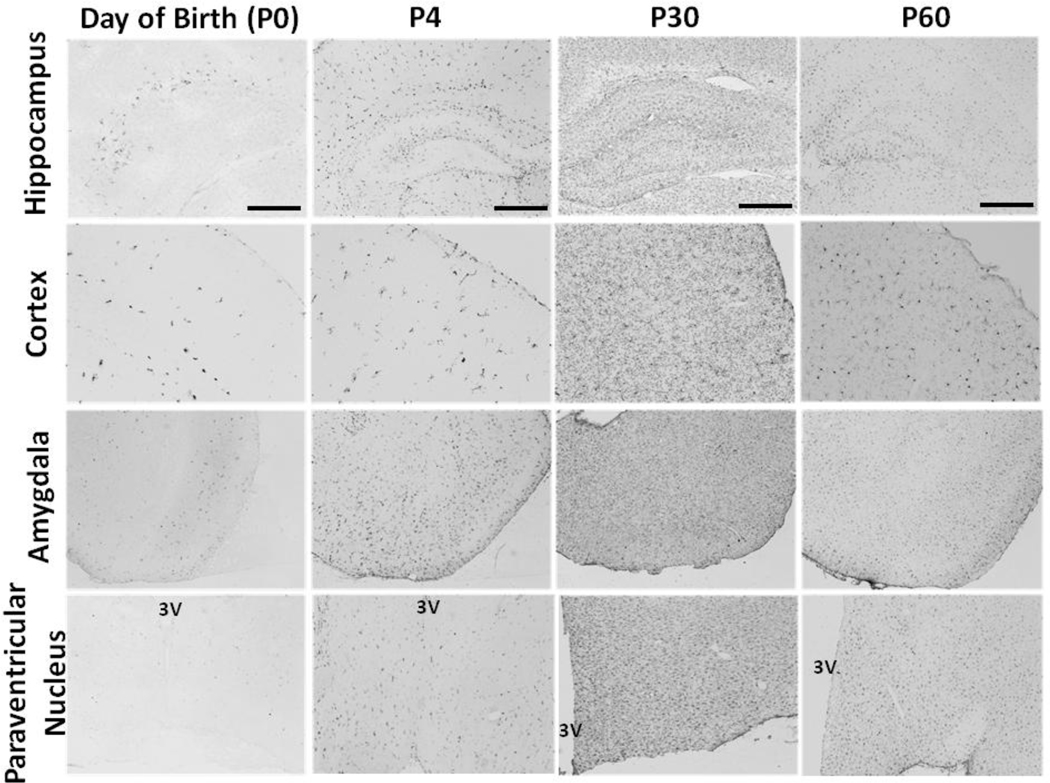
Similar to other developmental events (e.g., myelination and synaptogenesis), microglia colonize different brain regions at remarkably different rates. In our studies the hippocampus, amygdala, and cortex, regions important for cognition and emotion, are among the first to be populated by glia in the rat (~embryonic day 17), whereas the paraventricular nucleus of the hypothalamus is not colonized until ~postnatal day [P]1–4, a considerable time frame in the developing rodent brain (Figure 2). Yet other regions including the striatum exhibit a sparse glial density early in development, with an increase in density much later around the juvenile period within the rodent brain (not shown, Schwarz et al., submitted data). Microglial colonization of the human brain shows similar regional differences, albeit with a longer time course, increasing in white matter and in areas surrounding blood vessels within the 2nd trimester (primarily amoeboid), with differentiation into gray matter occurring throughout the 3rd trimester and postnatally (Rezaie and Male, 2002). Notably, environmental factors can alter the colonization rate and density of microglia. For instance, prenatal stress accelerates the colonization of microglia into the CNS, such that the total number of ramified microglia is greater in the early postnatal brain compared to non-stressed controls (Gomez-Gonzalez and Escobar, 2010). Because amoeboid microglia may be important for synaptic pruning, engulfment of apoptotic cells, and other processes, a change in the number of infiltrating cells, or an accelerated progression of these cells from amoeboid to ramified may have a profound impact on neural development.
Figure 2. Colonization of the developing brain by microglia.
On the day of birth (first column), microglia first appear within the brain around the hippocampus (top row), cortex (second row), and amygdala (third row). By P4 (second column), microglia have begun to enter hypothalamic nuclei such as the paraventricular nucleus (fourth row), and density begins to peak in hippocampus. At P0 and P4, microglial morphology is either amoeboid or round with short, stout processes (best seen above in the cortex at P0 and P4). By P30 (third column) and P60 (fourth column) microglia reside in most brain regions (including striatum and others not shown here). At P30, many microglia still have a relatively immature morphology. Specifically, microglia at this time have thicker, longer processes than microglia at P60. By P60, microglia have matured and are maintained in the healthy brain in a ramified state characterized by thin, long processes. This timeline is broadly representative of the pattern in both males and females, though there are sex differences in relative glial density at distinct stages (see Figure 3). (Scale bar = 500 µm and applies to all)
Sex Differences
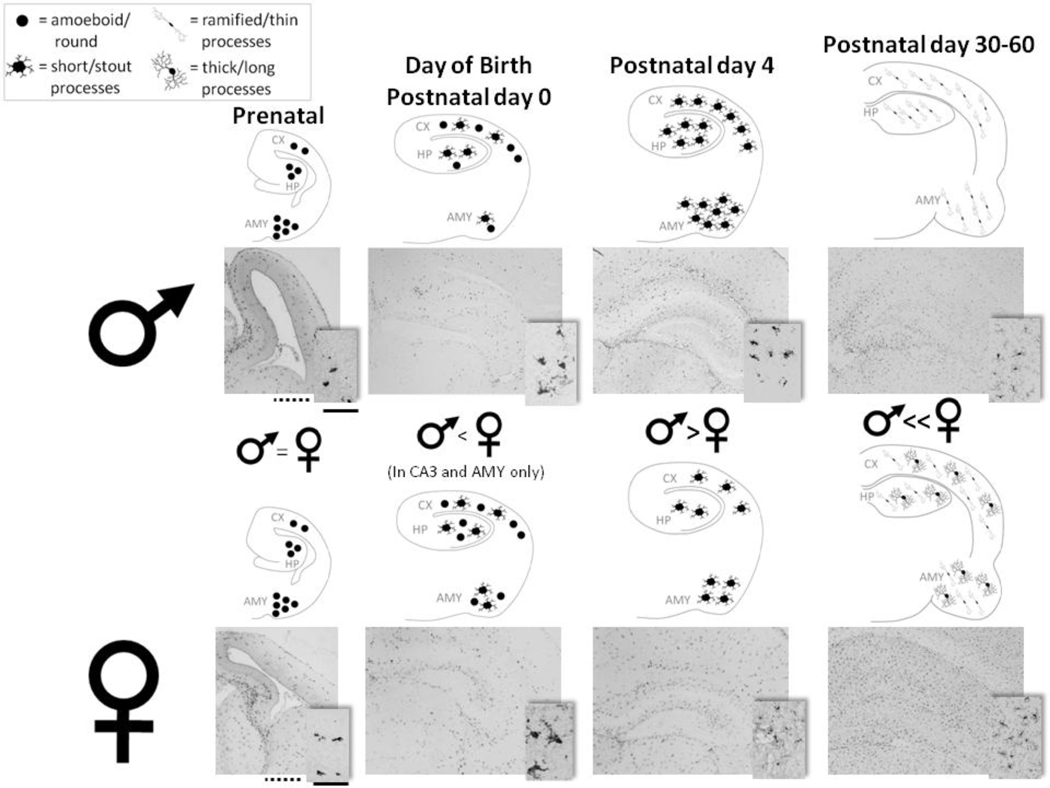
There is also a striking sex difference in the time course of microglial colonization in the rat brain (Figure 3), which may be informative in the context of the well-known sex bias in the presentation of many developmental and neuropsychiatric disorders. While the time course outlined in the previous section on regional differences holds true for both sexes, we have recently determined in rodents that glial numbers are several-fold higher in males than females during the early postnatal period (P4), suggesting that males may be more sensitive than females to an early-life immune challenge. In support of this hypothesis, the presentation and diagnosis of many early-onset neurodevelopmental disorders in humans (e.g., autism, ADHD) is higher in boys than in girls (Taylor and Rutter, 1985). Notably the sex difference only emerges after the prenatal spike in testosterone that occurs around E18. In contrast, females are more vulnerable to conditions that often emerge during adolescence, including depression, anxiety, and eating disorders (Taylor and Rutter, 1985). Intriguingly, we have also found that the sex difference in microglial number reverses at the time of adolescence and is maintained into adulthood, suggesting that females are more sensitive to immune dysregulation and thus cognitive dysfunction during this time than males (Schwarz et al., submitted data).
Figure 3. The density and morphology of microglia within cognitive regions of the brain are significantly affected by sex and age.
At embryonic day 17 (Prenatal; first column from left), males (top row of representative coronal sections) and females (bottom row) have a similar number of microglia within the hippocampus (HP), cortex (CX), and the amygdala (AMY.) At birth (Postnatal day 0), females (bottom series of representative coronal sections) have slightly more microglia than males (top series of representative coronal sections) within the CA3 region of HP and AMY, though not within the CX. As the brain develops and microglia continue to enter the brain, this sex difference reverses. By Postnatal day 4, males have significantly more microglia within the CX, all subregions of the HP, and AMY than females. Beginning around adolescence (postnatal day 30) and into adulthood (postnatal day 60) the sex difference reverses again. At this age, microglia generally appear within all brain regions in a ramified state, represented by thin, long processes. However, at postnatal day 30 and 60, females have a significantly greater population of microglia with thicker, longer processes than males in all three regions, suggestive of a differential function between the sexes at this age. One representative light micrograph is displayed below each diagram, which schematize cell density. Scale bars (in the first column from left): dotted line = 100 µm (for representative pictures at 4× for all ages), solid line = 50 µm (for inset pictures at 20× for all ages). One schematized coronal section is equivalent to 10 sections analyzed for cell counts and morphology at all ages. 1 cell shown above at postnatal day 0 and postnatal day 4 represents approximately 10 counted cells. 1 cell shown above at postnatal day 30 – 60 represents approximately 200 counted cells.
Early-Life Infection Alters Glial and Cognitive Function for Life
Infections are thought to contribute to 40% of pre-term births, and up to 70% of very pre-term births (Greene et al., 2008), for which incidences are increasing by 10% every decade (March of Dimes). A significant proportion of children also experience infections during the neonatal or early childhood years when neural circuits are still developing. Of these, bacterial infections represent the number one cause of infections worldwide (Osrin et al., 2004; Skogstrand et al., 2008). Major recent advances in maternal and perinatal medicine have greatly increased survival rates among these populations in developed countries. However, it remains to be determined what the total impact of infection or other types of inflammatory events during the perinatal period may have on subsequent physiology and behavior in individuals. The bulk of evidence from the animal literature suggests that regions important for cognition (i.e., HP and cortex) may be especially vulnerable to early-life disruption by immune activation (Table 1). The human literature supports this as well, as cognitive impairment, including learning, memory, and attention disorders, is one of the most consistent consequences of preterm birth, perinatal infection, or other complications such as trauma (Isaacs et al., 2000; Msall, 2004; Aylward, 2005; Rose et al., 2005a; Rose et al., 2005b; Eriksen et al., 2009).
Table 1.
E, embryonic; P, postnatal.
| Species | Challenge | Time | Impact on neural and cognitive outcomes in adulthood* |
Reference(s): |
|---|---|---|---|---|
| Rat | LPS | E8–12 | Decreased synaptophysin in CA1 of HP; impaired spatial learning | (Hao et al., 2010) |
| Mouse | Poly IC | E9.5 | Increased dendritic spine density along with decreased synaptophysin in cerebral cortex | (Soumiya et al., 2011) |
| Mouse | Poly IC | E12.5 | Impaired synaptic transmission in CA1 of HP; abnormal object information processing | (Ito et al., 2010) |
| Mouse | Poly IC | E12–17 | Impaired pre-pulse inhibition (PPI) and novel object recognition performance in adult but not juvenile offspring | (Ozawa et al., 2006) |
| Rats | Poly IC | E15 | Impaired PPI; impaired latent inhibition; altered hippocampal morphology | (Zuckerman et al., 2003; Zuckerman and Weiner, 2003, 2005; Wolff and Bilkey, 2008) |
| Rat | LPS | E15–16 | Decreased dentate gyrus neurogenesis at P14; altered hippocampal synaptic transmission at P20–25; impaired PPI | (Fortier et al., 2007; Lowe et al., 2008; Cui et al., 2009) |
| Mouse | LPS | E17 | Altered hippocampal morphology, impaired learning and object recognition | (Golan et al., 2005) |
| Mouse | Poly IC | E17 | Potentiated locomotor activity and altered NMDA receptor subunit expression | (Meyer et al., 2008) |
| Rat | LPS | E17 | Altered hippocampal morphology, decreased learning & memory | (Golan et al., 2005) |
| Rat | LPS | E18–19 | Impaired PPI | (Fortier et al., 2007) |
| Rat | Borna Disease Virus | P0 | Locomotor hyperactivity & hippocampal damage; spatial learning & memory deficits; stereotypy and hyperactivity | (Hornig and Lipkin, 2001; Hornig et al., 2001) |
| Rat | E. coli | P4 | Altered NMDA receptor subunit expression in HP; decreased BDNF; LPS-induced memory impairment | (Bilbo et al., 2005a; Bilbo et al., 2008; Bilbo, 2010) |
| Rat | LPS | P4–5 | Impaired avoidance learning | (Kohman et al., 2008) |
| Rat | LPS | P5 | Impaired passive avoidance learning and memory; axonal injury and neuron loss in the hippocampus | (Fan et al., 2008) |
| Rat | IL-1β | P5 | Impaired myelination; neuronal loss in CA1 of HP; impaired performance in passive avoidance task at P21 | (Fan et al., 2010) |
| Rat | LPS | P14 | Altered NMDA receptor subunit expression in HP; reduced novel object exploration | (Spencer et al., 2005; Harre et al., 2008) |
All phenotypes are in adulthood unless otherwise noted.
Alterations in neurogenesis, migration, axon growth, myelination, synaptogenesis, and dendritic spine density/function are all candidate mechanisms for inducing long-term changes in cognition (see Table 1). However, we believe the long lifespan of microglia and their pattern of CNS colonization during development also has particular significance for the fields of developmental programming and neuroinflammation, as diverse early-life events may exert enduring impacts on the brain and behavior of organisms via an influence on these resident immune cells, both by impacting their own intrinsic function as well as their interactions with ongoing developmental processes. In order to explore these questions, we developed a model of early-life infection in rats that enables the assessment of acute and long-term impacts on brain, neuroimmune function, and behavior.
Early-Life Infection Affects Adult Learning and Memory
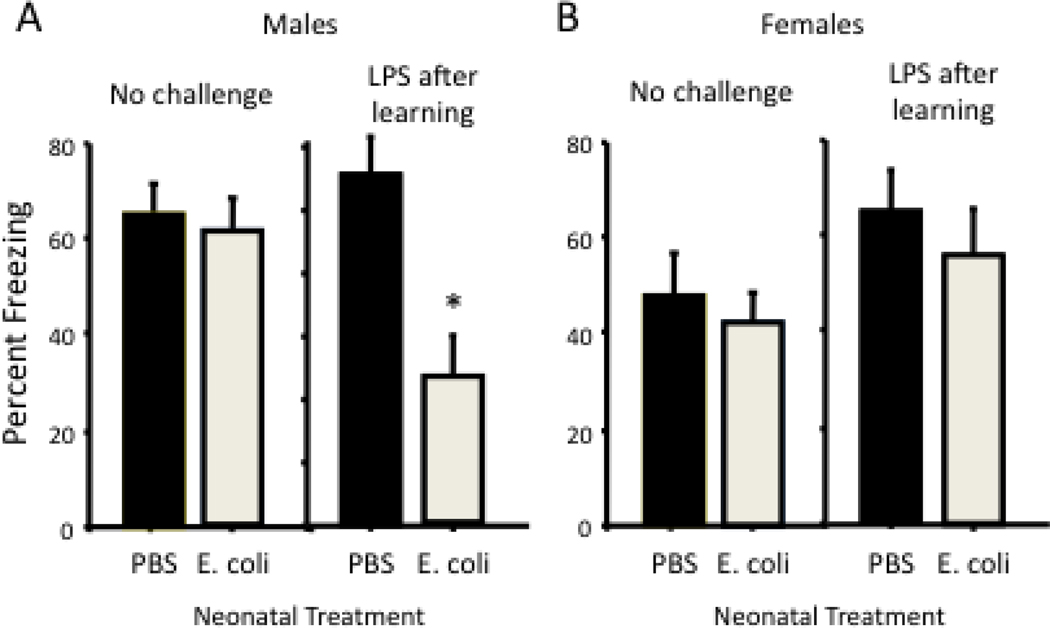
In this model, male rats are injected subcutaneously on P4, a developmental time point comparable to the early 3rd trimester in humans, with either PBS or a non-lethal dose of live Escherichia coli. When tested for memory as adults, rats infected neonatally with E. coli exhibit profound memory impairments; however, this impairment is dependent on receiving a second inflammatory challenge (peripheral LPS) around the time of learning. LPS is a potent inducer of IL-1β, and exaggerated or prolonged expression of this cytokine can impair synaptic processes and HP-dependent memories as discussed previously (Vereker et al., 2000; Barrientos et al., 2002). Consistent with this possibility, neonatally-infected rats exhibit prolonged IL-1β responses to LPS within the brain compared to control rats, and preventing the synthesis of IL-1β completely prevents the memory impairment (Bilbo et al., 2005a). Importantly, the same LPS challenge has no influence on memory in controls, suggesting that only an exaggerated IL-1β response like that measured in neonatally-infected rats causes memory impairments. Moreover, an infection later in development, on P30, does not lead to LPS-induced memory impairment later in life, indicating the vulnerability to glial and cognitive dysfunction is specifically a developmental effect, and not a general sensitizing event that can occur at any time (Bilbo et al., 2006); see (Bilbo and Schwarz, 2009) for review). Finally, females do not exhibit the same long-term memory impairment vulnerability as males following infection on P4 (Figure 4), consistent with our hypothesis that females may exhibit a different sensitive period.
Figure 4. Early-life bacterial infection leads to LPS-induced memory impairment in adult males but not females.
Male and female rat pups injected with PBS or E. coli on postnatal day 4 were tested for memory as adults using a contextual fear-conditioning paradigm. Half of the rats in each neonatal group were injected with LPS immediately after learning. (A) Freezing to the context 48 h later was significantly reduced in adult males injected with LPS after learning, but only if they had also received E. coli as neonates (from Bilbo et al., 2006; *p<0.05). (B) In contrast to males, LPS had no effect on memory in females from either neonatal treatment.
Early-Life Infection Leads to Enduring Changes in Microglial Function
Microglia are the primary producers of IL-1β within the brain (Streit et al., 2005). Microglial proliferation and density begin to peak within the HP and cortex during the first postnatal week in rodents (Figure 2). Thus, we initially hypothesized that these regions may be particularly vulnerable to long-term changes in microglial cell number or function at this time. Consistent with this hypothesis, microglia show a marked increase in activation marker expression (CD11b) within the male neonatal HP in response to the peripheral infection (Bilbo et al., 2005a). Notably this increase occurs with the same time course as an increase in IL-1β protein and several IL-1 pathway-specific genes (e.g., IL-1 type 1 receptor, caspase 1) (Schwarz and Bilbo, 2011). Moreover, the increase in CD11b is sustained into adulthood, as well as exaggerated in response to a systemic adult injection with LPS in neonatally-infected compared to control rats (Bilbo et al., 2005a; Bilbo et al., 2007). However, an increase in activation marker expression could indicate an increase in cell number, or a change in their reactivity. To distinguish between these two possibilities, we performed cell counts of glia in adulthood following E. coli infection at P4. The total number of microglia, astrocytes, & neurons within the adult HP did not differ as a consequence of neonatal treatment (assessed using unbiased stereology) (Bland et al., 2010). However, the morphology of microglia in neonatally-infected rats was very different in adulthood; cell volumes were larger with shorter, thicker processes (Bland et al., 2010).
More recently we have confirmed and extended this characterization of glia using flow cytometric analysis of rapidly isolated microglia from rats in each condition. To do this, rats from each neonatal treatment were injected as adults with SAL or LPS, and the HP was removed 24 h later following saline perfusion. Microglia were rapidly isolated as described in detail previously (Frank et al.; Henry et al., 2009). Next, isolated glia were stained for APC-conjugated CD11b. Cell size (forward light scatter) and CD11b+ expression were assessed using flow cytometry (Figure 5A). Microglia were both larger and exhibited increased CD11b+ expression on a per cell basis in neonatally-infected rats (Figure 5B), consistent with our previous in situ findings (Bland et al., 2010). Finally, rapidly isolated microglia from neonatally-infected rats express more IL-1β on a per cell basis compared to controls (Bilbo et al., submitted data). These data are schematized in Figure 5C. These collective data illustrate that changes in the function of microglia underlie the increased reactivity observed in adult neonatally-infected rats.
Figure 5. Bacterial infection in neonatal male rats alters long-term microglial morphology, surface antigen expression, and function.
(A) Male rats treated on P4 with PBS or live E. coli were injected systemically as adults with saline (SAL) or 25 µg/kg lipopolysaccharide (LPS), and microglia were rapidly isolated from whole HP 24 h later following cold saline perfusion to eliminate infiltrating cells as described in detail previously (Frank et al.; Henry et al., 2009). Isolated microglia were stained for APC-conjugated CD11b. Cell size (forward light scatter) and CD11b+ expression were assessed using a FACSCanto™ II flow cytometer (Becton, Dickinson and Co.) and FlowJo™ software (Treestar, Inc.). The gating strategy and representative contour plot are shown. (B) There is a greater proportion of large, CD11bbright microglia in adult rats infected neonatally with E. coli. Mean fluorescence of CD11b+ staining in gated cells was also greater on a per cell basis in rats infected neonatally with E. coli. *Overall effect of E. coli for each, p<0.01. (C) Sensitized/primed microglia in neonatally-infected rats produce more IL-1β in response to LPS in adulthood compared to controls (Bilbo et al., 2005a; 2007; Bilbo et al., submitted data).
Taken together, neonatal infection appears to induce a life-long vulnerability to cognitive disruption by increasing microglial reactivity within the HP long-term. The mechanism(s) by which IL-1β impairs memory is currently unknown. However, LPS transiently suppresses HP brain-derived-neurotrophic-factor (BDNF) (Barrientos et al., 2004), a molecule critical for LTP and memory consolidation, and this decrease is again exaggerated in neonatally-infected rats, possibly falling below a critical threshold necessary for normal memory consolidation (Bilbo et al., 2008).
Glial Priming, Aging, and Neurodegeneration
The pattern of initial activation and enduring sensitization just described is well in accord with the concept of “glial priming” that has been described in recent years within the aging and neurodegeneration literatures. “Priming” is a term first borrowed from the tissue macrophage literature (Johnson et al., 1983; Pace et al., 1983; Martinez et al., 2008), and has been implicated in AD, Parkinson‘s, and Huntington‘s disease (Perry et al., 2003). The characteristics of priming are not well defined, though primed glia have been characterized in situ by an activated morphology with enlarged cell bodies and short, thick processes. An important feature of priming is that these cells do not constitutively over-produce pro-inflammatory mediators within the brain. Rather, the pro-inflammatory response produced by primed glia to a subsequent challenge (e.g., systemic infection) is significantly exaggerated when compared to resting/quiescent glia that receive the same challenge (Perry et al., 2003). This exaggerated response can markedly impact cognitive function and other health outcomes (Barrientos et al.). Thus, it is hypothesized that primed glia adopt a prolonged sensitized state, presumably following initial activation by insult or injury (Streit and Xue, 2009; Perry et al., 2010). Note that there is also evidence that microglia become dystrophic in contrast to sensitized with age, which exhibit stripped or disembodied processes and are impaired in their normal homeostatic functions (Streit et al., 2009; Streit and Xue, 2009). In either case, because microglia are believed to be very long-lived, glial pathology has the capacity to permanently alter neural function and behavior, perhaps over the entire lifespan.
Early-Life Infection Alters Cognitive Aging
Aging is considered a glial priming event (Barrientos et al., 2006; Frank et al., 2006; Godbout and Johnson, 2009; Bilbo, 2010; Lynch, 2010). Aged rodents exhibit increased basal glial activation marker expression (CD11b, MHC II) compared to young animals. However, aged rodents do not necessarily exhibit cognitive dysfunction when compared to young animals. In contrast, peripheral infection or inflammatory challenge in aged (but not young) rodents leads to cognitive impairments (Barrientos et al.; Buchanan et al., 2008; Chen et al., 2008; Godbout et al., 2008; Rosczyk et al., 2008). These data have parallels to the clinical literature, in which systemic infection or surgery can precipitate a sharp decline in function in presumably healthy elderly populations (Perry et al., 2007; Perry et al., 2010). These data, together with our own observations, led to the prediction that early-life infection may result in less successful aging via an impact on glia. To test this possibility, rats were treated as before on P4 with PBS or E. coli, and then tested for learning and memory at 2 or 16 months of age. Notably, these memory tests were performed independent of an LPS challenge, as we hypothesized that age itself would be a “second hit”, akin to LPS in young adult rats. Consistent with this idea, neonatally-infected rats exhibited memory impairments in both a fear-conditioning task and a water maze task, but only at 16 month. Thus, neonatal infection alone did not impair memory in young rats, consistent with our previous findings, yet produced a cognitive deficit with age. Moreover, neonatally-infected rats exhibited greater aging-induced increases in glial markers (CD11b and MHC II on microglia, and GFAP on astrocytes) within the hippocampus (Bilbo, 2010). Taken together, these data indicate that early-life infection leads to less successful cognitive aging, which may be due to exaggerated increases in glial reactivity.
Potential Mechanisms of Glial Priming
Innate Immune Memory
The mechanisms underlying long-term priming remain largely unknown. In vitro studies using enriched populations of microglia suggest that the prior experience of microglia can dictate the response to a subsequent insult, indicating an innate immune memory capacity (Schwartz et al., 2006). For instance, the treatment of pure cultures of microglia in vitro with IL-4/IL-13 vs. interferon (IFN)/LPS elicit two distinct phenotypes (based on surface antigen expression and pro-inflammatory mediator production) that are theoretically associated in vivo with pro-inflammation or repair, respectively (Gensel et al., 2009). These are known as “classical”/M1 vs. “alternative”/M2 phenotypes, respectively, similar to tissue macrophages (Martinez et al., 2008). Importantly, the phenotypic class adopted may depend on prior inflammatory experience. For instance, pre-incubation of microglia with IL-4 elicits a partially neuroprotective (M2) phenotype in response to subsequent LPS, which is typically M1-inducing (Butovsky et al., 2005). Such in vitro data are intriguing given in vivo evidence in murine models of AD of a so-called “acquired deactivation” that appears functionally mid-way between M1 and M2 phenotypes (Colton and Wilcock, 2010). What remains unknown is the duration of these phenotypic changes, and whether previously activated microglia maintain the same dynamic capacity to alter their phenotype as naïve cells (reviewed in (Schwartz et al., 2006). In the case of AD, it appears that deactivated microglia have lost their plasticity in this regard, which contributes to pathology. Importantly, our data suggest that microglial phenotype can be set during an early sensitive period, resulting in a similar loss of plasticity that endures into adulthood, and is then exacerbated with increasing age.
Glial-Neuronal Interactions
In vivo, microglia appear to function within an “activation continuum” that is determined by multiple intrinsic and extrinsic signals within the microenvironment (Colton and Wilcock, 2009). For instance, microglial reactivity is strongly modulated by nearby neurons and their secreted immunoregulatory factors. CD200 is a neuronal membrane protein that maintains microglia in a quiescent state via direct cell-cell contact with its receptor, CD200R (on all myeloid cells including microglia (Barclay et al., 2002)). Similarly, fractalkine (CX3CL1) is a chemokine expressed by neurons that influences microglia via binding to its receptor, CX3CR1, on microglia (Harrison et al., 1998). A reduction in either of these factors at the ligand or receptor level is associated with neuroinflammation (Lue et al., 2010; Lynch, 2010). Thus, the increase in glial activation with age may be driven in large part by a loss of neuronal inhibition by CD200 and fractalkine (Jurgens and Johnson, 2010).
Very little, if anything, is known about the role of neuronal factors including CD200 and fractalkine in brain development, or about the conditions necessary for acute or chronic disruption of these factors. However, any insult that alters long-term neuronal structure or function, even independent of changes in number, may have the capacity to markedly alter glial reactivity throughout the brain, and thus cognitive function, via an impact on neuronal-glial crosstalk. For instance, a model of neonatal seizures in mice induces long-term changes in dendritic complexity within the DG in adulthood (Pugh et al., 2011). Though the authors did not explore microglial function in this study, one may hypothesize that this could impact glial reactivity due to decreased neuronal CD200 and therefore a de-inhibition of glia.
Glial-T cell Interactions
Microglia may be the primary antigen-presenting cells (APC) within the CNS; upon activation they upregulate the co-stimulatory molecules CD40, CD80, and CD86, which equip them for antigen presentation to T cells (Becher et al., 2006). Indeed, T cells can and regularly do enter the CNS where they interact with microglia. Importantly, T cells are not only active within the CNS in disease; as mentioned previously, CNS-autoreactive T cells appear to be important for normal adult neurogenesis and learning in mice, and this depends on interactions with microglia (Ziv et al., 2006). However, a common characteristic of many neuroinflammatory disorders is a combination of lymphocyte infiltration and microglial activation. What remains less clear are the circumstances under which glia initiate disease as opposed to peripheral immune cells, such as T cells. In experimental autoimmune encephalitis (EAE), an animal model of multiple sclerosis, encephalogenic T cells migrate across the blood brain barrier and enter the parenchyma, where they are widely believed to promote disease, in part via the activation of microglia (Murphy et al., 2010). Similarly, amyotrophic lateral sclerosis (ALS; a.k.a. Lou Gehrig‘s Disease) is characterized by infiltrating T cells and the progressive destruction of motoneurons, most notably in the spinal cord. In contrast to EAE, however, T cells may be playing a protective role. For instance, in studies using transgenic mice expressing an ALS-associated Cu2+/Zn2+ superoxide dismutase, the SOD1G93A mouse, T cell deficiency accelerates disease progression, whereas the passive transfer of CD4+ T cells slows deterioration and prolongs survival (Banerjee et al., 2008).
Importantly, there is increasing evidence that microglial-T cell interactions may be key to determining disease promotion vs. protection; for instance, Th1 cytokines promote M1 microglia and Th2 or Th17/Treg cytokines promote M2 microglia in a model of ALS (Chiu et al., 2008). It is possible that microglia may “instruct” T cell function within the brain in the opposite direction as well; if true, then long-term changes in microglial function/priming may carry consequences for the adaptive immune response as well. Virtually nothing is known about the potential role of lymphocytes in the type of fetal programming of innate immunity that we have described in this review. The thymus rapidly develops for 3–4 weeks after birth in rodents, and thymectomy during this time (and during the first postnatal week in humans) profoundly suppresses immunity throughout life (Murphy et al., 2008; Sauce et al., 2009). Thus, the capacity exists for an immune challenge during the perinatal period to impact thymic development or selection, potentially long-term, and therefore microglial reactivity indirectly via an impact on T cell function. This possibility certainly warrants future research, particularly as the manipulation of a peripheral lymphocyte population with the capacity to impact brain function is an attractive therapeutic target.
Conclusions
Taken together, immune factors are critical for normal brain function, but are increasingly implicated in brain pathology. The role of microglia in neurodegenerative and cognitive disorders has come under intense investigation in recent years. However, deciphering the cause of pathology, and whether microglia are bystanders vs. instigators in the midst of neuronal dysfunction and inflammation (e.g., in disorders such as AD) has proven very difficult. We propose that a consideration of the developmental history of the individual may be key to understanding functional changes in microglia and their role in brain and behavior.
Though we have focused in this review on the impact of early-life infection and a case for glial priming, we believe the important role of immune molecules in brain development has implications for a wide number of insults that activate the immune system either directly or indirectly, and thereby exert enduring effects on neural function. Increasing evidence suggests the function of some TLRs, including TLR2 and 4, extends beyond that of pathogen associated molecular pattern (PAMP) recognition (e.g., LPS) to “danger” associated molecular patterns (DAMPs) more broadly (Matzinger, 2002). DAMPs include endogenous “alarmins” that are released in response to cellular or tissue distress or damage (Bianchi, 2007). There are many putative alarmins, including IL-1α, hyaluronan, HMGB1, and heat shock proteins (see (Bianchi, 2007) for review). Interestingly, HMGB1 is important for TLR4-dependent changes in HP LTP and cognitive dysfunction following peripheral inflammatory challenge (Costello et al., 2011).
Moreover, there is growing evidence that diverse environmental stimuli can trigger TLR signaling, either directly or indirectly via an alarmin pathway, including toxins such as diesel exhaust (Inoue et al., 2006b; Inoue et al., 2006a), drugs of abuse such as morphine (Hutchinson et al., 2010), and even dietary fatty acids (Schaeffler et al., 2009). If such factors are present during development (or in the case of fatty acids, in abnormal concentrations), they may have the capacity to program neuroimmune function long-term, similar to infection, via this common TLR mechanism. Indeed, we have recently demonstrated that TLR4 is upregulated on microglia within the newborn brains of rats as a consequence of their mothers‘ high fat diet, and that they exhibit exaggerated neuroinflammation to peripheral LPS as adults (Bilbo and Tsang, 2010).
In closing, the capacity of resident CNS immune cells to recognize and respond to a wide array of endogenous and exogenous signals may have profound implications for the environmental origins of developmental programming within the immune system, and thus the brain and behavior. Importantly, the recognition that immune molecules are necessary for neural development and life-long function provides a plethora of novel targets within the immune system, and has recently (Wang et al., 2007) and should continue to lead to innovative and effective treatments for a wide number of neural disorders.
Acknowledgments
Supported by NIH R01 MH083698 and R01 DA025978 to S.D.B.
Footnotes
No disclaimers or disclosures
References
- Abraham WC, Williams JM. Properties and mechanisms of LTP maintenance. Neuroscientist. 2003;9:463–474. doi: 10.1177/1073858403259119. [DOI] [PubMed] [Google Scholar]
- Adler MW, Geller EB, Chen X, Rogers TJ. Viewing chemokines as a third major system of communication in the brain. AAPS J. 2005;7:E865–E870. doi: 10.1208/aapsj070484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akira S, Takeda K. Toll-like receptor signalling. Nat Rev Immunol. 2004;4:499–511. doi: 10.1038/nri1391. [DOI] [PubMed] [Google Scholar]
- Anderson KV, Jurgens G, Nusslein-Volhard C. Establishment of dorsal-ventral polarity in the Drosophila embryo: genetic studies on the role of the Toll gene product. Cell. 1985;42:779–789. doi: 10.1016/0092-8674(85)90274-0. [DOI] [PubMed] [Google Scholar]
- Araque A, Parpura V, Sanzgiri RP, Haydon PG. Tripartite synapses: glia, the unacknowledged partner. Trends Neurosci. 1999;22:208–215. doi: 10.1016/s0166-2236(98)01349-6. [DOI] [PubMed] [Google Scholar]
- Aylward GP. Neurodevelopmental outcomes of infants born prematurely. J Dev Behav Pediatr. 2005;26:427–440. doi: 10.1097/00004703-200512000-00008. [DOI] [PubMed] [Google Scholar]
- Banerjee R, Mosley RL, Reynolds AD, Dhar A, Jackson-Lewis V, Gordon PH, Przedborski S, Gendelman HE. Adaptive immune neuroprotection in G93A-SOD1 amyotrophic lateral sclerosis mice. PLoS One. 2008;3:e2740. doi: 10.1371/journal.pone.0002740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barclay AN, Wright GJ, Brooke G, Brown MH. CD200 and membrane protein interactions in the control of myeloid cells. Trends Immunol. 2002;23:285–290. doi: 10.1016/s1471-4906(02)02223-8. [DOI] [PubMed] [Google Scholar]
- Barrientos RM, Frank MG, Watkins LR, Maier SF. Memory impairments in healthy aging: Role of aging-induced microglial sensitization. Aging Dis. 1:212–231. [PMC free article] [PubMed] [Google Scholar]
- Barrientos RM, Higgins EA, Sprunger DB, Watkins LR, Rudy JW, Maier SF. Memory for context is impaired by a post context exposure injection of interleukin-1 beta into dorsal hippocampus. Behav Brain Res. 2002;134:291–298. doi: 10.1016/s0166-4328(02)00043-8. [DOI] [PubMed] [Google Scholar]
- Barrientos RM, Sprunger DB, Campeau S, Watkins LR, Rudy JW, Maier SF. BDNF mRNA expression in rat hippocampus following contextual learning is blocked by intrahippocampal IL-1beta administration. J Neuroimmunol. 2004;155:119–126. doi: 10.1016/j.jneuroim.2004.06.009. [DOI] [PubMed] [Google Scholar]
- Barrientos RM, Higgins EA, Biedenkapp JC, Sprunger DB, Wright-Hardesty KJ, Watkins LR, Rudy JW, Maier SF. Peripheral infection and aging interact to impair hippocampal memory consolidation. Neurobiology of aging. 2006;27:723–732. doi: 10.1016/j.neurobiolaging.2005.03.010. [DOI] [PubMed] [Google Scholar]
- Beattie EC, Stellwagen D, Morishita W, Bresnahan JC, Ha BK, Von Zastrow M, Beattie MS, Malenka RC. Control of synaptic strength by glial TNFalpha. Science. 2002;295:2282–2285. doi: 10.1126/science.1067859. [DOI] [PubMed] [Google Scholar]
- Becher B, Bechmann I, Greter M. Antigen presentation in autoimmunity and CNS inflammation: how T lymphocytes recognize the brain. J Mol Med. 2006;84:532–543. doi: 10.1007/s00109-006-0065-1. [DOI] [PubMed] [Google Scholar]
- Bell MJ, Hallenbeck JM. Effects of intrauterine inflammation on developing rat brain. J Neurosci Res. 2002;70:570–579. doi: 10.1002/jnr.10423. [DOI] [PubMed] [Google Scholar]
- Ben Menachem-Zidon O, Avital A, Ben-Menahem Y, Goshen I, Kreisel T, Shmueli EM, Segal M, Ben Hur T, Yirmiya R. Astrocytes support hippocampal-dependent memory and long-term potentiation via interleukin-1 signaling. Brain Behav Immun. 2011;25:1008–1016. doi: 10.1016/j.bbi.2010.11.007. [DOI] [PubMed] [Google Scholar]
- Bennet L, Gunn A. The Fetal Origins of Adult Mental Illness. In: Wintour-Coghlan M, Owens J, editors. Early Life Origins of Health and Disease (Advances in Experimental Medicine and Biology. New York: Springer; 2006. pp. 204–211. [Google Scholar]
- Bianchi ME. DAMPs, PAMPs and alarmins: all we need to know about danger. J Leukoc Biol. 2007;81:1–5. doi: 10.1189/jlb.0306164. [DOI] [PubMed] [Google Scholar]
- Bilbo SD. Early-life infection is a vulnerability factor for aging-related glial alterations and cognitive decline. Neurobiol Learn Mem. 2010;94:57–64. doi: 10.1016/j.nlm.2010.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bilbo SD, Schwarz JM. Early-life programming of later-life brain and behavior: a critical role for the immune system. Front Behav Neurosci. 2009;3:14. doi: 10.3389/neuro.08.014.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bilbo SD, Tsang V. Enduring consequences of maternal obesity for brain inflammation and behavior of offspring. FASEB J. 2010;24:2104–2115. doi: 10.1096/fj.09-144014. [DOI] [PubMed] [Google Scholar]
- Bilbo SD, Rudy JW, Watkins LR, Maier SF. A behavioural characterization of neonatal infection-facilitated memory impairment in adult rats. Behav Brain Res. 2006;169:39–47. doi: 10.1016/j.bbr.2005.12.002. [DOI] [PubMed] [Google Scholar]
- Bilbo SD, Biedenkapp JC, Der-Avakian A, Watkins LR, Rudy JW, Maier SF. Neonatal infection-induced memory impairment after lipopolysaccharide in adulthood is prevented via caspase-1 inhibition. J Neurosci. 2005a;25:8000–8009. doi: 10.1523/JNEUROSCI.1748-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bilbo SD, Levkoff LH, Mahoney JH, Watkins LR, Rudy JW, Maier SF. Neonatal infection induces memory impairments following an immune challenge in adulthood. Behav Neurosci. 2005b;119:293–301. doi: 10.1037/0735-7044.119.1.293. [DOI] [PubMed] [Google Scholar]
- Bilbo SD, Newsum NJ, Sprunger DB, Watkins LR, Rudy JW, Maier SF. Differential effects of neonatal handling on early life infection-induced alterations in cognition in adulthood. Brain Behav Immun. 2007;21:332–342. doi: 10.1016/j.bbi.2006.10.005. [DOI] [PubMed] [Google Scholar]
- Bilbo SD, Barrientos RM, Eads AS, Northcutt A, Watkins LR, Rudy JW, Maier SF. Early-life infection leads to altered BDNF and IL-1beta mRNA expression in rat hippocampus following learning in adulthood. Brain Behav Immun. 2008;22:451–455. doi: 10.1016/j.bbi.2007.10.003. [DOI] [PubMed] [Google Scholar]
- Bland ST, Beckley JT, Young S, Tsang V, Watkins LR, Maier SF, Bilbo SD. Enduring consequences of early-life infection on glial and neural cell genesis within cognitive regions of the brain. Brain Behav Immun. 2010;24:329–338. doi: 10.1016/j.bbi.2009.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchanan JB, Sparkman NL, Chen J, Johnson RW. Cognitive and neuroinflammatory consequences of mild repeated stress are exacerbated in aged mice. Psychoneuroendocrinology. 2008;33:755–765. doi: 10.1016/j.psyneuen.2008.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butovsky O, Talpalar AE, Ben-Yaakov K, Schwartz M. Activation of microglia by aggregated beta-amyloid or lipopolysaccharide impairs MHC-II expression and renders them cytotoxic whereas IFN-gamma and IL-4 render them protective. Mol Cell Neurosci. 2005;29:381–393. doi: 10.1016/j.mcn.2005.03.005. [DOI] [PubMed] [Google Scholar]
- Cai Z, Pan ZL, Pang Y, Evans OB, Rhodes PG. Cytokine induction in fetal rat brains and brain injury in neonatal rats after maternal lipopolysaccharide administration. Pediatr Res. 2000;47:64–72. doi: 10.1203/00006450-200001000-00013. [DOI] [PubMed] [Google Scholar]
- Carpentier PA, Palmer TD. Immune influence on adult neural stem cell regulation and function. Neuron. 2009;64:79–92. doi: 10.1016/j.neuron.2009.08.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J, Buchanan JB, Sparkman NL, Godbout JP, Freund GG, Johnson RW. Neuroinflammation and disruption in working memory in aged mice after acute stimulation of the peripheral innate immune system. Brain Behav Immun. 2008;22:301–311. doi: 10.1016/j.bbi.2007.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiu IM, Chen A, Zheng Y, Kosaras B, Tsiftsoglou SA, Vartanian TK, Brown RH, Jr, Carroll MC. T lymphocytes potentiate endogenous neuroprotective inflammation in a mouse model of ALS. Proc Natl Acad Sci U S A. 2008;105:17913–17918. doi: 10.1073/pnas.0804610105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christopherson KS, Ullian EM, Stokes CC, Mullowney CE, Hell JW, Agah A, Lawler J, Mosher DF, Bornstein P, Barres BA. Thrombospondins are astrocyte-secreted proteins that promote CNS synaptogenesis. Cell. 2005;120:421–433. doi: 10.1016/j.cell.2004.12.020. [DOI] [PubMed] [Google Scholar]
- Colton CA, Wilcock DM. Assessing Activation States in Microglia. CNS Neurol Disord Drug Targets. 2009 doi: 10.2174/187152710791012053. [DOI] [PubMed] [Google Scholar]
- Colton CA, Wilcock DM. Assessing activation states in microglia. CNS Neurol Disord Drug Targets. 2010;9:174–191. doi: 10.2174/187152710791012053. [DOI] [PubMed] [Google Scholar]
- Corriveau RA, Huh GS, Shatz CJ. Regulation of class I MHC gene expression in the developing and mature CNS by neural activity. Neuron. 1998;21:505–520. doi: 10.1016/s0896-6273(00)80562-0. [DOI] [PubMed] [Google Scholar]
- Costello DA, Watson MB, Cowley TR, Murphy N, Murphy Royal C, Garlanda C, Lynch MA. Interleukin-1alpha and HMGB1 mediate hippocampal dysfunction in SIGIRR-deficient mice. J Neurosci. 2011;31:3871–3879. doi: 10.1523/JNEUROSCI.6676-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui K, Ashdown H, Luheshi GN, Boksa P. Effects of prenatal immune activation on hippocampal neurogenesis in the rat. Schizophr Res. 2009;113:288–297. doi: 10.1016/j.schres.2009.05.003. [DOI] [PubMed] [Google Scholar]
- Dantzer R, Kelley KW. Twenty years of research on cytokine-induced sickness behavior. Brain Behav Immun. 2007;21:153–160. doi: 10.1016/j.bbi.2006.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derecki NC, Cardani AN, Yang CH, Quinnies KM, Crihfield A, Lynch KR, Kipnis J. Regulation of learning and memory by meningeal immunity: a key role for IL-4. J Exp Med. 2010;207:1067–1080. doi: 10.1084/jem.20091419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deverman BE, Patterson PH. Cytokines and CNS development. Neuron. 2009;64:61–78. doi: 10.1016/j.neuron.2009.09.002. [DOI] [PubMed] [Google Scholar]
- Dziegielewska KM, Moller JE, Potter AM, Ek J, Lane MA, Saunders NR. Acute-phase cytokines IL-1beta and TNF-alpha in brain development. Cell Tissue Res. 2000;299:335–345. doi: 10.1007/s004419900157. [DOI] [PubMed] [Google Scholar]
- Eriksen W, Sundet JM, Tambs K. Register data suggest lower intelligence in men born the year after flu pandemic. Ann Neurol. 2009;66:284–289. doi: 10.1002/ana.21702. [DOI] [PubMed] [Google Scholar]
- Eroglu C, Barres BA. Regulation of synaptic connectivity by glia. Nature. 2010;468:223–231. doi: 10.1038/nature09612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eroglu C, Allen NJ, Susman MW, O'Rourke NA, Park CY, Ozkan E, Chakraborty C, Mulinyawe SB, Annis DS, Huberman AD, Green EM, Lawler J, Dolmetsch R, Garcia KC, Smith SJ, Luo ZD, Rosenthal A, Mosher DF, Barres BA. Gabapentin receptor alpha2delta-1 is a neuronal thrombospondin receptor responsible for excitatory CNS synaptogenesis. Cell. 2009;139:380–392. doi: 10.1016/j.cell.2009.09.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan LW, Tien LT, Mitchell HJ, Rhodes PG, Cai Z. Alpha-phenyl-n-tert-butyl-nitrone ameliorates hippocampal injury and improves learning and memory in juvenile rats following neonatal exposure to lipopolysaccharide. Eur J Neurosci. 2008;27:1475–1484. doi: 10.1111/j.1460-9568.2008.06121.x. [DOI] [PubMed] [Google Scholar]
- Fan LW, Tien LT, Zheng B, Pang Y, Rhodes PG, Cai Z. Interleukin-1beta-induced brain injury and neurobehavioral dysfunctions in juvenile rats can be attenuated by alpha-phenyl-n-tert-butyl-nitrone. Neuroscience. 2010;168:240–252. doi: 10.1016/j.neuroscience.2010.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fee D, Grzybicki D, Dobbs M, Ihyer S, Clotfelter J, Macvilay S, Hart MN, Sandor M, Fabry Z. Interleukin 6 promotes vasculogenesis of murine brain microvessel endothelial cells. Cytokine. 2000;12:655–665. doi: 10.1006/cyto.1999.0599. [DOI] [PubMed] [Google Scholar]
- Fortier ME, Luheshi GN, Boksa P. Effects of prenatal infection on prepulse inhibition in the rat depend on the nature of the infectious agent and the stage of pregnancy. Behav Brain Res. 2007;181:270–277. doi: 10.1016/j.bbr.2007.04.016. [DOI] [PubMed] [Google Scholar]
- Frank MG, Barrientos RM, Watkins LR, Maier SF. Aging sensitizes rapidly isolated hippocampal microglia to LPS ex vivo. J Neuroimmunol. 226:181–184. doi: 10.1016/j.jneuroim.2010.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank MG, Barrientos RM, Biedenkapp JC, Rudy JW, Watkins LR, Maier SF. mRNA upregulation of MHC II and pivotal pro-inflammatory genes in normal brain aging. Neurobiology of aging. 2006;27:717–722. doi: 10.1016/j.neurobiolaging.2005.03.013. [DOI] [PubMed] [Google Scholar]
- Gadient RA, Otten U. Expression of interleukin-6 (IL-6) and interleukin-6 receptor (IL-6R) mRNAs in rat brain during postnatal development. Brain Res. 1994;637:10–14. doi: 10.1016/0006-8993(94)91211-4. [DOI] [PubMed] [Google Scholar]
- Gallo P, Frei K, Rordorf C, Lazdins J, Tavolato B, Fontana A. Human immunodeficiency virus type 1 (HIV-1) infection of the central nervous system: an evaluation of cytokines in cerebrospinal fluid. J Neuroimmunol. 1989;23:109–116. doi: 10.1016/0165-5728(89)90029-5. [DOI] [PubMed] [Google Scholar]
- Garay PA, McAllister AK. Novel roles for immune molecules in neural development: implications for neurodevelopmental disorders. Front Synaptic Neurosci. 2010;2:136. doi: 10.3389/fnsyn.2010.00136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia O, Torres M, Helguera P, Coskun P, Busciglio J. A role for thrombospondin-1 deficits in astrocyte-mediated spine and synaptic pathology in Down's syndrome. PLoS One. 2010;5:e14200. doi: 10.1371/journal.pone.0014200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garden GA, Moller T. Microglia biology in health and disease. J Neuroimmune Pharmacol. 2006;1:127–137. doi: 10.1007/s11481-006-9015-5. [DOI] [PubMed] [Google Scholar]
- Gensel JC, Nakamura S, Guan Z, van Rooijen N, Ankeny DP, Popovich PG. Macrophages promote axon regeneration with concurrent neurotoxicity. J Neurosci. 2009;29:3956–3968. doi: 10.1523/JNEUROSCI.3992-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ginhoux F, Greter M, Leboeuf M, Nandi S, See P, Gokhan S, Mehler MF, Conway SJ, Ng LG, Stanley ER, Samokhvalov IM, Merad M. Fate mapping analysis reveals that adult microglia derive from primitive macrophages. Science. 2010;330:841–845. doi: 10.1126/science.1194637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giulian D, Young DG, Woodward J, Brown DC, Lachman LB. Interleukin-1 is an astroglial growth factor in the developing brain. J Neurosci. 1988;8:709–714. doi: 10.1523/JNEUROSCI.08-02-00709.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godbout JP, Johnson RW. Age and neuroinflammation: a lifetime of psychoneuroimmune consequences. Immunol Allergy Clin North Am. 2009;29:321–337. doi: 10.1016/j.iac.2009.02.007. [DOI] [PubMed] [Google Scholar]
- Godbout JP, Moreau M, Lestage J, Chen J, Sparkman NL, J OC, Castanon N, Kelley KW, Dantzer R, Johnson RW. Aging exacerbates depressive-like behavior in mice in response to activation of the peripheral innate immune system. Neuropsychopharmacology. 2008;33:2341–2351. doi: 10.1038/sj.npp.1301649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golan HM, Lev V, Hallak M, Sorokin Y, Huleihel M. Specific neurodevelopmental damage in mice offspring following maternal inflammation during pregnancy. Neuropharmacology. 2005;48:903–917. doi: 10.1016/j.neuropharm.2004.12.023. [DOI] [PubMed] [Google Scholar]
- Gomez-Gonzalez B, Escobar A. Prenatal stress alters microglial development and distribution in postnatal rat brain. Acta Neuropathol. 2010;119:303–315. doi: 10.1007/s00401-009-0590-4. [DOI] [PubMed] [Google Scholar]
- Goshen I, Kreisel T, Ounallah-Saad H, Renbaum P, Zalzstein Y, Ben-Hur T, Levy-Lahad E, Yirmiya R. A dual role for interleukin-1 in hippocampal-dependent memory processes. Psychoneuroendocrinology. 2007;32:1106–1115. doi: 10.1016/j.psyneuen.2007.09.004. [DOI] [PubMed] [Google Scholar]
- Greene MF, Creasy RK, Resnik R, Iams JD, Lockwood CJ, Moore T. Creasy and Resnik's Maternal-Fetal Medicine: Principles and Practice. 6th Edition. Philadelphia: Saunders; 2008. [Google Scholar]
- Griffin WS, Stanley LC, Ling C, White L, MacLeod V, Perrot LJ, White CL, 3rd, Araoz C. Brain interleukin 1 and S-100 immunoreactivity are elevated in Down syndrome and Alzheimer disease. Proc Natl Acad Sci U S A. 1989;86:7611–7615. doi: 10.1073/pnas.86.19.7611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hao LY, Hao XQ, Li SH, Li XH. Prenatal exposure to lipopolysaccharide results in cognitive deficits in age-increasing offspring rats. Neuroscience. 2010;166:763–770. doi: 10.1016/j.neuroscience.2010.01.006. [DOI] [PubMed] [Google Scholar]
- Harre EM, Galic MA, Mouihate A, Noorbakhsh F, Pittman QJ. Neonatal inflammation produces selective behavioural deficits and alters N-methyl-D-aspartate receptor subunit mRNA in the adult rat brain. Eur J Neurosci. 2008;27:644–653. doi: 10.1111/j.1460-9568.2008.06031.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison JK, Jiang Y, Chen S, Xia Y, Maciejewski D, McNamara RK, Streit WJ, Salafranca MN, Adhikari S, Thompson DA, Botti P, Bacon KB, Feng L. Role for neuronally derived fractalkine in mediating interactions between neurons and CX3CR1-expressing microglia. Proc Natl Acad Sci U S A. 1998;95:10896–10901. doi: 10.1073/pnas.95.18.10896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haydon PG, Carmignoto G. Astrocyte control of synaptic transmission and neurovascular coupling. Physiol Rev. 2006;86:1009–1031. doi: 10.1152/physrev.00049.2005. [DOI] [PubMed] [Google Scholar]
- Henneberger C, Papouin T, Oliet SH, Rusakov DA. Long-term potentiation depends on release of D-serine from astrocytes. Nature. 2010;463:232–236. doi: 10.1038/nature08673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henry CJ, Huang Y, Wynne AM, Godbout JP. Peripheral lipopolysaccharide (LPS) challenge promotes microglial hyperactivity in aged mice that is associated with exaggerated induction of both pro-inflammatory IL-1beta and anti-inflammatory IL-10 cytokines. Brain Behav Immun. 2009;23:309–317. doi: 10.1016/j.bbi.2008.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hornig M, Lipkin WI. Infectious and immune factors in the pathogenesis of neurodevelopmental disorders: epidemiology, hypotheses, and animal models. Ment Retard Dev Disabil Res Rev. 2001;7:200–210. doi: 10.1002/mrdd.1028. [DOI] [PubMed] [Google Scholar]
- Hornig M, Weissenbock H, Horscroft N, Lipkin WI. An infection-based model of neurodevelopmental damage. Proc Natl Acad Sci U S A. 1999;96:12102–12107. doi: 10.1073/pnas.96.21.12102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hornig M, Solbrig M, Horscroft N, Weissenbock H, Lipkin WI. Borna disease virus infection of adult and neonatal rats: models for neuropsychiatric disease. Curr Top Microbiol Immunol. 2001;253:157–177. doi: 10.1007/978-3-662-10356-2_8. [DOI] [PubMed] [Google Scholar]
- Hsiao EY, Patterson PH. Activation of the maternal immune system induces endocrine changes in the placenta via IL-6. Brain Behav Immun. 2011;25:604–615. doi: 10.1016/j.bbi.2010.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Husband AJ. The immune system and integrated homeostasis. Immunology and cell biology. 1995;73:377–382. doi: 10.1038/icb.1995.58. [DOI] [PubMed] [Google Scholar]
- Hutchinson MR, et al. Evidence that opioids may have toll-like receptor 4 and MD-2 effects. Brain Behav Immun. 2010;24:83–95. doi: 10.1016/j.bbi.2009.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inoue K, Takano H, Yanagisawa R, Hirano S, Kobayashi T, Ichinose T, Yoshikawa T. Effects of organic chemicals derived from ambient particulate matter on lung inflammation related to lipopolysaccharide. Arch Toxicol. 2006a;80:833–838. doi: 10.1007/s00204-006-0105-1. [DOI] [PubMed] [Google Scholar]
- Inoue K, Takano H, Yanagisawa R, Hirano S, Ichinose T, Shimada A, Yoshikawa T. The role of toll-like receptor 4 in airway inflammation induced by diesel exhaust particles. Arch Toxicol. 2006b;80:275–279. doi: 10.1007/s00204-005-0040-6. [DOI] [PubMed] [Google Scholar]
- Isaacs EB, Lucas A, Chong WK, Wood SJ, Johnson CL, Marshall C, Vargha-Khadem F, Gadian DG. Hippocampal volume and everyday memory in children of very low birth weight. Pediatr Res. 2000;47:713–720. doi: 10.1203/00006450-200006000-00006. [DOI] [PubMed] [Google Scholar]
- Ito HT, Smith SE, Hsiao E, Patterson PH. Maternal immune activation alters nonspatial information processing in the hippocampus of the adult offspring. Brain Behav Immun. 2010;24:930–941. doi: 10.1016/j.bbi.2010.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jakubs K, Bonde S, Iosif RE, Ekdahl CT, Kokaia Z, Kokaia M, Lindvall O. Inflammation regulates functional integration of neurons born in adult brain. J Neurosci. 2008;28:12477–12488. doi: 10.1523/JNEUROSCI.3240-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson WJ, Marino PA, Schreiber RD, Adams DO. Sequential activation of murine mononuclear phagocytes for tumor cytolysis: differential expression of markers by macrophages in the several stages of development. J Immunol. 1983;131:1038–1043. [PubMed] [Google Scholar]
- Jurgens HA, Johnson RW. Dysregulated neuronal-microglial cross-talk during aging, stress and inflammation. Exp Neurol. 2010 doi: 10.1016/j.expneurol.2010.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohman RA, Tarr AJ, Sparkman NL, Bogale TM, Boehm GW. Neonatal endotoxin exposure impairs avoidance learning and attenuates endotoxin-induced sickness behavior and central IL-1beta gene transcription in adulthood. Behav Brain Res. 2008;194:25–31. doi: 10.1016/j.bbr.2008.06.018. [DOI] [PubMed] [Google Scholar]
- Lieberam I, Agalliu D, Nagasawa T, Ericson J, Jessell TM. A Cxcl12-CXCR4 chemokine signaling pathway defines the initial trajectory of mammalian motor axons. Neuron. 2005;47:667–679. doi: 10.1016/j.neuron.2005.08.011. [DOI] [PubMed] [Google Scholar]
- Lowe GC, Luheshi GN, Williams S. Maternal infection and fever during late gestation are associated with altered synaptic transmission in the hippocampus of juvenile offspring rats. Am J Physiol Regul Integr Comp Physiol. 2008;295:R1563–R1571. doi: 10.1152/ajpregu.90350.2008. [DOI] [PubMed] [Google Scholar]
- Lu M, Grove EA, Miller RJ. Abnormal development of the hippocampal dentate gyrus in mice lacking the CXCR4 chemokine receptor. Proc Natl Acad Sci U S A. 2002;99:7090–7095. doi: 10.1073/pnas.092013799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lue LF, Kuo YM, Beach T, Walker DG. Microglia activation and anti-inflammatory regulation in Alzheimer's disease. Mol Neurobiol. 2010;41:115–128. doi: 10.1007/s12035-010-8106-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch MA. Age-related neuroinflammatory changes negatively impact on neuronal function. Front Aging Neurosci. 2010 doi: 10.3389/neuro.24.006.2009. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maier SF, Watkins LR. Cytokines for psychologists: implications of bidirectional immune-to-brain communication for understanding behavior, mood, and cognition. Psychol Rev. 1998;105:83–107. doi: 10.1037/0033-295x.105.1.83. [DOI] [PubMed] [Google Scholar]
- Martinez FO, Sica A, Mantovani A, Locati M. Macrophage activation and polarization. Front Biosci. 2008;13:453–461. doi: 10.2741/2692. [DOI] [PubMed] [Google Scholar]
- Matzinger P. The danger model: a renewed sense of self. Science. 2002;296:301–305. doi: 10.1126/science.1071059. [DOI] [PubMed] [Google Scholar]
- McAfoose J, Baune BT. Evidence for a cytokine model of cognitive function. Neuroscience and biobehavioral reviews. 2009;33:355–366. doi: 10.1016/j.neubiorev.2008.10.005. [DOI] [PubMed] [Google Scholar]
- Merrill JE. Tumor necrosis factor alpha, interleukin 1 and related cytokines in brain development: normal and pathological. Dev Neurosci. 1992;14:1–10. doi: 10.1159/000111642. [DOI] [PubMed] [Google Scholar]
- Meyer U, Feldon J, Schedlowski M, Yee BK. Immunological stress at the maternal-foetal interface: a link between neurodevelopment and adult psychopathology. Brain Behav Immun. 2006;20:378–388. doi: 10.1016/j.bbi.2005.11.003. [DOI] [PubMed] [Google Scholar]
- Meyer U, Nyffeler M, Yee BK, Knuesel I, Feldon J. Adult brain and behavioral pathological markers of prenatal immune challenge during early/middle and late fetal development in mice. Brain Behav Immun. 2008;22:469–486. doi: 10.1016/j.bbi.2007.09.012. [DOI] [PubMed] [Google Scholar]
- Meyers CA. Neurocognitive dysfunction in cancer patients. Oncology (Huntingt) 2000;14:75–79. discussion 79, 81–72, 85. [PubMed] [Google Scholar]
- Mignini F, Streccioni V, Amenta F. Autonomic innervation of immune organs and neuroimmune modulation. Auton Autacoid Pharmacol. 2003;23:1–25. doi: 10.1046/j.1474-8673.2003.00280.x. [DOI] [PubMed] [Google Scholar]
- Miller LC, Isa S, LoPreste G, Schaller JG, Dinarello CA. Neonatal interleukin-1 beta, interleukin-6, and tumor necrosis factor: cord blood levels and cellular production. The Journal of pediatrics. 1990;117:961–965. doi: 10.1016/s0022-3476(05)80145-3. [DOI] [PubMed] [Google Scholar]
- Miller RJ, Rostene W, Apartis E, Banisadr G, Biber K, Milligan ED, White FA, Zhang J. Chemokine action in the nervous system. J Neurosci. 2008;28:11792–11795. doi: 10.1523/JNEUROSCI.3588-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Msall ME. Developmental vulnerability and resilience in extremely preterm infants. JAMA. 2004;292:2399–2401. doi: 10.1001/jama.292.19.2399. [DOI] [PubMed] [Google Scholar]
- Muller CM, Best J. Ocular dominance plasticity in adult cat visual cortex after transplantation of cultured astrocytes. Nature. 1989;342:427–430. doi: 10.1038/342427a0. [DOI] [PubMed] [Google Scholar]
- Murphy AC, Lalor SJ, Lynch MA, Mills KH. Infiltration of Th1 and Th17 cells and activation of microglia in the CNS during the course of experimental autoimmune encephalomyelitis. Brain Behav Immun. 2010;24:641–651. doi: 10.1016/j.bbi.2010.01.014. [DOI] [PubMed] [Google Scholar]
- Murphy K, Travers P, Walport M. Janeway's Immunobiology. Seventh Edition. New York: Garland Science; 2008. [Google Scholar]
- Nelson KB, Willoughby RE. Infection, inflammation and the risk of cerebral palsy. Curr Opin Neurol. 2000;13:133–139. doi: 10.1097/00019052-200004000-00004. [DOI] [PubMed] [Google Scholar]
- Nimmerjahn A, Kirchhoff F, Helmchen F. Resting microglial cells are highly dynamic surveillants of brain parenchyma in vivo. Science. 2005;308:1314–1318. doi: 10.1126/science.1110647. [DOI] [PubMed] [Google Scholar]
- Osrin D, Vergnano S, Costello A. Serious bacterial infections in newborn infants in developing countries. Current opinion in infectious diseases. 2004;17:217–224. doi: 10.1097/00001432-200406000-00008. [DOI] [PubMed] [Google Scholar]
- Ozawa K, Hashimoto K, Kishimoto T, Shimizu E, Ishikura H, Iyo M. Immune activation during pregnancy in mice leads to dopaminergic hyperfunction and cognitive impairment in the offspring: a neurodevelopmental animal model of schizophrenia. Biol Psychiatry. 2006;59:546–554. doi: 10.1016/j.biopsych.2005.07.031. [DOI] [PubMed] [Google Scholar]
- Pace JL, Russell SW, Torres BA, Johnson HM, Gray PW. Recombinant mouse gamma interferon induces the priming step in macrophage activation for tumor cell killing. J Immunol. 1983;130:2011–2013. [PubMed] [Google Scholar]
- Pang Y, Cai Z, Rhodes PG. Disturbance of oligodendrocyte development, hypomyelination and white matter injury in the neonatal rat brain after intracerebral injection of lipopolysaccharide. Brain Res Dev Brain Res. 2003;140:205–214. doi: 10.1016/s0165-3806(02)00606-5. [DOI] [PubMed] [Google Scholar]
- Perry VH, Newman TA, Cunningham C. The impact of systemic infection on the progression of neurodegenerative disease. Nat Rev Neurosci. 2003;4:103–112. doi: 10.1038/nrn1032. [DOI] [PubMed] [Google Scholar]
- Perry VH, Cunningham C, Holmes C. Systemic infections and inflammation affect chronic neurodegeneration. Nat Rev Immunol. 2007;7:161–167. doi: 10.1038/nri2015. [DOI] [PubMed] [Google Scholar]
- Perry VH, Nicoll JA, Holmes C. Microglia in neurodegenerative disease. Nat Rev Neurol. 2010;6:193–201. doi: 10.1038/nrneurol.2010.17. [DOI] [PubMed] [Google Scholar]
- Pocock JM, Kettenmann H. Neurotransmitter receptors on microglia. Trends Neurosci. 2007;30:527–535. doi: 10.1016/j.tins.2007.07.007. [DOI] [PubMed] [Google Scholar]
- Pugh CR, Nguyen KT, Gonyea JL, Fleshner M, Wakins LR, Maier SF, Rudy JW. Role of interleukin-1 beta in impairment of contextual fear conditioning caused by social isolation. Behav Brain Res. 1999;106:109–118. doi: 10.1016/s0166-4328(99)00098-4. [DOI] [PubMed] [Google Scholar]
- Pugh P, Adlaf E, Zhao CS, Markwardt S, Gavin C, Wadiche J, Overstreet-Wadiche L. Enhanced integration of newborn neurons after neonatal insults. Front Neurosci. 2011;5:45. doi: 10.3389/fnins.2011.00045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rakic S, Zecevic N. Programmed cell death in the developing human telencephalon. Eur J Neurosci. 2000;12:2721–2734. doi: 10.1046/j.1460-9568.2000.00153.x. [DOI] [PubMed] [Google Scholar]
- Rantakallio P, Jones P, Moring J, Von Wendt L. Association between central nervous system infections during childhood and adult onset schizophrenia and other psychoses: a 28-year follow-up. Int J Epidemiol. 1997;26:837–843. doi: 10.1093/ije/26.4.837. [DOI] [PubMed] [Google Scholar]
- Rezaie P, Male D. Colonisation of the developing human brain and spinal cord by microglia: a review. Microsc Res Tech. 1999;45:359–382. doi: 10.1002/(SICI)1097-0029(19990615)45:6<359::AID-JEMT4>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- Rezaie P, Male D. Mesoglia & microglia--a historical review of the concept of mononuclear phagocytes within the central nervous system. J Hist Neurosci. 2002;11:325–374. doi: 10.1076/jhin.11.4.325.8531. [DOI] [PubMed] [Google Scholar]
- Rice D, Barone S., Jr Critical periods of vulnerability for the developing nervous system: evidence from humans and animal models. Environ Health Perspect. 2000;108 Suppl 3:511–533. doi: 10.1289/ehp.00108s3511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson-Burns SM, Tyler KL. Regional differences in viral growth and central nervous system injury correlate with apoptosis. J Virol. 2004;78:5466–5475. doi: 10.1128/JVI.78.10.5466-5475.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rolls A, Shechter R, London A, Ziv Y, Ronen A, Levy R, Schwartz M. Toll-like receptors modulate adult hippocampal neurogenesis. Nat Cell Biol. 2007;9:1081–1088. doi: 10.1038/ncb1629. [DOI] [PubMed] [Google Scholar]
- Rosczyk HA, Sparkman NL, Johnson RW. Neuroinflammation and cognitive function in aged mice following minor surgery. Exp Gerontol. 2008;43:840–846. doi: 10.1016/j.exger.2008.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose SA, Feldman JF, Jankowski JJ. Recall memory in the first three years of life: a longitudinal study of preterm and term children. Dev Med Child Neurol. 2005a;47:653–659. doi: 10.1017/S0012162205001349. [DOI] [PubMed] [Google Scholar]
- Rose SA, Feldman JF, Jankowski JJ, Van Rossem R. Pathways from prematurity and infant abilities to later cognition. Child Dev. 2005b;76:1172–1184. doi: 10.1111/j.1467-8624.2005.00843.x. [DOI] [PubMed] [Google Scholar]
- Ross FM, Allan SM, Rothwell NJ, Verkhratsky A. A dual role for interleukin-1 in LTP in mouse hippocampal slices. J Neuroimmunol. 2003;144:61–67. doi: 10.1016/j.jneuroim.2003.08.030. [DOI] [PubMed] [Google Scholar]
- Santello M, Bezzi P, Volterra A. TNFalpha controls glutamatergic gliotransmission in the hippocampal dentate gyrus. Neuron. 2011;69:988–1001. doi: 10.1016/j.neuron.2011.02.003. [DOI] [PubMed] [Google Scholar]
- Sauce D, Larsen M, Fastenackels S, Duperrier A, Keller M, Grubeck-Loebenstein B, Ferrand C, Debre P, Sidi D, Appay V. Evidence of premature immune aging in patients thymectomized during early childhood. J Clin Invest. 2009;119:3070–3078. doi: 10.1172/JCI39269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaeffler A, Gross P, Buettner R, Bollheimer C, Buechler C, Neumeier M, Kopp A, Schoelmerich J, Falk W. Fatty acid-induced induction of Toll-like receptor-4/nuclear factor-kappaB pathway in adipocytes links nutritional signalling with innate immunity. Immunology. 2009;126:233–245. doi: 10.1111/j.1365-2567.2008.02892.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitz T, Chew LJ. Cytokines and myelination in the central nervous system. The Scientific World Journal. 2008;8:1119–1147. doi: 10.1100/tsw.2008.140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz M, Kipnis J. A conceptual revolution in the relationships between the brain and immunity. Brain Behav Immun. 2010 doi: 10.1016/j.bbi.2010.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz M, Butovsky O, Bruck W, Hanisch UK. Microglial phenotype: is the commitment reversible? Trends Neurosci. 2006;29:68–74. doi: 10.1016/j.tins.2005.12.005. [DOI] [PubMed] [Google Scholar]
- Schwarz JM, Bilbo SD. LPS elicits a much larger and broader inflammatory response than Escherichia coli infection within the hippocampus of neonatal rats. Neurosci Lett. 2011 doi: 10.1016/j.neulet.2011.04.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi L, Fatemi SH, Sidwell RW, Patterson PH. Maternal influenza infection causes marked behavioral and pharmacological changes in the offspring. J Neurosci. 2003;23:297–302. doi: 10.1523/JNEUROSCI.23-01-00297.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sierra A, Encinas JM, Deudero JJ, Chancey JH, Enikolopov G, Overstreet-Wadiche LS, Tsirka SE, Maletic-Savatic M. Microglia shape adult hippocampal neurogenesis through apoptosis-coupled phagocytosis. Cell Stem Cell. 2010;7:483–495. doi: 10.1016/j.stem.2010.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skogstrand K, Hougaard DM, Schendel DE, Bent NP, Svaerke C, Thorsen P. Association of preterm birth with sustained postnatal inflammatory response. Obstetrics and gynecology. 2008;111:1118–1128. doi: 10.1097/AOG.0b013e31817057fb. [DOI] [PubMed] [Google Scholar]
- Smith SE, Li J, Garbett K, Mirnics K, Patterson PH. Maternal immune activation alters fetal brain development through interleukin-6. J Neurosci. 2007;27:10695–10702. doi: 10.1523/JNEUROSCI.2178-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soumiya H, Fukumitsu H, Furukawa S. Prenatal immune challenge compromises development of upper-layer but not deeper-layer neurons of the mouse cerebral cortex. J Neurosci Res. 2011 doi: 10.1002/jnr.22636. [DOI] [PubMed] [Google Scholar]
- Spencer SJ, Heida JG, Pittman QJ. Early life immune challenge--effects on behavioural indices of adult rat fear and anxiety. Behav Brain Res. 2005;164:231–238. doi: 10.1016/j.bbr.2005.06.032. [DOI] [PubMed] [Google Scholar]
- Spulber S, Mateos L, Oprica M, Cedazo-Minguez A, Bartfai T, Winblad B, Schultzberg M. Impaired long term memory consolidation in transgenic mice overexpressing the human soluble form of IL-1ra in the brain. J Neuroimmunol. 2009 doi: 10.1016/j.jneuroim.2009.01.010. [DOI] [PubMed] [Google Scholar]
- Stanley LC, Mrak RE, Woody RC, Perrot LJ, Zhang S, Marshak DR, Nelson SJ, Griffin WS. Glial cytokines as neuropathogenic factors in HIV infection: pathogenic similarities to Alzheimer's disease. J Neuropathol Exp Neurol. 1994;53:231–238. doi: 10.1097/00005072-199405000-00003. [DOI] [PubMed] [Google Scholar]
- Stellwagen D, Malenka RC. Synaptic scaling mediated by glial TNF-alpha. Nature. 2006;440:1054–1059. doi: 10.1038/nature04671. [DOI] [PubMed] [Google Scholar]
- Stevens B, Allen NJ, Vazquez LE, Howell GR, Christopherson KS, Nouri N, Micheva KD, Mehalow AK, Huberman AD, Stafford B, Sher A, Litke AM, Lambris JD, Smith SJ, John SW, Barres BA. The classical complement cascade mediates CNS synapse elimination. Cell. 2007;131:1164–1178. doi: 10.1016/j.cell.2007.10.036. [DOI] [PubMed] [Google Scholar]
- Streit WJ. Microglia and macrophages in the developing CNS. Neurotoxicology. 2001;22:619–624. doi: 10.1016/s0161-813x(01)00033-x. [DOI] [PubMed] [Google Scholar]
- Streit WJ. Microglial activation and neuroinflammation in Alzheimer's disease: a critical examination of recent history. Front Aging Neurosci. 2010;2:22. doi: 10.3389/fnagi.2010.00022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Streit WJ, Xue QS. Life and death of microglia. J Neuroimmune Pharmacol. 2009;4:371–379. doi: 10.1007/s11481-009-9163-5. [DOI] [PubMed] [Google Scholar]
- Streit WJ, Mrak RE, Griffin WS. Microglia and neuroinflammation: a pathological perspective. J Neuroinflammation. 2004;1:14. doi: 10.1186/1742-2094-1-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Streit WJ, Braak H, Xue QS, Bechmann I. Dystrophic (senescent) rather than activated microglial cells are associated with tau pathology and likely precede neurodegeneration in Alzheimer's disease. Acta Neuropathol. 2009;118:475–485. doi: 10.1007/s00401-009-0556-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Streit WJ, Conde JR, Fendrick SE, Flanary BE, Mariani CL. Role of microglia in the central nervous system's immune response. Neurol Res. 2005;27:685–691. doi: 10.1179/016164105X49463a. [DOI] [PubMed] [Google Scholar]
- Taylor E, Rutter M. Sex differences in neurodevelopmental and psychiatric disorders: One explanation or many? Behavioral and Brain Sciences. 1985;8:460. [Google Scholar]
- Tran PB, Miller RJ. Chemokine receptors: signposts to brain development and disease. Nat Rev Neurosci. 2003;4:444–455. doi: 10.1038/nrn1116. [DOI] [PubMed] [Google Scholar]
- Tremblay ME, Majewska AK. A role for microglia in synaptic plasticity? Commun Integr Biol. 2011;4:220–222. doi: 10.4161/cib.4.2.14506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tremblay ME, Lowery RL, Majewska AK. Microglial interactions with synapses are modulated by visual experience. PLoS Biol. 2010;8:e1000527. doi: 10.1371/journal.pbio.1000527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ullian EM, Sapperstein SK, Christopherson KS, Barres BA. Control of synapse number by glia. Science. 2001;291:657–661. doi: 10.1126/science.291.5504.657. [DOI] [PubMed] [Google Scholar]
- Urakubo A, Jarskog LF, Lieberman JA, Gilmore JH. Prenatal exposure to maternal infection alters cytokine expression in the placenta, amniotic fluid, and fetal brain. Schizophr Res. 2001;47:27–36. doi: 10.1016/s0920-9964(00)00032-3. [DOI] [PubMed] [Google Scholar]
- Vereker E, Campbell V, Roche E, McEntee E, Lynch MA. Lipopolysaccharide inhibits long term potentiation in the rat dentate gyrus by activating caspase-1. J Biol Chem. 2000;275:26252–26258. doi: 10.1074/jbc.M002226200. [DOI] [PubMed] [Google Scholar]
- Vitkovic L, Bockaert J, Jacque C. "Inflammatory" cytokines: neuromodulators in normal brain? Journal of neurochemistry. 2000;74:457–471. doi: 10.1046/j.1471-4159.2000.740457.x. [DOI] [PubMed] [Google Scholar]
- Wake H, Moorhouse AJ, Jinno S, Kohsaka S, Nabekura J. Resting microglia directly monitor the functional state of synapses in vivo and determine the fate of ischemic terminals. J Neurosci. 2009;29:3974–3980. doi: 10.1523/JNEUROSCI.4363-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H, Li W, Goldstein R, Tracey KJ, Sama AE. HMGB1 as a potential therapeutic target. Novartis Found Symp. 2007;280:73–85. discussion 85–91, 160–164. [PubMed] [Google Scholar]
- Whitney NP, Eidem TM, Peng H, Huang Y, Zheng JC. Inflammation mediates varying effects in neurogenesis: Relevance to the pathogenesis of brain injury and neurodegenerative disorders. Journal of neurochemistry. 2009 doi: 10.1111/j.1471-4159.2009.05886.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Widera D, Holtkamp W, Entschladen F, Niggemann B, Zanker K, Kaltschmidt B, Kaltschmidt C. MCP-1 induces migration of adult neural stem cells. Eur J Cell Biol. 2004;83:381–387. doi: 10.1078/0171-9335-00403. [DOI] [PubMed] [Google Scholar]
- Wolff AR, Bilkey DK. Immune activation during mid-gestation disrupts sensorimotor gating in rat offspring. Behav Brain Res. 2008;190:156–159. doi: 10.1016/j.bbr.2008.02.021. [DOI] [PubMed] [Google Scholar]
- Wrona D. Neural-immune interactions: an integrative view of the bidirectional relationship between the brain and immune systems. J Neuroimmunol. 2006;172:38–58. doi: 10.1016/j.jneuroim.2005.10.017. [DOI] [PubMed] [Google Scholar]
- Yang Y, Ge W, Chen Y, Zhang Z, Shen W, Wu C, Poo M, Duan S. Contribution of astrocytes to hippocampal long-term potentiation through release of D-serine. Proc Natl Acad Sci U S A. 2003;100:15194–15199. doi: 10.1073/pnas.2431073100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yirmiya R, Goshen I. Immune modulation of learning, memory, neural plasticity and neurogenesis. Brain Behav Immun. 2011;25:181–213. doi: 10.1016/j.bbi.2010.10.015. [DOI] [PubMed] [Google Scholar]
- Yoon BH, Romero R, Kim CJ, Jun JK, Gomez R, Choi JH, Syn HC. Amniotic fluid interleukin-6: a sensitive test for antenatal diagnosis of acute inflammatory lesions of preterm placenta and prediction of perinatal morbidity. American journal of obstetrics and gynecology. 1995;172:960–970. doi: 10.1016/0002-9378(95)90028-4. [DOI] [PubMed] [Google Scholar]
- Yu HM, Yuan TM, Gu WZ, Li JP. Expression of glial fibillary acidic protein in developing rat brain after intrauterine infection. Neuropathology. 2004;24:136–143. doi: 10.1111/j.1440-1789.2003.00539.x. [DOI] [PubMed] [Google Scholar]
- Zhao C, Teng EM, Summers RG, Jr, Ming GL, Gage FH. Distinct morphological stages of dentate granule neuron maturation in the adult mouse hippocampus. J Neurosci. 2006;26:3–11. doi: 10.1523/JNEUROSCI.3648-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziv Y, Ron N, Butovsky O, Landa G, Sudai E, Greenberg N, Cohen H, Kipnis J, Schwartz M. Immune cells contribute to the maintenance of neurogenesis and spatial learning abilities in adulthood. Nat Neurosci. 2006;9:268–275. doi: 10.1038/nn1629. [DOI] [PubMed] [Google Scholar]
- Zuckerman L, Weiner I. Post-pubertal emergence of disrupted latent inhibition following prenatal immune activation. Psychopharmacology (Berl) 2003;169:308–313. doi: 10.1007/s00213-003-1461-7. [DOI] [PubMed] [Google Scholar]
- Zuckerman L, Weiner I. Maternal immune activation leads to behavioral and pharmacological changes in the adult offspring. J Psychiatr Res. 2005;39:311–323. doi: 10.1016/j.jpsychires.2004.08.008. [DOI] [PubMed] [Google Scholar]
- Zuckerman L, Rehavi M, Nachman R, Weiner I. Immune activation during pregnancy in rats leads to a postpubertal emergence of disrupted latent inhibition, dopaminergic hyperfunction, and altered limbic morphology in the offspring: a novel neurodevelopmental model of schizophrenia. Neuropsychopharmacology. 2003;28:1778–1789. doi: 10.1038/sj.npp.1300248. [DOI] [PubMed] [Google Scholar]