Abstract
Low serum bilirubin levels have been associated with increased risk for cardiovascular disease, and recent data suggest that lower body fat and reductions in weight are associated with higher bilirubin levels. However, it is unknown if exercise training can increase bilirubin levels and whether a higher dose of exercise will further increase bilirubin levels compared to a lower dose.
Purpose
The primary aim of our current report is to examine whether exercise dose affects bilirubin levels in obese postmenopausal women from the Dose Response to Exercise in Women (DREW) trial. In addition, we evaluated whether changes in fitness, insulin sensitivity, and waist circumference associated with exercise training were associated with change in bilirubin levels.
Methods
Participants (n= 419) were randomized to the control group or 4, 8 and 12 kilocalories per kilogram per week (KKW) of exercise training at an intensity of 50% of aerobic capacity. Total bilirubin levels were evaluated at baseline and at follow-up.
Results
Exercise training significantly increased serum bilirubin levels only in the 12 KKW group (0.044 mg/dL, p=0.026) compared to control (0.004 mg/dL). Subgroup analyses showed that there was a significant increase in bilirubin levels in participants in the 12 KKW group (0.076 mg/dL) who were classified as insulin resistant (HOMA> 2.6), compared to insulin resistant control participants (0.018 mg/dL) (p=0.028).
Conclusion
Our findings suggest that high doses of exercise training are necessary to significantly increase bilirubin levels in previously sedentary postmenopausal women especially in individuals with impaired glucose metabolism.
Keywords: Antioxidant capacity, physical activity, dose-response, postmenopausal
Introduction
Low serum total bilirubin concentrations are associated with increased risk of cardiovascular disease (CVD) (9, 18, 26), metabolic syndrome (11), and type-2 diabetes (12). Conversely, mild hyperbilirubinemia within the normal clinical range has been associated with reduced risk (9, 11, 12, 18, 26). Bilirubin modulates CVD risk by reducing lipid peroxidation, decreasing inflammation, and may be a reflection of heme-oxygenase activity (10). Therefore, an increase in bilirubin levels should have clinical implications in postmenopausal women who present with elevated CVD risk (14). There is evidence that lower body fat (8) and reductions in weight (3) are associated with elevated bilirubin levels. Since aerobic exercise training has beneficial effects on body composition, it is plausible that aerobic exercise training can increase total bilirubin levels.
To our knowledge, there is only one published report examining the effect of aerobic exercise training on serum bilirubin levels. Devries et al. (8) found that there was no significant change in total bilirubin level following 3 months of cycling training in lean (men: −0.4 mmol/L, women: 0.3 mmol/L) and obese men and women (men: 1.3 mmol/L, women: −0.5 mmol/L), but did note a significant inverse correlation between body fat and bilirubin level before and after training (r= −0.374, p= 0.005). However, since one standardized dose of exercise was evaluated in this study, it is unknown if there is a threshold dose of exercise necessary to significantly increase bilirubin levels. In addition, since there was a relatively small sample size (n=29), this may have limited their ability to detect a significant change in bilirubin levels following exercise training. A primary objective of the Dose Response to Exercise in Women trial (DREW) was to evaluate exercise levels 50% below and 50% above public health recommendations in order to evaluate whether a lower dose provides any clinical benefit, and whether the higher dose provides proportionally greater clinical benefit than the standard recommended exercise level (20). Thus, the purpose of this secondary analysis is to evaluate the effect of three standardized doses of aerobic exercise training at 50% of VO2 peak on serum bilirubin levels in overweight and obese postmenopausal women from the DREW trial. In addition, we evaluated the effect of exercise training related changes in weight, waist circumference, fitness and insulin resistance on serum bilirubin levels.
Methods
Study design and participants
The full design and methodology for the DREW trial has been published previously (20). In brief, the study was a randomized controlled trial evaluating the dose-response of aerobic exercise training with increasingly higher doses of energy expenditure in 464 sedentary postmenopausal women aged 45-75 with elevated blood pressure. The research protocol was reviewed and approved annually by the Cooper Institute institutional review board, and subsequently approved by Pennington Biomedical Research Center for continued analysis. Written informed consent was obtained from all participants prior to screening. Women recruited for this study were healthy, postmenopausal, overweight or obese (Body Mass Index (BMI) 25.0-43.0 kg/m2), sedentary (no participation in greater than 20 minutes of exercise 3 times a week), elevated systolic blood pressure (120-159.0 mmHg), and were physically capable of participating in an exercise program. Exclusion criteria included the presence of CVD, significant medical conditions, elevated LDL (≥ 3.36 mmol/L), or weight loss of more than 9.1 kg in the previous year (20). From this population, a subset of 419 participants had available total serum bilirubin levels at baseline and follow-up.
Fitness testing
Testing was performed using a Lode Excalibur Sport rate-independent cycle ergometer (Groningen, the Netherlands). Participants cycled at 30 watts (W) for 2 minutes, 50 W for 4 minutes, followed by increases of 20 W every 2 minutes until they could no longer maintain a pedal cadence of 50 revolutions per minute. Respiratory gases were measured using a Parvomedics Truemax 2400 Metabolic Measurement Cart (Sandy, UT). Volume and gas calibrations were conducted before each test. Gas-exchange variables (VO2, VCO2 production, minute ventilation, and respiratory exchange ratio [RER]) were collected breath-by-breath and averaged into 15-second intervals. Two fitness tests were performed on separate days at baseline, and two fitness tests performed at follow-up. The VO2 peak values presented are the average obtained at baseline (baseline fitness test# 1 and baseline fitness test #2) and follow up: (follow-up fitness test#1 and follow-up fitness test#2).
Weight and waist circumference
Weight was measured on an electronic scale (Siemens Medical Solutions, Malvern, PA), which was calibrated on a weekly basis. Waist circumference was obtained using the recommendations of the Airlie Conference (17) .
Total serum bilirubin measurements
Total serum bilirubin was measured at baseline and follow-up in the fasting state (20), and was analyzed using a Dimension Vista 1500 Intelligent Lab System (Siemens Healthcare Diagnostics, Deerfield, IL) . Samples were obtained in the morning at approximately the same time of day at baseline and follow-up, and were analyzed within 24 hours.
Glucose, insulin, and calculation of the homeostasis model (HOMA)
During the baseline and the follow-up visit, we collected standard fasting (12 hr) blood chemistries inclusive of insulin and glucose. Additionally, we also calculated the homeostatic model assessment for insulin resistance (HOMA) as a surrogate marker of impaired glucose metabolism ([Glucose × insulin]/22.5) (19).
Participant randomization
Following completion of all baseline testing, participants were randomized as described previously to the 4, 8, or 12 KKW, or the non-exercise control group (20). There were a total of 419 participants in the analysis with 94,142, 89 and 94 randomized to the control, 4,8 and 12 KKW groups respectively. The over-recruitment of the 4 KKW group in DREW was decided a priori to increase power due to the smaller anticipated changes in the 4 KKW group (20).
Exercise training
We calculated the exercise energy expenditure for women in the DREW age range associated with meeting the consensus public health recommendations (21) as previously reported (20). Details of these calculations are presented in the DREW design and methods report (20). Exercising women participated in 3 or 4 training sessions each week for 6 months with training intensity at the heart rate associated with 50% of each woman’s peak VO2. During the first week, each group expended 4 kcal/kg per week (KKW). Those assigned to that treatment arm (4 KKW group) continued to expend 4 KKW for 6 months. All the other groups increased their energy expenditure by 1 KKW until they reached the energy expenditure required for their group. All exercise sessions were performed under the supervision of the exercise laboratory staff with complete and strict monitoring of the amount of exercise completed in each session. Participants were weighed each week and their weight was multiplied by their exercise dosage to determine the number of calories to be expended for the week. Women in the exercise groups alternated training sessions on semi-recumbent cycle ergometers and treadmills. Adherence to exercise training during the entire 6-month period was calculated for each individual by dividing the kilocalories expended during the exercise training by the kilocalories prescribed for the training period × 100%. There was excellent adherence in the DREW study, and this secondary analysis had similar compliance to the parent study (98% of the target 6 month caloric expenditure) (7). A full report of training related data (exercise duration, kcals expended per week, METS per week, sessions per week) has been previously reported in our main outcomes paper (7) .
Women in the non-exercise control group were asked to maintain their level of activity during the 6-month study period.
Blinding
There were separate intervention and assessment teams, and assessment staff members were blinded to the randomization of study participants. Additionally, the exercise testing core and exercise training laboratories were on separate floors of the building. Participants were reminded regularly not to discuss their randomization with assessment team members.
Statistical procedure
All statistical analyses were conducted using SAS version 9.1 (Cary, NC). Descriptive data were tabulated as means (standard deviation (SD)). A one-way analysis of variance was performed to evaluate for significant differences of continuous baseline measurements. A chisquared test was used to test for differences in hormone therapy (HT) between groups. Changes in bilirubin, fitness, waist circumference, and weight in response to exercise training were analyzed using an analysis of covariance (ANCOVA), and were adjusted for age, baseline value, and ethnicity. Results are presented as adjusted least-square means with 95% confidence intervals. HT was included as a covariate in the primary analysis to explore if it affected the change in bilirubin following exercise training. Spearman correlation coefficients were used to evaluate the relationship between baseline bilirubin and baseline weight, waist circumference, and HOMA score. Additionally, we evaluated the change in bilirubin in tertiles of changes in HOMA, fitness, weight and waist circumference following exercise training in exercisers (n= 325). A trend analysis was conducted using multiple linear regression. For confirmation, the same analysis was performed in all participants (n=419). For all analyses, a p value less than 0.05 was used to reject the null hypothesis.
Since lower bilirubin levels have been associated with conditions associated with impaired glucose metabolism (11-13, 15) and CVD (9, 18, 26), we evaluated the effect of aerobic exercise training on bilirubin levels in participants that were classified as insulin resistant (HOMA score >2.6) (n=176) and insulin sensitive at baseline (HOMA score < 2.6) (n= 243). The criteria for insulin resistance was based on previous data in the literature (4). In the insulin sensitive sub-group there were 57, 81, 52, and 53 in the control, 4, 8 and 12 KKW groups respectively. Similarly in the insulin resistant group, there were 37, 61, 37 and 41 in the control, 4, 8 and 12 KKW groups respectively. A 2 × 4 ANCOVA was performed to test for significant differences in the change in bilirubin levels following exercise training. Multiple linear regression was performed to analyze the trend for the change in bilirubin across exercise dose in participants who were classified as insulin resistant and insulin sensitive.
Results
Demographic data are presented in Table 1. The study sample had a mean ((SD)) age of 57.3 (6.4) years; a mean weight of 84.3 kg (11.8); a mean BMI (Body Mass Index) of 32.0 (5.1) kg/m2; and approximately 64% were Caucasian. The mean total bilirubin was 0.39 mg/dL at baseline. There were no significant differences in baseline characteristics across groups. Similarly, whether participants were on hormone therapy between groups was not different between groups at baseline (p=0.445). Baseline bilirubin was significantly associated with baseline fasting glucose (r= −0.13, p=0.008), insulin (r= −0.13, p= 0.013) and HOMA score (r= −0.14, p=0.007), but was not associated with fitness (r=0.00, p= 0.930), BMI (r= −0.06, p= 0.236) or waist circumference (r= −0.07, p= 0.135).
Table 1.
Baseline participant characteristics- Shown are the baseline characteristics of 419 postmenopausal women from the Dose Response to Exercise in women (DREW). Data are presented as mean (SD). KKW: kilocalorie per kilogram of body weight per week BMI: Body Mass Index, HOMA: The Homeostasis Model, HT: Hormone therapy
| Control | 4 KKW | 8 KKW | 12 KKW | |
|---|---|---|---|---|
| n | 94 | 142 | 89 | 94 |
| Age (yrs) | 57.3 (5.9) | 57.9 (6.5) | 56.8 (6.6) | 56.9 (6.4) |
| HRT (%) | 52.7 | 42.5 | 42.7 | 46.2 |
| BMI | 32.3 (3.8) | 32.3 (6.9) | 32.3 (4.0) | 31.1 (3.6) |
| Weight (Kg) | 85.9 (12.3) | 83.8 (11.5) | 85.0 (12.3) | 83.0 (11.1) |
| Height (Cm) | 162.9 (6.2) | 162.9 (5.9) | 162.3 (5.2) | 163.3 (5.3) |
| Total Bilirubin (mg/dL) | 0.4 (0.2) | 0.4 (0.2) | 0.4 (0.1) | 0.4 (0.2) |
| VO2 peak (mL·kg-1·min −1) | 15.5 (3.0) | 15.4 (3.0) | 15.0 (2.4) | 16.0 (3.0) |
| Systolic Blood Pressure (mmHg) | 141.5 (12.1) | 138.9 (13.3) | 140.2 (13.6) | 137.9 (13.1) |
| Diastolic Blood Pressure (mmHg) | 80.7 (7.8) | 80.7 (9.0) | 81.2 (8.4) | 80.8 (8.7) |
| HOMA | 3.1 (2.4) | 3.0 (1.8) | 3.0 (1.8) | 2.8 (1.8) |
| Exercise time (min/week) | - | 71.4 (12.6) | 130.3 (17.6) | 169.0 (29.9) |
The effect of exercise training on fitness
Participants were sedentary and had low fitness with a mean VO2 peak of 15.5 (2.9) ml.kg−1.min−1. Similar to the main outcomes paper (7), there was a significant increase in VO2 peak in the 4 (0.51 mL·kg−1·min −1, CI: 0.17, 0.85), 8 (1.1 mL·kg−1·min −1, CI: 0.67, 1.5) and 12 (1.3 mL·kg−1·min −1 , CI: 0.93, 1.8) KKW groups compared to control (−0.35 mL·kg−1, CI:0.93, 1.77). Additionally, there was a significant difference in fitness between each group with the exception of the 8 and 12 KKW groups (p=0.276). Waist circumference was significantly reduced in the 4 (−3.1 cm, CI:−4.5, −1.7), 8 (−2.7 cm, CI: −4.3 −1.1) and 12 (−3.0 cm, CI: −4.7, −1.3) compared to control (0.4 cm, CI: −1.2, 2.0). There was no significant change in weight in the 4 (−1.2 kg, CI:−1.8, −0.5), 8 (−1.7 kg, CI:−2.5, −0.9) and 12 (−1.2 kg, CI:−2.0, −0.37) KKW groups compared to control (−1.0 kg, CI: −1.8, −0.2).
The effect of exercise training on bilirubin
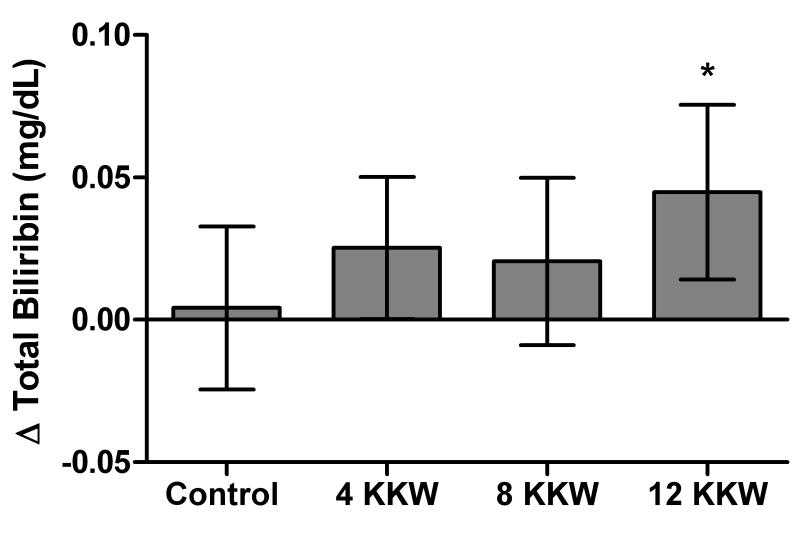
As shown in figure 1, there was an increase in total bilirubin levels in the 12 KKW group (0.044 mg/dL, CI: 0.01, 0.08) compared to control (0.004 mg/dL, CI: −0.02, 0.03), but not in the 4 (0.025 mg/dL, CI: 0.00,0.05) and 8 KKW (0.020 mg/dL, CI: 0.01, 0.08) groups. HT status at baseline did not affect the change in bilirubin with exercise, as it was not significant (p= 0.713) when it was included as a covariate in the main analysis.
Figure 1.
The effect of different doses of aerobic exercise training on total serum bilirubin levels. Shown are the effect of exercise training on total serum bilirubin levels in 419 participants from the DREW trail. Analysis was adjusted for baseline value, ethnicity and age. Results are presented as adjusted least-square means with 95% confidence intervals.
Tertiles of fitness, HOMA, and waist circumference on bilirubin levels
Results of trend analysis among tertiles of change in weight, waist circumference, and HOMA following exercise training are shown in table 2. There were significant trends observed for baseline HOMA score (p-trend=0.018) and change in HOMA score (p-trend=0.002) with change in total bilirubin following exercise training. There were no significant trends observed for change in bilirubin across tertiles of change in fitness, waist circumference or weight following exercise training (p-trend> 0.05). Similar results were observed when all participants (n=419) were included in the analysis (Data not shown).
Table 2.
Changes in serum bilirubin based on tertiles of fitness, weight, waist circumference and insulin resistance- Shown are the change in bilirubin levels across tertiles of baseline HOMA and change in fitness, weight and waist circumference in exercising subjects from the DREW trial. displayed-trend was determined with multiple linear regression. For all analyses a p values < 0.05 was used to reject the null hypothesis. Values are presented as mean (SD).
| Tertile 1 | Tertile 2 | Tertile 3 | ||
|---|---|---|---|---|
| Variable | Bilirubin change | Bilirubin Change | Bilirubin Change | P- trend |
| HOMA change | 0.063 (0.13) | 0.015 (0.14) | −0.023 (0.13) | 0.002 |
| Vo2 peak change (ml/kg/min) | 0.002 (0.14) | 0.020 (0.14) | 0.018 (0.14) | 0.645 |
| Weight change (kg) | 0.025 (0.14) | 0.007 (0.14) | 0.005 (0.14) | 0.728 |
| Waist Circumference change (cm) | 0.033 (0.15) | −0.007 (0.14) | 0.012 (0.13) | 0.200 |
The effect of insulin resistance on change in serum total bilirubin
Baseline bilirubin levels were not significantly different between participants who were classified as insulin sensitive (0.41 mg/dL) compared to those classified as insulin resistant (0.39 mg/dL) (p=0.104). As shown in figure 2, individuals classified as insulin sensitive had no significant increase in bilirubin level following exercise training at any dose of exercise compared to control participants who were classified as insulin sensitive (all p-values > 0.05). However, there was a significant increase in bilirubin in individuals classified as insulin resistant in the 12 KKW (0.076 mg/dL), group compared to control participants classified as insulin resistant (p = 0.028). In addition, this change was significantly greater than insulin sensitive participants in the 12 KKW group (0.018, p < 0.03). The interaction term was not significant (p=0.418). In addition, there was a significant trend found for the change in bilirubin in individuals classified as insulin resistant (p-trend=0.040), but not for participants classified as insulin sensitive (p-trend=0.556).
Figure 2.
The effect of different doses of exercise on total serum bilirubin levels in insulin sensitive versus insulin resistant participants. Shown are the effect of exercise training on serum bilirubin levels in participants classified as insulin resistant (HOMA score >2.6) (n=176) and insulin sensitive at baseline (HOMA score < 2.6) (n= 276). Analysis was adjusted for baseline value, ethnicity and age. Results are presented as adjusted least-square means with 95% confidence intervals.
Discussion
The novel finding of this investigation is that the highest dose of exercise training (12 KKW) resulted in a modest but significant elevation in serum bilirubin levels in previously sedentary postmenopausal women. Furthermore, the increase in bilirubin was greater in participants classified as insulin resistant at baseline at the highest dose of exercise compared to insulin sensitive individuals. The mean baseline bilirubin level of our study population was low (0.39 mg/dL) and likely associated with elevated risk, as previous data suggest that a bilirubin level below 0.7-0.8 mg/L corresponds to a greatest risk of CVD (9) and diabetes (12). The findings may have important clinical implications as low serum bilirubin levels have been associated with elevated CVD risk (9, 18, 26).
Based on previous epidemiological data the elevation in bilirubin in the 12 KKW group in the present study (0.044 mg/dL) is associated with approximately a 2.6% decreased risk of peripheral vascular disease (24), a 4.0% reduced risk of stroke (25), and a 4.9% decreased risk of CVD (23). However, exercise training increased bilirubin levels almost two fold in individuals classified as insulin resistant at baseline at the highest dose of exercise (0.076 mg/dL, 20% change from baseline). Similarly, when change in HOMA was divided into tertiles, we observed a significant trend with reduced HOMA score and higher baseline bilirubin levels. This may have clinical implications as reduced serum bilirubin levels have been associated with insulin resistant states such as metabolic syndrome (13) and type-2 diabetes (12), which tend to be more prevalent in postmenopausal women (6, 28). There were lower baseline bilirubin levels which approached significance in individuals who were classified as insulin resistant compared to insulin sensitive individuals. In addition, we found a significant correlation between baseline HOMA score and baseline bilirubin levels. Therefore, we speculate that the larger magnitude of improvement following exercise training could reflect a normalization of bilirubin levels.
In contrast to previous data (8), we observed no significant associations between body composition measures and bilirubin (baseline or change following exercise training). Devries et al. (8) found that body fat was inversely correlated with bilirubin before and after training. Differences between studies may be due to a smaller range in body composition, as we recruited overweight and obese women, while Devries et al. (8) included both obese and lean participants. However, our results support Andersson et al. (3) who found that in obese and overweight individuals undergoing sibutramine treatment, the relationship between weight loss and elevation in bilirubin levels did not become significant until participants lost more than 2% of their total body weight (3). The average amount of weight loss in the 12 KKW group was −1.4 kg (1.6% of total body weight), therefore it may require larger amounts of overall weight loss to significantly increase bilirubin levels. Additionally, Andersson et al. (3) found that change in fasting glucose and reductions in insulin medication usage were significantly associated with higher bilirubin levels in women (3). This supports our findings that exercise related increase in bilirubin level observed in the present study are associated with improved glucose metabolism.
Although we did not examine specific mechanisms responsible for the elevation in bilirubin with exercise training, evidence suggests that insulin resistant states may impair heme-oxygnase-1 activity (HO-1) (1, 5) (the rate limiting enzyme responsible for the conversion of biliverin to bilirubin), and induction of HO-1 has been shown to increase insulin sensitivity in animal studies (16, 22). Since exercise training may increase the activity of HO-1 system (2), it is possible that this could lead to an increase in bilirubin production. Another plausible mechanism is elevated heme catabolism via exercise induced hemolysis due to increased heel strike, elevated core temperature, and oxidative stress during aerobic training (27). Since heme is the pre-cursor to bilirubin production (10), greater heme bio-availability may promote elevated bilirubin levels downstream. Moreover, women in the highest dose of exercise (12 KKW group) had the greatest exposure to factors associated with exercise induced hemolysis. However, we saw no significant changes in hemoglobin or hematocrit following exercise training (Data not shown). Thus, the specific mechanisms responsible for the elevation in bilirubin levels with aerobic exercise training require further study, and cannot be determined from the present investigation.
The strengths of the present investigation are that DREW is a randomized controlled study with a large study population and strict monitoring of exercise training dose. An important limitation is that we measured total bilirubin, therefore we cannot determine if there were elevations in free, conjugated or unconjugated bilirubin. However, much of the epidemiological data associating low bilirubin levels with cardiovascular risk are based on total bilirubin levels and not on its components (9, 11, 12, 18, 26). Another limitation of the present study is that women in DREW exercised at 50% of VO2 peak, therefore other exercise intensities may produce different results. Lastly, since HOMA-IR is a surrogate measure of insulin resistance, future research should investigate if similar relationships with bilirubin are found when the classification of insulin resistance is based on the results of a hyperinsulinemic euglycemic clamp.
In conclusion, the results of the present study found that only the highest dose of exercise training (150% of recommended levels) significantly increased bilirubin levels in overweight and obese postmenopausal women. Additionally, within the 12 KKW group, the increase in bilirubin was significantly greater in participants classified as insulin resistant at baseline compared to insulin sensitive individuals. Our results suggest that individuals who have insulin resistance may have greater elevations in total bilirubin with high dose exercise training compared to individuals with normal glucose tolerance. In addition, our results have public health implications as they suggests that a high dose of exercise training may have a greater impact on the reduction of cardiovascular risk compared to low dose exercise training due an increase in antioxidant capacity (via an elevation in bilirubin levels) along with a larger increase in cardiorespiratory fitness (7). Future studies should evaluate the effect of exercise training on bilirubin levels in individuals with greater insulin resistance (i.e.type-2 diabetes), and the specific mechanisms responsible for the increase in bilirubin with exercise training.
Acknowledgements
This work was performed at The Cooper Institute, and the staff is especially commended for their efforts. We thank The Cooper Institute Scientific Advisory Board and the DREW participants. In addition, we would like to thank the NIH T-32 postdoctoral fellowship (Obesity from Genes to man) awarded to Pennington Biomedical Research Center. This work was supported by National Institute of Health [grant number HL66262] and unrestricted research support from Coca-Cola. The results of the present study do not constitute an endorsement by ACSM.
Funding: This work was supported by National Institute of Health [grant number HL66262] and unrestricted research support from Coca-Cola.
Footnotes
Conflict of Interest: The authors of the present work have no conflict of interest to declare
This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Abraham NG. Heme Oxygenase: A Target Gene for Anti-Diabetic and Obesity. Curr Pharm Des. 2008;14:412–21. doi: 10.2174/138161208783597371. [DOI] [PubMed] [Google Scholar]
- 2.Abraham NG, Kappas A. Pharmacological and Clinical Aspects of Heme Oxygenase. Pharmacol Rev. 2008;60(1):79–127. doi: 10.1124/pr.107.07104. [DOI] [PubMed] [Google Scholar]
- 3.Andersson C, Weeke P, Fosbøl EL, et al. Acute effect of weight loss on levels of total bilirubin in obese, cardiovascular high-risk patients: an analysis from the lead-in period of the Sibutramine Cardiovascular Outcome trial. Metabolism. 2009;58(8):1109–15. doi: 10.1016/j.metabol.2009.04.003. [DOI] [PubMed] [Google Scholar]
- 4.Ascaso JF, Pardo S, Real JT, Lorente RI, Priego A, Carmena R. Diagnosing insulin resistance by simple quantitative methods in subiects with normal glucose metabolism. Diabetes Care. 2003;26(12):3320–5. doi: 10.2337/diacare.26.12.3320. [DOI] [PubMed] [Google Scholar]
- 5.Bruce CR, Carey AL, Hawley JA, Febbraio MA. Intramuscular Heat Shock Protein 72 and Heme Oxygenase-1 mRNA Are Reduced in Patients With Type 2 Diabetes. Diabetes. 2003;52(9):2338–45. doi: 10.2337/diabetes.52.9.2338. [DOI] [PubMed] [Google Scholar]
- 6.Carr MC. The Emergence of the Metabolic Syndrome with Menopause. J Clin Endocrinol Metab. 2003;88(6):2404–11. doi: 10.1210/jc.2003-030242. [DOI] [PubMed] [Google Scholar]
- 7.Church TS, Earnest CP, Skinner JS, Blair SN. Effects of Different Doses of Physical Activity on Cardiorespiratory Fitness Among Sedentary, Overweight or Obese Postmenopausal Women With Elevated Blood Pressure. JAMA. 2007;297(19):2081–91. doi: 10.1001/jama.297.19.2081. [DOI] [PubMed] [Google Scholar]
- 8.Devries MC, Samjoo IA, Hamadeh MJ, Tarnopolsky MA. Effect of Endurance Exercise on Hepatic Lipid Content, Enzymes, and Adiposity in Men and Women. Obesity. 2008;16(10):2281–8. doi: 10.1038/oby.2008.358. [DOI] [PubMed] [Google Scholar]
- 9.Djoussé L, Levy D, Cupples LA, Evans JC, D’Agostino RB, Ellison RC. Total serum bilirubin and risk of cardiovascular disease in the Framingham offspring study. The American Journal of Cardiology. 2001;87(10):1196–200. doi: 10.1016/s0002-9149(01)01494-1. [DOI] [PubMed] [Google Scholar]
- 10.Franchini M, Targher G, Lippi G. Advances in Clinical Chemistry. Vol 50. Elsevier Academic Press Inc; San Diego: 2010. Serum bilirubin levels and cardiovascular disease risk: a janus bifrons? pp. 47–63. [DOI] [PubMed] [Google Scholar]
- 11.Giral P, Ratziu V, Couvert P, et al. Plasma bilirubin and gamma-glutamyltransferase activity are inversely related in dyslipidemic patients with metabolic syndrome: Relevance to oxidative stress. Atherosclerosis. 2010;210(2):607–13. doi: 10.1016/j.atherosclerosis.2009.12.026. [DOI] [PubMed] [Google Scholar]
- 12.Han SS, Na KY, Chae D-W, Kim YS, Kim S, Chin HJ. High serum bilirubin is associated with the reduced risk of diabetes mellitus and diabetic nephropathy. The Tohoku Journal Of Experimental Medicine. 2010;221(2):133–40. doi: 10.1620/tjem.221.133. [DOI] [PubMed] [Google Scholar]
- 13.Hwang H-J, Kim S-H. Inverse relationship between fasting direct bilirubin and metabolic syndrome in Korean adults. Clin Chim Acta. 2010;411(19-20):1496–501. doi: 10.1016/j.cca.2010.06.003. [DOI] [PubMed] [Google Scholar]
- 14.Kannel WB, Hjortland MC, McNamara PM, Gordon T. Menopause and Risk of Cardiovascular Disease. Ann Intern Med. 1976;85(4):447–52. doi: 10.7326/0003-4819-85-4-447. [DOI] [PubMed] [Google Scholar]
- 15.Kim S-H, Lee J-W, Im J-A, Hwang H-J. Increased γ-glutamyltransferase and decreased total bilirubin are associated with metabolic syndrome in Korean postmenopausal women. Clin Chem Lab Med. 2010;48(11):1623–8. doi: 10.1515/CCLM.2010.302. [DOI] [PubMed] [Google Scholar]
- 16.Li M, Kim DH, Tsenovoy PL, et al. Treatment of Obese Diabetic Mice With a Heme Oxygenase Inducer Reduces Visceral and Subcutaneous Adiposity, Increases Adiponectin Levels, and Improves Insulin Sensitivity and Glucose Tolerance. Diabetes. 2008;57(6):1526–35. doi: 10.2337/db07-1764. [DOI] [PubMed] [Google Scholar]
- 17.Lohman T. Antropometric standardization reference manual. Human Kinetics; Champaign: 1988. pp. 44–6. [Google Scholar]
- 18.Madhavan M, Wattigney WA, Srinivasan SR, Berenson GS. Serum bilirubin distribution and its relation to cardiovascular risk in children and young adults. Atherosclerosis. 1997;131(1):107–13. doi: 10.1016/s0021-9150(97)06088-7. [DOI] [PubMed] [Google Scholar]
- 19.Matthews D, Hosker J, Rudenski A, Naylor B, Treacher D, Turner R. Homeostasis model assessment: insulin resistance and β-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28(7):412–9. doi: 10.1007/BF00280883. [DOI] [PubMed] [Google Scholar]
- 20.Morss GM, Jordan AN, Skinner JS, et al. Dose-Response to Exercise in Women Aged 45-75 yr (DREW): Design and Rationale. Med Sci Sports Exerc. 2004;36(2):336–44. doi: 10.1249/01.MSS.0000113738.06267.E5. [DOI] [PubMed] [Google Scholar]
- 21.National Institute of Health Physical Activity and Cardiovascular Health: NIH Consensus Development Panel on Physical Activity and Cardiovascular Health. JAMA. 1996;276(3):241–6. [PubMed] [Google Scholar]
- 22.Ndisang JF, Lane N, Syed N, Jadhav A. Up-Regulating the Heme Oxygenase System with Hemin Improves Insulin Sensitivity and Glucose Metabolism in Adult Spontaneously Hypertensive Rats. Endocrinology. 2010;151(2):549–60. doi: 10.1210/en.2009-0471. [DOI] [PubMed] [Google Scholar]
- 23.Novotny L, Vitek L. Inverse Relationship Between Serum Bilirubin and Atherosclerosis in Men: A Meta-Analysis of Published Studies. Exp Biol Med. 2003;228(5):568–71. doi: 10.1177/15353702-0322805-29. [DOI] [PubMed] [Google Scholar]
- 24.Perlstein TS, Pande RL, Beckman JA, Creager MA. Serum Total Bilirubin Level and Prevalent Lower-Extremity Peripheral Arterial Disease: National Health and Nutrition Examination Survey (NHANES) 1999 to 2004. Arterioscler Thromb Vasc Biol. 2008;28(1):166–72. doi: 10.1161/ATVBAHA.107.153262. [DOI] [PubMed] [Google Scholar]
- 25.Perlstein TS, Pande RL, Creager MA, Weuve J, Beckman JA. Serum Total Bilirubin Level, Prevalent Stroke, and Stroke Outcomes: NHANES 1999-2004. The American Journal of Medicine. 2008;121(9):781–8. e1. doi: 10.1016/j.amjmed.2008.03.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schwertner H, Jackson W, Tolan G. Association of low serum concentration of bilirubin with increased risk of coronary artery disease. Clin Chem. 1994;40(1):18–23. [PubMed] [Google Scholar]
- 27.Sentürk üK, Gündüz F, Kuru O, et al. Exercise-induced oxidative stress leads hemolysis in sedentary but not trained humans. J Appl Physiol. 2005;99(4):1434–41. doi: 10.1152/japplphysiol.01392.2004. [DOI] [PubMed] [Google Scholar]
- 28.Sowers JR. Diabetes Mellitus and Cardiovascular Disease in Women. Arch Intern Med. 1998;158(6):617–21. doi: 10.1001/archinte.158.6.617. [DOI] [PubMed] [Google Scholar]