Abstract
Background
Side effects of lifetime immunosuppression for cell transplants often outweigh the benefits, therefore, induction of transplant tolerance is needed. We have shown that cotransplantation with myeoid-derived suppressor cells (MDSC) effectively protect islet allografts from rejection without requirement of immunosuppression. This study was to investigate the underlying mechanisms.
Methods
MDSC were generated by addition of hepatic stellate cells (HpSC) from various stain mice into dendritic cell (DC) culture. The quality of MDSC was monitored by phenotype and function analyses. MDSC mixed with islet allografts were transplanted into diabetic recipients. T cell response was analyzed following transplant by flow and histochemical analyses, and compared to islet alone and islet/DC transplant groups. B7-H1 knockout mice were used to determine the role of B7-H1 on MDSC in regulation of T cell response.
Results
Cotransplantation with MDSC (not DC) effectively protected islet allografts without requirement of immunosuppression. This is associated with attenuation of CD8 T cells in the grafts and marked expansion of T regulatory (Treg) cells, which contributed to MDSC-induced T cell hyporesponsiveness. Antigen-specific Treg cells were prone to accumulate in lymphoid organs close to the grafts. Both in vitro and in vivo data demonstrated that B7-H1 was absolutely required for MDSC to exert immune regulatory activity and induction of Treg cells.
Conclusion
The described approach holds great clinical application potential, and may overcome the limitation of requiring chronic administration of immunosuppression in cell transplants. Understanding the underlying mechanisms will facilitate the development of this novel therapeutic strategy.
Keywords: myeloid-derived suppressor cells, hepatic stellate cells, islet transplant, T regulatory cells, B7-H1
Introduction
Organ transplantation has been extensively applied for decades, but cellular transplants, including islets and hepatocytes, remain largely experimental, because the side effects of immunosuppression often outweigh the benefits. Therefore, it is crucial to develop an approach to reduce or avoid immunosupression. We have noted that liver transplants often achieve spontaneously acceptance in many species (1-3), but hepatocyte allografts are acutely rejected (4), suggesting an immune regulatory activity in liver non-parenchymal cells, and demonstrated that hepatic stellate cells (HpSC), well known for storing vitamin A and participating in fibrogenesis, are immunosuppressive (5). Allogeneic islets cotransplanted with HpSC achieves long-term survival in mice via inhibition of T cell response and marked expansion of regulatory T (Treg) cells and myeloid-derived suppressor cells (MDSC) (6,7).
MDSC were initially found to accumulate in cancer patients as key contributors to tumor immune evasion (8), and recently recognized as a subset of innate immune cells capable of regulating adaptive immunity (8,9). MDSC contain heterogeneous cell populations including dendritic cells (DC), monocytes, macrophages, and granulocytes, but share many common characteristics: immature phenotype, inability to become mature, high expression of arginase 1, iNOS, and immune suppressive activity (9). MDSC can be induced by coculture of immune competent cells with tumor cell lines in vitro (10,11). We have shown HpSC are potent MDSC inducers. Addition of small amounts of HpSC into DC culture (derived from bone marrow [BM] cells in mice) leads to generation of MDSC, cotransplantation with which effectively protects islet allografts without immunosuppression (7). We reported here that MDSC protected islet allografts via attenuation of T cell response by enhancement of antigen-specific Treg cells which was mediated by B7-H1 pathway.
Results
Generation of MDSC
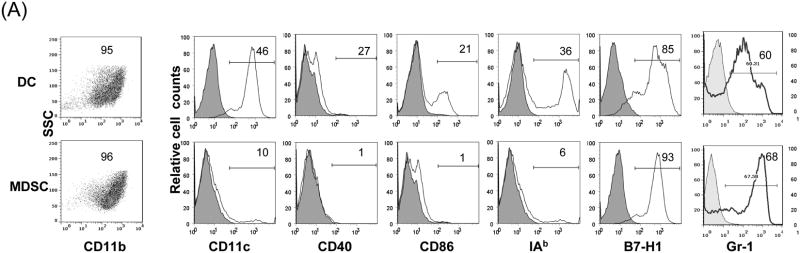
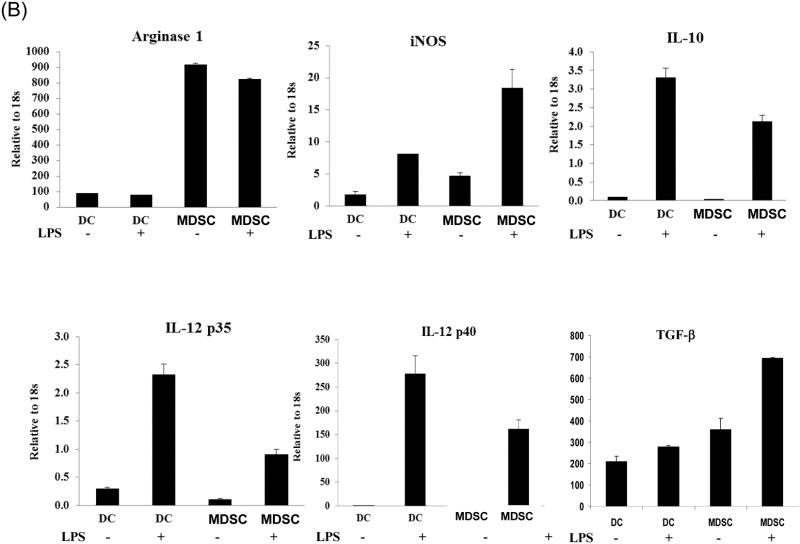
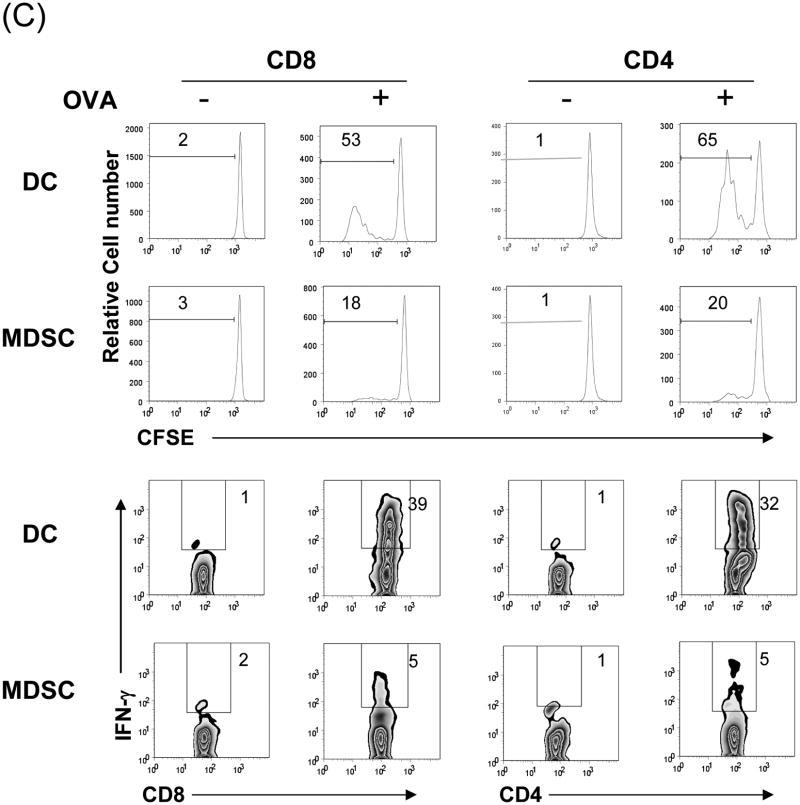
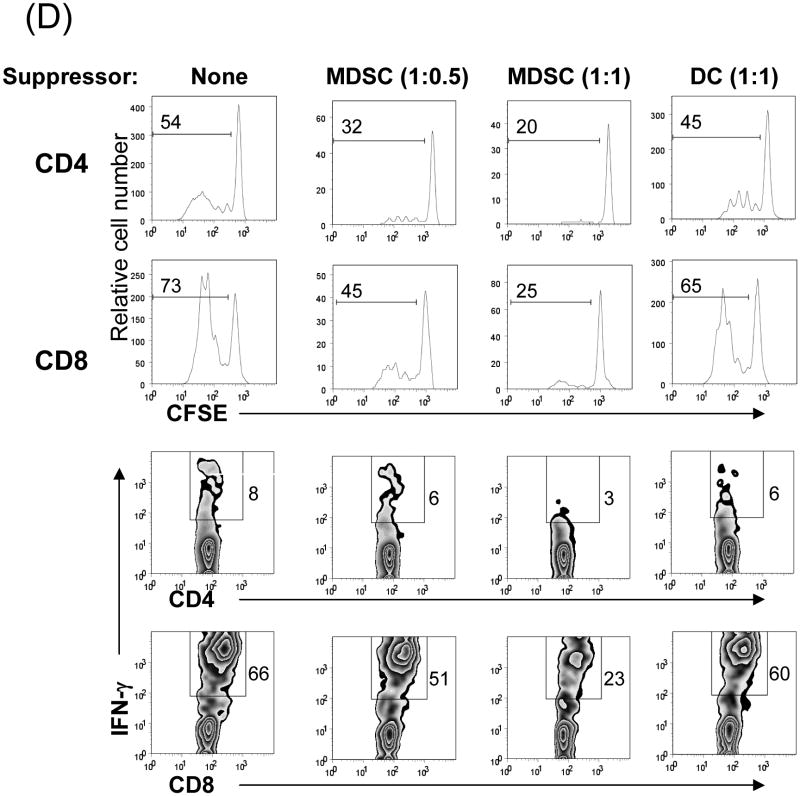
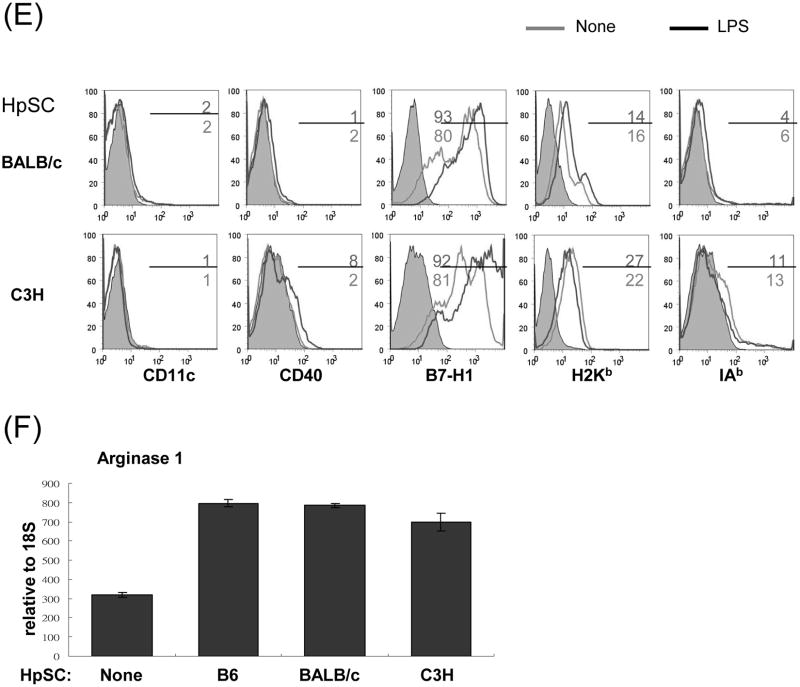
The MDSC were generated by addition of HpSC isolated from B6 mouse liver into BM cell-derived DC culture as previously described (7,12). The quality of MDSC was monitored by analyses for phenotype and function. Flow analysis gated on myeloid cells (CD11b+) showed that HpSC markedly suppressed the generation of CD11c+ cells (declined from 46% to 10%), while generated CD11c- cells with immature phenotype, expression of high Ly6C, Gr-1, and modest levels of F4/80 and B220 (Fig. 1A), reflecting heterogenous nature (8). Since expression of Gr-1 was high in both groups as previously reported (7), Gr-1 was not an appropriate marker to distinguish MDSC in this experimental setting. CD11b+ cells were then purified by beads positive sorting (purity >95%), and hereafter referred as DC (without HpSC) and MDSC (with HpSC), respectively. Upon LPS stimulation, DC expressed high IL-12 and IL-10, while MDSC expressed low IL-12, but high arginase-1 and iNOS and TGF-β (Fig. 1B). The immune stimulatory activity was evaluated by culture of irradiated DC or MDSC with CFSE labeled OVA-specific CD4+ or CD8+ T cells at a ratio of 1:20 in the presence of OVA protein. Compared to DC that elicited robust response, MDSC stimulated very low T cell proliferative response and IFN-γ production (Fig. 1C). To examine the immune suppressive activity, a graded number of MDSC (B6) were added into a one-way MLR culture, resulting in markedly suppressed T-cell response in a dose related manner. This was not due to over-crowd of cells since addition of the same number of DC did not suppress T cell response (Fig. 1D). HpSC from BALB/c or C3H mice generated similar levels of MDSC compared to B6 HpSC, with immature phenotype, resistance to maturation upon LPS stimulation (Fig. 1E), and expressing high arginase 1 (Fig. 1F), indicating that induction of MDSC by HpSC is not MHC restricted.
Figure 1. Generation of MDSC.
To generate MDSC, B6 HpSC were added at the beginning of B6 BM cell culture (2 × 106/well) at a ratio of 1:80 in the presence of mouse rGM-CSF (8 ng/ml) for 5 days (MDSC). Absence of HpSC served as control (DC). (A) The floating cells were harvested for flow analysis following staining with anti-CD11b, -CD11c and indicated key surface molecules, expressed as histograms gated on CD11b+ cells (filled area is isotype control); (B) CD11b+ cells were purified by positive selection with magnetic beads. Expression of arginase 1, iNOS, IL-10, IL12p35, IL-12p40 and TGF-β with or without LPS stimulation was determined by qPCR. (C) MDSC stimulate low T cell response. CFSE labeled OVA specific CD4+ or CD8+ T cells from spleens of OT II and OT I mice, respectively, were cultured for 3 days with irradiated B6 DC or MDSC at the ratio of 20:1 with or without OVA protein (100 μg/ml). The CFSE dilution and expression of IFN-γ (intracellular staining) were analyzed by flow cytometry. (D) MDSC suppress T cell response. CFSE labeled BALB/c spleen T cells were cultured with irradiated B6 DC at a ratio of 20:1 for 3 days. The generated MDSC (B6) were added at the beginning into the culture at an DC: MDSC ratio of 1:0.5 or 1:1. Addition of DC at a ratio of 1:1 served as control. The number is percentage of dividing cells or IFN-γ positive cells. (E) and (F) Induction of MDSC by HpSC is not MHC-restricted. The HpSC from B6, BALB/c or C3H mice were added into B6 BM cell culture with or without LPS stimulation as described above. The floating cells were harvested, and analyzed by flow cytometry for expression of key surface molecules on MDSC, and displayed as histograms gated on CD11b+ cells. The number is percentage of positive cells (E). The CD11b+ cells were purified and analyzed for expression of arginase 1 by qPCR (F). The data are representative of three separated experimental
Cotransplantation with MDSC effectively protects islet allografts
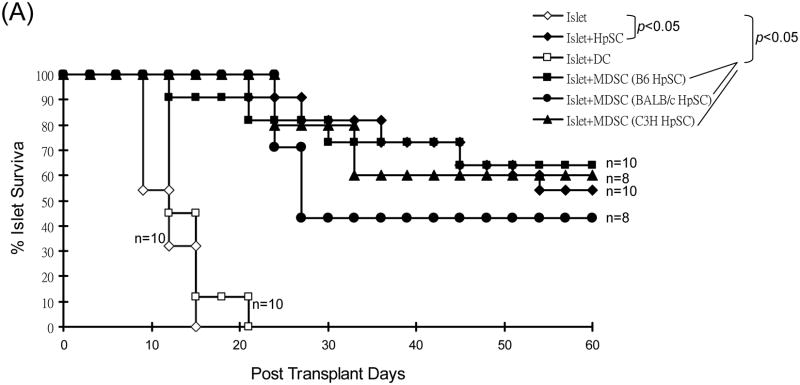
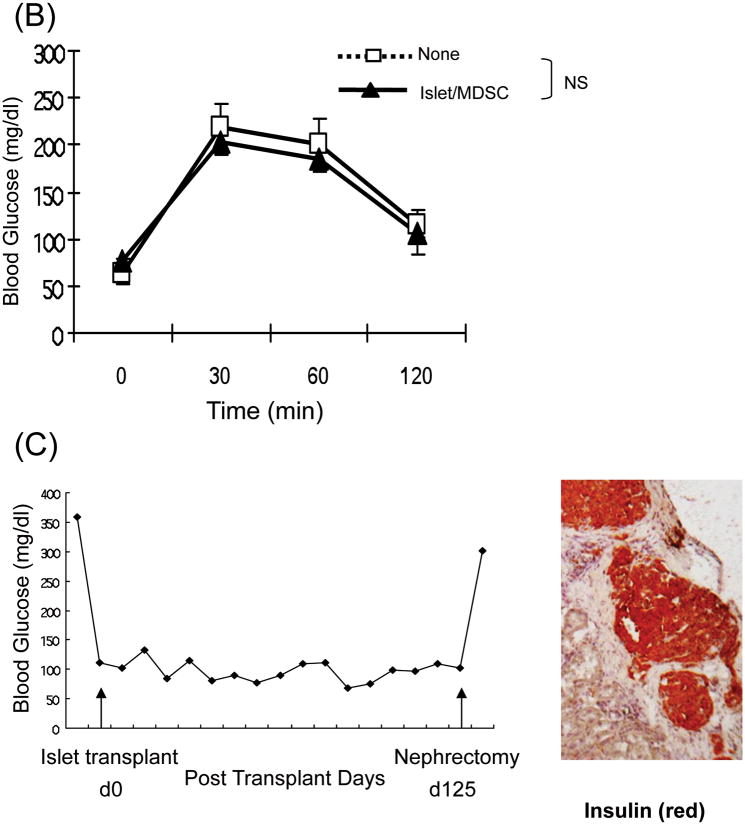
When 2.5 × 106 of the in vitro-generated B6 MDSC (optimal number as shown in our previous study [7]) induced by B6, BALB/c or C3H HpSC were mixed with 300 BALB/c islets, and transplanted into diabetic B6 mice, survival of islet allografts was markedly prolonged (p<0.05, vs. islet only control), similar to that cotansplanted with HpSC (2.5 × 105, p>0.05), while cotransplantation with same number of DC did not prolong islet graft survival (p>0.05, vs. islet only control, Fig. 2A). The recipients bearing long-term survived islet grafts well responded to glucose challenge in a glucose tolerance test comparable to normal mice (Fig. 2B), indicating a normal function of islet grafts. All recipients with long-term survived grafts underwent nephrectomy (to remove islet grafts), and resulted in prompt restore of hyperglycemia. Histochemical staining showed positive for insulin (Fig. 2C), indicating the functional islet grafts.
Figure 2. Cotransplantation with MDSC protects islet allografs.
(A) Impact of cotransplantation with MDSC on survival of islet allografts. 300 islets (BALB/c) mixed with 2.5 × 106 MDSC generated by B6, BALB/c or C3H HpSC were transplanted into diabetic B6 recipients (islet allografting alone and cotransplanted with B6 HpSC or B6 DC served as controls). Cotransplantation with HpSC or DC (both from B6), and transplantation of islet allografts alone served as controls. (B) Randomly selected 3 recipients with euglycemia for >60 days (normal B6 mice served as controls) underwent glucose tolerance test as described in Methods, and the data were demonstrated as mean glucose (mg/dl) ±1SD. (C) The kidney bearing islet allografts was removed in a recipient with euglycemia for 125 days, resulting in prompt hyperglycemia. The islet grafts were stained for insulin (red). (D)
Cotransplantation with MDSC Expands Treg cells
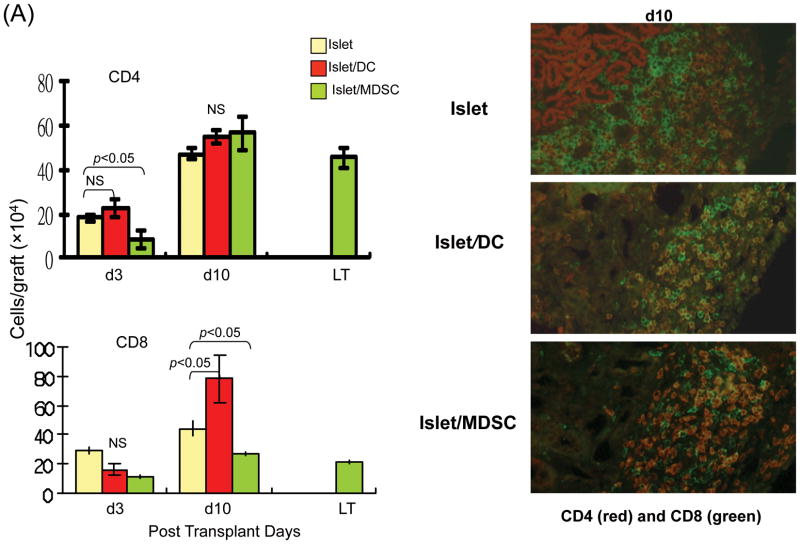
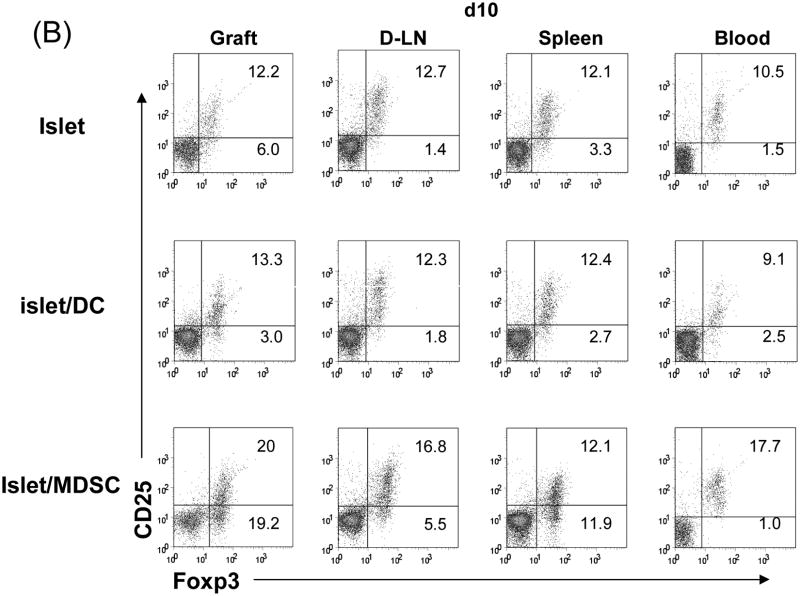
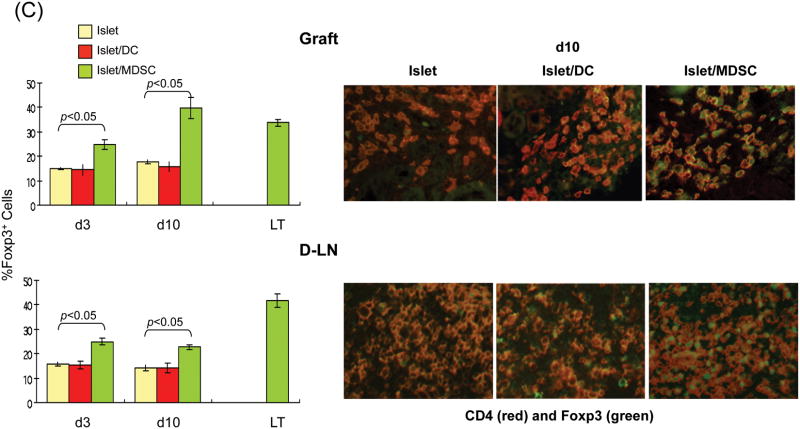
To understand the underlying mechanisms, the subsets of T cells in the islet grafts were studied at various time points post transplant by both flow and immunohistochemical analyses. Flow analysis revealed that the number of CD4+ cells were slightly reduced in islet/MDSC grafts on POD3, but quickly rebounded to the levels similar to islet/DC and islet alone grafts on POD10, and remained high in long-term survived grafts. The number of CD8+ cells that were markedly reduced in islet/MDSC grafts at POD 10, and kept consistently low in islet/MDSC grafts (Fig. 3A, left panels), This tendency was confirmed in immunohistochemical analysis (Fig. 3A, right panels), suggesting that MDSC cotransplantation reduces CD8 effector T cells. To assess Treg activity, lymphocytes from islet grafts, draining lymph node (D-LN), spleen and peripheral blood at various time points were analyzed for expression of CD25 and Foxp3 by flow cytometry gated on CD4+ cells. The frequency of CD25+Foxp3+ cells was marked increased in grafts, D-LN and blood in islet/MDSC recipients, compared to islet alone and islet/DC groups, while CD25-Foxp3+ cells increased in spleen, as well as in grafts and D-LN, (Fig. 3B). CD25-Foxp3+ cells have been recognized as a reservoir of Treg cells whose CD25 expression can be regained upon stimulation (13). Kinetic analysis showed consistent increase of Foxp3+ cells in islet/MDSC recipients; ∼40% CD4+ cells in the grafts and D-LN were Foxp3+ (Fig. 3C, left panels). This bias was also demonstrated in the immunohistochemical analysis (Fig. 3C, right panels), indicating that islet/MDSC cotransplantation is associated with expansion of Treg cells.
Figure 3. Impact of cotransplantation with MDSC on T cell response.
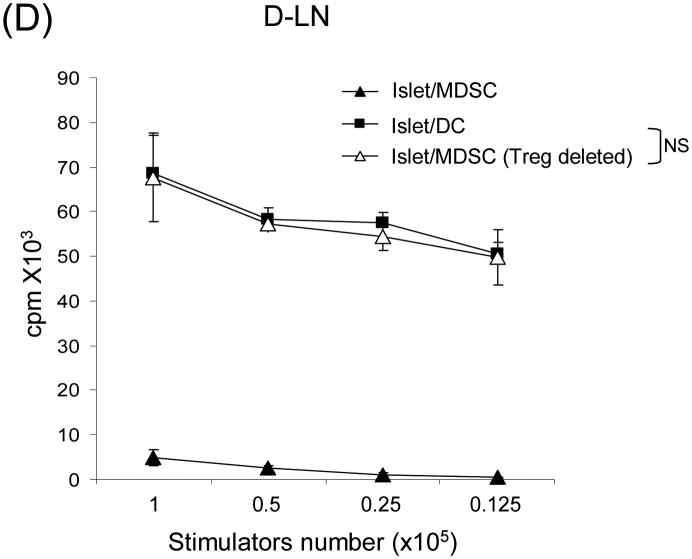
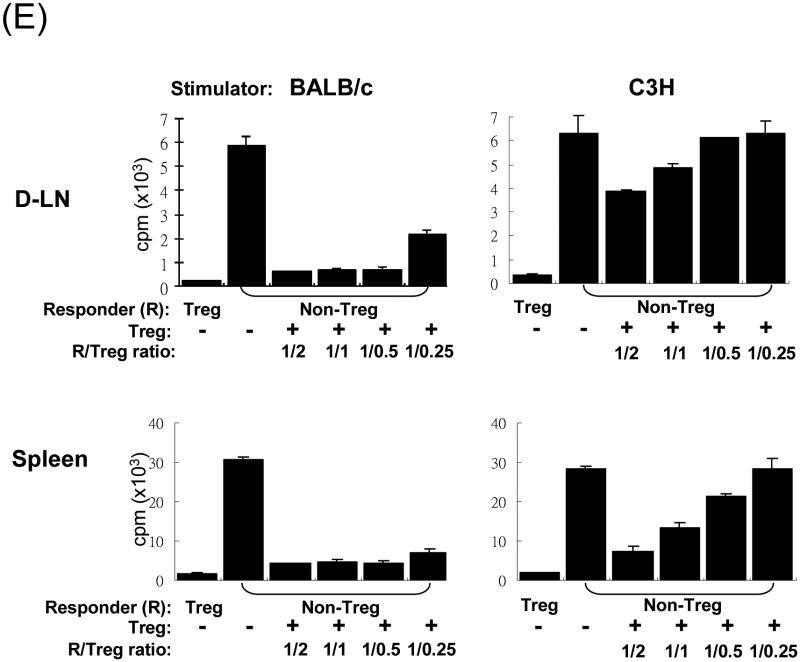
The islet grafts, D-LN, spleen and blood were collected from the recipients of islet alone, islet/DC or islet/MDSC on post operative day (POD) 3, 10, or >90 (LT) for immunohistochemical and flow analyses. (A) Cotransplantation with MDSC reduces CD8+ T cells. Left panels: The number of CD4+ and CD8+ T cells in islet grafts was calculated based on flow analysis on POD 3, 10 and >90, and expressed as mean/graft±1SD. Right panels: Sections of islet allograft on POD 10 were stained with anti-CD4 (red) and anti-CD8 (green) mAb. (B and C) Cotransplantation with MDSC enhances Treg cells. (B) Cotransplantation with MDSC enhances Foxp3+ Treg cells. Isolated cells (POD 10) from indicated compartments were stained for CD4, CD25 and Foxp3, and analyzed by flow cytometry gated on CD4+ cells. The number is percentage of positive cells. (C) left panels: The frequencies of Foxp3+ cells in islet grafts and D-LN kinetically analyzed by flow cytometry, and expressed as mean±1SD. Right panels: Sections of islet grafts and D-LN (POD 10) were stained for CD4 (red) and Foxp3 (green). (D) CD4+CD25+ cells contribute to T cell hyporesponsiveness. T cells isolated from recipient D-LN (POD 10) were stimulated by graded number of irradiated BALB/c splenocytes for 3 days. T cells from islet/MDSC recipients showed markedly lower proliferative response, which was restored to the levels similar to islet/DC group when the CD4+CD25+ T cells were deleted (Treg cell deleted) by magnetic bead (Militenyi Biotech) sorting. (E) Suppressive activity of CD4+CD25+ cells is Ag-specific. T cells isolated from D-LN or spleen in islet/MDSC recipients (POD 10) were separated (using the CD4+CD25+ cell isolation kit) into Treg (CD4+CD25+) and non-Treg (CD25-) portions. The function of these cell populations were studied in a one-way MLR culture. To test suppressive activity, graded numbers of CD4+CD25+ cells (Treg) were added at beginning into an MLR culture, in which CD4+CD25- T-cells (Non-Treg) were stimulated by irradiated BALB/c or C3H splenocytes for 5 days. T cell proliferation was determine by thymidine uptake. The data are representative of three separate experiments.
MDSC induce T cell hyporesponsiveness which is mediated by Treg cells
The response of T cells isolated from D-LN of islet/MDSC recipients were subjected to a recall stimulation with donor (BALB/c) spleen cells, and showed markedly low proliferative response compared to islet/DC group (Fig. 3D), demonstrating an MDSC-induced T cell hyponresponsiveness. To determine the role of Treg cells in T cell inhibition, Treg cells were depleted using the CD4+CD25+ cell isolation kit (Miltenyi Biotec). CD4+Foxp3+ cells were reduced to <2% compared to >20% in non-treated group (data not shown), indicating the Treg cells were efficiently depleted, which is expected since most of CD25+ cells in D-LN were Foxp3+ (see Fig. 3B). Following the depletion of Treg cells, T cell proliferative response restored to the levels similar to islet/DC group (Fig. 3D), suggesting that Treg cells contribute to T cell hyporesponsiveness.
MDSC induce antigen-specific Treg cells
T cells isolated from D-LN or spleen in islet/MDSC recipients (POD 10) were separated (using the CD4+CD25+ cell isolation kit) into Treg (CD4+CD25+) and non-Treg (CD4+CD25-) portions. The function of these cells were studied in a one-way MLR culture. As shown in Fig. 3E, Treg (CD4+CD25+) cells stimulated by donor (BALB/c) or third party (C3H) spleen cells demonstrated minimal proliferative response, exhibiting anergic feature as expected. Whereas, non-Treg cells robustly responded to either BALB/c or C3H stimulation, which was significantly suppressed by addition of the Treg cells, either from D-LN or spleen, in a dose related manner. Importantly, the Treg cells isolated from D-LN only modestly suppressed the response of non-Treg cells stimulated by third party antigens (C3H), not as effectively as the Treg cells from spleen (Fig. 3E, right panels), indicating that Treg cells in D-LN demonstrate more antigen-specific, but less non-antigen specific, suppressive activity than that in spleen, suggesting that MDSC induced antigen-specific Treg cells intend to accumulate in lymphoid organs close to the inflammatory site (graft).
B7-H1 on MDSC is required for expansion of Treg cells
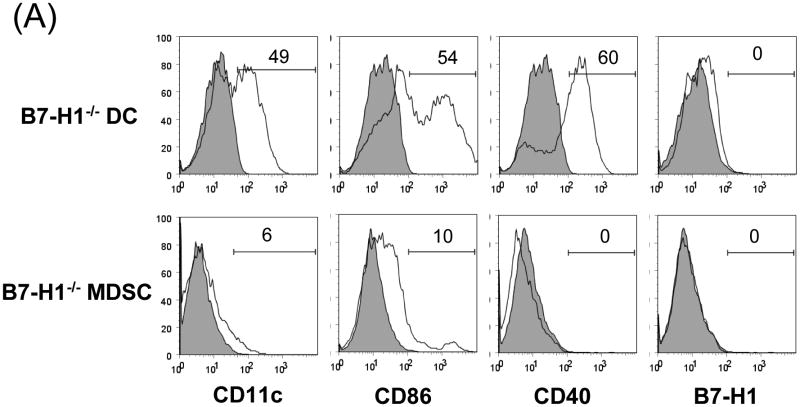
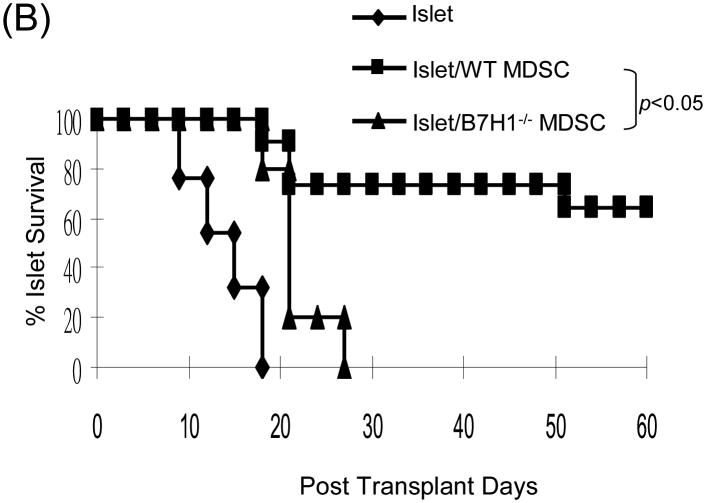
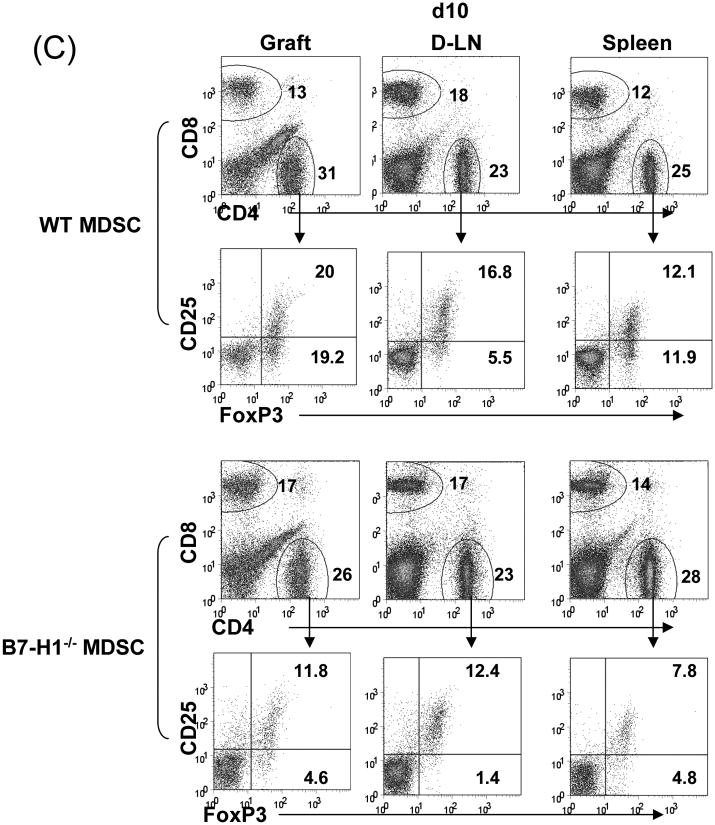
To determine the role of B7-H1 on MDSC, we propagated DC and MDSC from the B7-H1-/- mice. Flow analysis showed that null B7-H1 did not affect expression of other key surface molecules on either DC or MDSC (Fig. 4A). Cotransplantation with B7-H1-/- MDSC largely lost their ability to protect islet allografts, compared to wild type (WT) MDSC (p<0.05, Fig 4B), suggesting a critical role of B7-H1. Lymphocytes isolated from the grafts, D-LN and spleen were analyzed by flow cytometry. The incidence of CD8+ cells in islet/B7-H1-/- MDSC recipients was comparable to WT MDSC group. Whereas, the frequencies of CD4+Foxp3+ cells were markedly decreased in all tested compartments in B7-H1-/- MDSC recipients, compared to WT MDSC group (Fig. 4C), suggesting that B7-H1 in unlikely involved in MDSC-induced attenuation of CD8+ cells, but play an important role in MDSC-induced expansion of Treg cells.
Figure 4. Induction of Treg cells requires B7-H1.
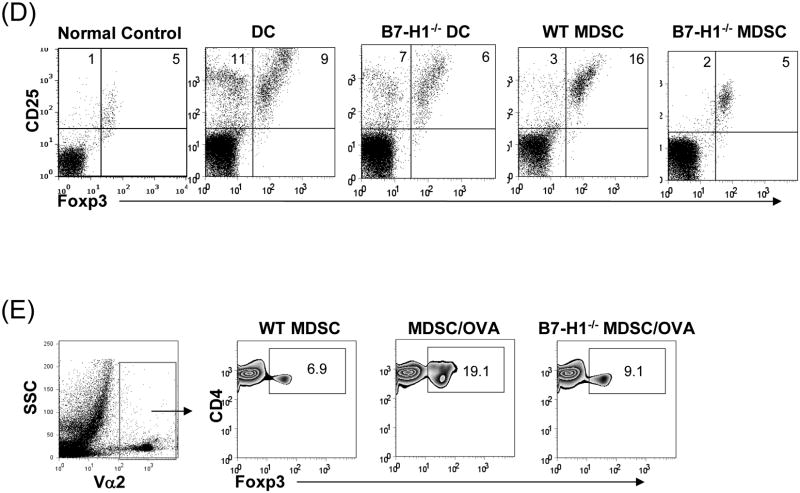
(A) Phenotype of MDSC generated from B7-H1-/- mice. B7-H1-/- DC and MDSC were stained for CD11b, CD11c and the key surface molecules, analyzed by flow cytometry gated on CD11b+ cells, and expressed as hitograms (filled area is isotype control). (B) B7-H1-/- MDSC lose ability to protect islet allografts and expand Treg cells. 2 × 106 B7-H1-/- or wild type (WT) MDSC mixed with 300 BALB/c islets were transplanted into diabetic B6 recipients (islet grafting alone served as control). Left panel: islet graft survival. Right panel: Cells isolated from the islet grafts, D-LN and spleen on POD 10 were stained for CD4, CD8, CD25 and Foxp3. Expression of Foxp3 and CD25 were analyzed by flow cytometry gated on CD4+ cells. (C) Failure of B7-H1-/- MDSC to expand Treg cells in vitro. B6 DC or MDSC (either WT or B7-H1-/-) were cultured with BALB/c spleen T-cells [at a ratio of 1:20, and in the presence of IL-2 (10U/ml)] for 3 days. Treg cells were analyzed by flow cytometry gated on CD4+ cells. (D) Failure of B7-H1-/- MDSC to expand Treg cells in vivo. Normal B6 mice were adoptive transferred (i.v.) with 107 OVA specific CD4+ T cells (from OT II mice), while footpad was injected with 5 × 105 B7-H1-/- or wild type (WT) MDSC pulsed with OVA protein (100 μl/ml overnight). Footpad injection with WT MDSC without antigen pulse served as control. Lymphocytes were isolated from the popliteal lymph nodes (injected side) 3 days later, and analyzed by flow for expression of CD4, Foxp3 gated on Vα2+ (OT II phenotypic) cells. The data are representative of two separate experiments. The number is percentage of positive cells.
Direct evidence of the role of B7-H1 on MDSC in induction of Treg cells
To directly study effect of B7-H1 on induction of Treg cells, DC or MDSC generated from WT or B7-H1-/- mice were added into the culture of BALB/c T-cells at a ratio of 1:20 with IL-2 for 3 days, and analyzed for CD4 T cells. Exposure to WT DC slightly enhanced the incidence of Foxp3+ cells (Treg, Tr), but markedly increased CD25+Foxp3- cells (T effector, Te), resulting in Tr:Te = 9:11= 0.8. WT MDSC expanded Foxp3+ cells, but slightly enhanced CD25+Foxp3- cells (Tr:Te = 16:3 = 5.3), indicating that MDSC expanded Treg cells (Fig 4D). MDSC deficient in B7-H1 markedly reduced capacity of inducing Treg cells, resulting in markedly lower ratio of Tr:Te=5:2=2.5 (Fig. 4D), suggesting requirement of B7-H1 for expansion of Treg cells. To test in vivo, OVA specific CD4+ T-cells were i.v. injected into naïve B6 mice followed by footpad injection with OVA-pulsed WT or B7-H1-/- MDSC. Lymphocytes isolated from the popliteal LN (injected side) 3 days later were analysis by flow gated on Vα2+ (OT II phenotypic) cells. Administration of OVA-pulsed MDSC stimulated expansion of CD4+Foxp3+ cells (19%) compared to 6.9% in without OVA control, while administration of B7-H1-/- MDSC stimulated less Treg expansion (9.1%) (Fig. 4E), providing in vivo evidence that expansion of Treg cells depends on B7-H1.
Discussion
The superior outcomes of whole organ transplants to cell transplants have led to an assumption that the organ accessory cells contribute to immune regulation. This was supported by observations in clinical islet transplants - graft survival was positively correlated with the contamination of ductal-epithelial cells in islet preparation (14), which is not due to containing islet progenitor cells, since β-cell renewal in adults does not originate from islet progenitors (15). This hypothesis was also supported by cotransplantation with Sertoli cells (stromal cells in testis) leading to long-term survival of islets in several animal models (16,17). We have recently showed that mouse islet allografts, when transplanted with HpSC, achieved long-term survival (5,6). Potent immunosuppressive activity of stromal cells in testis and liver is not surprising as both are immune privilege organs (18). This approach may overcome the limitation of requiring chronic administration of immunosuppression in cell transplants - only by reducing the need for such agents, will cellular transplantation be widely applied (19). However, the limited source of Sertoli cells and HpSC may hurdle wide application.
A solution appears emerged. We have shown that HpSC protect cell transplants via induction of MDSC. Large amounts of MDSC can be generated by addition of small number of HpSC into DC culture. Cotransplantation with the propagated MDSC protects islet allografts as effectively as HpSC (7). Our findings, in this study, demonstrated that induction of MDSC by HpSC was not MHC restricted since the HpSC from allogeneic and third party strains also induced similar levels of MDSC, which will definitely enhance the feasibility in clinical applications. To generate MDSC, HpSC from any source, such as surgical specimens or discarded liver donors, can be added into DC culture from patient peripheral blood mononuclear cells (PBMC), the protocol of which has well been established in cancer immunotherapy (20). It is also worthwhile to test whether human HpSC cell lines, such as LX-2, which was established by spontaneous immortalization in low serum condition (21), can be used for generation of MDSC.
Consistent with previous reportes, the present study demonstrated that cotransplantation with islet/MDSC suppressed T cell response via expansion Treg cells. It has however, been controversial whether MDSC-induced inhibition of T cells response is antigen-specific (22-26). Our data suggested that islet/MDSC cotransplantation enhanced both antigen-non-specific and-specific Treg cells, and the antigen-specific Treg cells were prone accumulated in the D-LN. Accumulating data have shown that the inducible Treg are usually antigen-specific, while expanded nature Treg are not. The suppressive activities of antigen-specific Treg cells are often superior to non-specific Treg cells (10,11,13). Distribution of more antigen-specific Treg cells in D-LN close to the battle field (the graft) is expected to more efficient suppress the inflammatory response.
This study demonstrated that B7-H1-/- MDSC lost their ability to protect islet allografts., There was a marked reduction of Treg cells in the recipients of islet/B7-H1-/- MDSC compared to WT MDSC controls, but deficient in B7-H1 on MDSC did not affect the decrease of CD8 T cells in islet grafts, suggesting a critical role of B7-H1 in induction of Treg cells by MDSC. This is in agreement with the studies showing that PD-1, B7-H1's receptor, is expressed on Treg cells in mice (27), and a profound defect in conversion of naive CD4 T cells into inducible Treg cells is seen in the absence of B7-H1 (28). However, B7-H1 is not consistently favoring induction of Treg cells. A study reported that B7-H1 ligation negatively regulated Treg cells in humans during chronic hepatitis C virus (HCV) infection, as blockade of B7-H1 enhanced IL-2-dependent proliferation of Treg cells in response to HCV antigens (29), reflect a complex effect of B7-H1 pathway on development, maintenance and function of Treg cells. The conflicting data may also suggest that other factor (s) may participate in B7-H1-mediated regulation of Treg cell development. TGF-β has been shown to be a key signaling factor for Treg development. Similar to Foxp3-/- mice, TGF-β deficient mice develop an autoimmune syndrome, which is associated with impaired proliferation of peripheral inducible Treg cells (30-32). B7-H1 and TGF-β also show a synergistic effect on promoting inducible Treg cell development (28,33,34). This may partially explain what we have seen in this study. DC expressed high B7-H1 (Fig. 1A) though, but failed to expand Treg cells, probably because they expressed low TGF-β, but produced large amount of IL-12 which promote Th1 differentiation. Moreover, a study revealed that TGF-β-induced Treg cells derived from diabetes-prone NOD mice were defective in suppressive activity, and found that a small group genes related to suppressive activity, including LRRC32, CTLA4 and CD73, formed a coregulated cluster which affected T cell differentiation (35), suggesting that multiple co-factors participate in regulation of Treg cell development.
Materials and Methods
Mice
Male C57BL/6 (B6; H-2b), BALB/c (H-2d), C3H (H-2k) and OT I, OT II (both H-2b) mice were purchased from the Jackson Laboratory (Bar Harbor, ME). B7-H1 Knockout (KO) mice were kindly provided by Dr. Lieping Chen (Johns Hopkins University Medical School, Baltimore, MD). All mice were maintained at specific pathogen-free animal facility and used under NIH guidelines.
Generation of MDSC
As previously described (7,12), HpSC isolated from the mouse liver were added at the beginning of culture of 2 × 106/well BM cells from B6 mice (HpSC:BM cell ratio of 1:50) in RPMI-1640 medium containing 10% FCS in the presence of mouse granulocyte-macrophage colony-stimulating factor (GM-CSF, 8 ng/ml, Schering-Plough, Kenilworth, NJ) for 5-7 days. The floating cells were harvested and CD11b+ cells were purified by positive sorting with MACS microbeads (Miltenyi Biotec). The cells cultured in the absence of HpSC were used as DC. For further stimulation, cells were exposed to LPS at 1μg/ml for the last 18 hours of culture.
Islet Transplantation
As previously described (5-7), 300 islets were transplanted under renal capsule of diabetic recipients induced by a single intraperitoneal (i.p.) injection of streptozotocin (STZ) (180 mg/kg). The first day of two consecutive readings of blood glucose greater than ≥350 mg/dL was defined as the date of graft failure. The kidney bearing islet grafts was removed in all recipients with euglycemia for >60 days to test restore of hyperglycemia. Glucose Tolerance Test: mouse fasting overnight was i.p. injected with 50% dextrose solution at 2g/kg. Blood glucose was measured before and after glucose injection at indicated time points.
Flow cytometric analysis
Anti-CD4, -CD8, -CD11b, -CD11c, -CD25, -CD40, -CD86, -IAb, -Gr-1, -IFN-γ, -Ly6C, -F4/80, -B220, and -Vα2 monocloncal antibodies (mAbs) were purchased from BD PharMingen (San Diego, CA), anti-B7-H1 from eBioscience (San Diego, CA). For intracellular IFN-γ staining, cells were pre-fixed using 2% paraformadehyde, permeabilized by 0.1% saponin in 0.1 % bovine serum albumin solution. Foxp3 was stained using fixation and permeabilization buffers in a FoxP3 kit (eBioscience).
Immunohistochemistry
The tissue cryostat sections were stained using the immunofluorescence protocols for CD4, CD8 (BD PharMingen) or Foxp3 (eBioscience). Insulin was identified using anti-insulin mAb as previously described (7).
Quantitative polymerase chain reaction (qPCR)
Total RNA was extracted with TRIzol Reagent. cDNA was synthesized with SuperScript II reverse transcriptase (Invitrogen, Carlsbad, CA). The mRNAs were quantified using Applied Biosystems 7500 Fast PCR System in duplicate. The expression levels were normalized to 18S mRNA.
Mixed leukocyte reaction (MLR)
Responder Nylon-eluted T cells (2 × 105/ well) were cultured in round-bottom 96-well with graded doses of γ-irradiated (20Gy) stimulator cells, maintained in RPMI-1640 complete medium, for 3-5 days in 5% CO2 in air. T cell proliferative response was determined by thymidine uptake ([3H]TdR (1 μCi/well), and expressed as mean counts per minute (cpm) ± 1 SD). In some experiments, CFSE dilution was used to evaluate proliferative response of CFSE labeled CD8+ and CD4+ cells. For suppressor assays, the tested cells were added at the beginning of the MLR culture at the indicated ratios.
Statistical Analysis
Graft survival was analyzed using the log-rank test. The parametric data were analyzed by Student t test (2-tailed). p< 0.05 was considered statistically significant.
Acknowledgments
We thank Dr. Lieping Chen (Johns Hopkins University Medical School) for providing B7-H1 KO mice; Kathleen Brown for performing immunohistochemistry. This study was supported in part by grants from NIH: DK084192 to LL and AI090468 to SQ.
Abbreviations
- BM
bone marrow
- CFSE
carboxyfluorescein diacetate succinimidyl ester
- DC
dendritic cells
- GM-CSF
granulocyte-macrophage colony-stimulating factor
- HpSC
hepatic stellate cells
- IFN
interferon
- IL
interleukin
- i.p.
intraperitoneal
- i.v.
intravenous
- KO
knockout
- LN
lymph node
- MLR
Mixed leukocyte reaction
- mAb
monocloncal antibody
- MDSC
Myeloid-derived Suppressor cells
- OVA
chicken oval albumin antigen
- POD
post operative day
- qPCR
quantitative polymerase chain reaction
- STZ
streptozotocin
- Treg
T regulatory (cells)
- WT
wild type
Footnotes
H-S. C. participated in research design, performed most experiments, analyzed the data, and wrote the manuscript; C-C H. performed propagation and analysis of MDSC; L.W. performed molecular biology studies; R.C and T.W. participated in manuscript writing; J.J.F. senior discussant and contributed to experimental design; S.Q. and L.L. participated in research design, wrote and finalized the manuscript, sponsored the project.
Disclosure: The authors of this manuscript have no conflict interests to disclose, and the manuscript was not funded by a commercial organization.
References
- 1.Calne RY, Sell RA, Pena JR, Davis DR, Millard PR, Binns RM, et al. Induction of immunological tolerance by porcine liver allografts. Nature. 1969;233:472–474. doi: 10.1038/223472a0. [DOI] [PubMed] [Google Scholar]
- 2.Kamada N, Brons G, Davies HS. Fully allogeneic liver grafting in rats induces a state of systemic nonreactivity to donor transplantation antigens. Transplantation. 1980;29:429–431. doi: 10.1097/00007890-198005000-00021. [DOI] [PubMed] [Google Scholar]
- 3.Qian S, Demetris AJ, Murase N, Rao AS, Fung JJ, Starzl TE. Murine liver allograft transplantation: Tolerance and donor cell chimerism. Hepatology. 1994;19:916–923. doi: 10.1002/hep.1840190418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bumgardner GL, Orosz CG. Unsual patterns of alloimmunity evoked by allogeneic liver parenchymal cells. Immunol Rev. 2000;175:260–279. doi: 10.1034/j.1600-0528.2002.017409.x. [DOI] [PubMed] [Google Scholar]
- 5.Chen CH, Kuo LM, Chang Y, et al. In vivo immune modulatory activity of hepatic stellate cells in mice. Hepatology. 2006;44:1171–1181. doi: 10.1002/hep.21379. [DOI] [PubMed] [Google Scholar]
- 6.Yang HR, Chou HS, Gu X et al. Mechanistic Insights into Immunomodulation by Hepatic Stellate Cells in Mice: A Critical Role of IFN-g Signaling. Hepatology. 2009;50:1981–1991. doi: 10.1002/hep.23202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chou HS, Hsieh CC, Yang HR, et al. Hepatic stellate cells regulate immune response via induction of myeloid suppressor cells in mice. Hepatology. 2011;53:1007–1019. doi: 10.1002/hep.24162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gabrilovich DI, Nagaraj S. Myeloid-derived suppressor cells as regulators of the immune system Nat Rev Immunol. 2009;9:162–174. doi: 10.1038/nri2506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Stewart TJ, Abrams SI. How tumours escape mass destruction. Oncogene. 2008;27:5894–5903. doi: 10.1038/onc.2008.268. [DOI] [PubMed] [Google Scholar]
- 10.Dumitriu IE, Dunbar DR, Howie SE, Sethi T, Gregory CD. Human dendritic cells produce TGF-beta 1 under the influence of lung carcinoma cells and prime the differentiation of CD4+CD25+Foxp3+ regulatory T cells. J Immunol. 2009;182:2795–2807. doi: 10.4049/jimmunol.0712671. [DOI] [PubMed] [Google Scholar]
- 11.Rodrigues JC, Gonzalez GC, Zhang L, et al. Normal human monocytes exposed to glioma cells acquire myeloid-derived suppressor cell-like properties. Neuro Oncol. 2010;12:351–365. doi: 10.1093/neuonc/nop023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yu MC, Chen CH, Liang X, et al. Inhibition of T-cell responses by hepatic stellate cells via B7-H1-mediated T-cell apoptosis in mice. Hepatology. 2004;40:1312–21. doi: 10.1002/hep.20488. [DOI] [PubMed] [Google Scholar]
- 13.Zelenay S, Lopes-Carvalho T, Caramalho I, Mores-Fontes MF, Rebelo M, Demengeot J. Foxp3+CD25-CD4+ T cells constitute a reservoir of committed regulatory cells that regain CD25 expression upon momerstatic expansion. PNAS. 2005;102:4091–4096. doi: 10.1073/pnas.0408679102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Street CN, Lakey JR, Shapiro AM, et al. Islet graft assessment in the Edmonton Protocol: implications for predicting long-term clinical outcome. Diabetes. 2004;53:3107–3114. doi: 10.2337/diabetes.53.12.3107. [DOI] [PubMed] [Google Scholar]
- 15.Sambanis A. Encapsulated islets in diabetes treatment. Diabtes Technol Ther. 2003;5:665–668. doi: 10.1089/152091503322250686. [DOI] [PubMed] [Google Scholar]
- 16.Selawry HP, Cameron DF. Sertoli cell-enriched fractions in successful islet cell transplantation. Cell Transplant. 1993;2:123–129. doi: 10.1177/096368979300200206. [DOI] [PubMed] [Google Scholar]
- 17.Korbutt GS, Elliott JF, Rajotte RV. Cotransplantation of allogeneic islets with allogeneic testicular cell aggregates allows long-term graft survival without systemic immunosuppression. Diabetes. 1997;46:317–322. doi: 10.2337/diab.46.2.317. [DOI] [PubMed] [Google Scholar]
- 18.Griffith TS, Brunner T, Fletcher SM, Green DR, Ferguson TA. Fas ligand-induced apoptosis as a mechanism of immune privilege. Science. 1995;270:1189–1192. doi: 10.1126/science.270.5239.1189. [DOI] [PubMed] [Google Scholar]
- 19.Ricordi C, Strom TB. Clinical islet transplantation: advances and immunological challenges. Nat Rev Immunol. 2004;4:259–268. doi: 10.1038/nri1332. [DOI] [PubMed] [Google Scholar]
- 20.Kulakova N, Urban B, McMichael AJ, Ho LP. Functional analysis of dendritic cell–T cell interaction in sarcoidosis. Clin Exp Immunol. 2010;159:82–86. doi: 10.1111/j.1365-2249.2009.04046.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Xu L, Hui AY, Albanis E, et al. Human hepatic stellate cell lines, LX-1 and LX-2: new tools for analysis of hepatic fibrosis. Gut. 2005;54:142–151. doi: 10.1136/gut.2004.042127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bronte V, Zanovello P. Regulation of immune responses by L-arginine metabolism. Nat Rev Immunol. 2005;5:641–654. doi: 10.1038/nri1668. [DOI] [PubMed] [Google Scholar]
- 23.Huang B, Pan PY, Li Q, et al. Gr-1+CD115+ immature myeloid suppressor cells mediate the development of tumor-induced T regulatory cells and T-cell anergy in tumor-bearing host. Cancer Res. 2006;66:1123–1131. doi: 10.1158/0008-5472.CAN-05-1299. [DOI] [PubMed] [Google Scholar]
- 24.Kusmartsev SY, Nefedova DY, Gabrilovich DI. Antigenspecific inhibition of CD8+ T cell response by immature myeloid cells in cancer is mediated by reactive oxygen species. J Immunol. 2004;172:989–999. doi: 10.4049/jimmunol.172.2.989. [DOI] [PubMed] [Google Scholar]
- 25.Kusmartsev SA, Li Y, Chen SH. Gr-1+ myeloid cells derived from tumor-bearing mice inhibit primary T cell activation induced through CD3/CD28 costimulation. J Immunol. 2000;165:779–785. doi: 10.4049/jimmunol.165.2.779. [DOI] [PubMed] [Google Scholar]
- 26.Zea AH, Rodriguez PC, Atkins MB, et al. Arginase-producing myeloid suppressor cells in renal cell carcinoma patients: a mechanism of tumor evasion. Cancer Res. 2005;65:3044–3048. doi: 10.1158/0008-5472.CAN-04-4505. [DOI] [PubMed] [Google Scholar]
- 27.Gavin MA, Clarks SR, Negrou E, Gallegos A, Rudensky A. Homostasis and anergy of CD4+CD25+ suppressor T cells in vivo. Nat Immunol. 2002;3:33–41. doi: 10.1038/ni743. [DOI] [PubMed] [Google Scholar]
- 28.Francisco LM, Salinas VH, Brown KE, et al. PD-L1 regulates the development, maintenance, and function of induced regulatory T cells. J Exp Med. 2009;206:3015–3029. doi: 10.1084/jem.20090847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Krupnick AS, Gelman AE, Barchet W, et al. Murine vascular endothelium activates and induces the generation of allogeneic CD4+25+Foxp3+ regulatory T cells. J Immunol. 2005;175:6265–6270. doi: 10.4049/jimmunol.175.10.6265. [DOI] [PubMed] [Google Scholar]
- 30.Marie JC, Letterio JJ, Gavin M, Rudensky AY. TGF-β1 maintains suppressor function and Foxp3 expression in CD4+CD25+ regulatory T cells. J Exp Med. 2005;201:1061–1067. doi: 10.1084/jem.20042276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fantini MC, Becker C, Monteleone G, Pallone F, Galle PR, Neurath MF. Cutting edge: TGF-β induces a regulatory phenotype in CD4+CD25– T cells through Foxp3 induction and down-regulation of Smad7. J Immunol. 2004;172:5149–5153. doi: 10.4049/jimmunol.172.9.5149. [DOI] [PubMed] [Google Scholar]
- 32.Chen W, Jin W, Hardegen N, et al. Conversion of peripheral CD4+CD25– naive T cells to CD4+CD25+ regulatory T cells by TGF-β induction of transcription factor Foxp3. J Exp Med. 2003;198:1875–1886. doi: 10.1084/jem.20030152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hamdi H, Godot V, Maillot MC, et al. Induction of antigen-specific regulatory T lymphocytes by human dendritic cells expressing the glucocorticoid-induced leucine zipper. Blood. 2007;110:211–219. doi: 10.1182/blood-2007-08-107896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chen ZM, O'Shaughnessy MJ, Gramaglia I. IL-10 and TGF-beta induce alloreactive CD4+CD25- T cells to acquire regulatory cell function. Blood. 2003;101:5076–5083. doi: 10.1182/blood-2002-09-2798. [DOI] [PubMed] [Google Scholar]
- 35.D'Alise AM, Ergun A, Hill JA, Mathis D, Benoist C. A cluster of coregulated genes determines TGF-β–induced regulatory T-cell (Treg) dysfunction in NOD mice. PNAS. 2011;108:8737–8742. doi: 10.1073/pnas.1105364108. [DOI] [PMC free article] [PubMed] [Google Scholar]