Abstract
BACKGROUND
FDA advisory-committees recently made some recommendations to address acetaminophen (APAP)-related toxicity.
OBJECTIVES
To study the proportion of APAP-users potentially consuming APAP over the currently recommended dose (4gm/day) and a toxic dose (10gm/day). To explore the impact of substituting the APAP-strength in combination-prescriptions to 325mg on potential APAP-overuse patterns.
METHODS
Using the 2001-2008 pharmacy claims from IMS LifeLink Health Plans, APAP potential maximum daily dose (PMDD), potential cumulative dose and potential average daily dose (PADD) were calculated annually for APAP-users. The proportion of users with potential APAP-use above 4gm/day and 10gm/day are reported. Analyses were repeated by substituting the maximum APAP-strength in combination-prescriptions to 325mg. Ordinary least squares regression was used to detect linear trends in APAP-use/overuse.
RESULTS
790,188 of 2,656,161 study subjects were prescribed acetaminophen in one or more years from 2001-2008. The proportions of adult APAP-users with PMDD >4gm/day and PADD >4gm/day significantly decreased (p=0.0020 and p=0.0024 respectively). If the maximum APAP-strength in combination-prescriptions was 325mg, the proportion of APAP-users with PMDD >4gm would be 14.08% in 2001 and 13.67% in 2008 while the proportion of those with PMDD >10gm would be 0.21% and 2.30%.
CONCLUSION
About 1 in 4 APAP-users have a PMDD >4gm/day while 2-3% have a PMDD >10gm based exclusively on prescription data which is concerning. These proportions could reduce by over half if the maximum APAP-strength in combination-prescriptions is 325mg. Additional monitoring of opioid prescription-patterns, physician and pharmacist cognizance in prescribing APAP-containing combination products and dose reduction strategies should be considered to reduce APAP-overuse.
INTRODUCTION
FDA advisory committees recently made a number of recommendations to address the toxicity of acetaminophen(APAP), a commonly used analgesic-antipyretic.1 In the US, acetaminophen over-dose accounts for approximately 56,000 emergency room visits, 26,000 hospitalizations and 458 deaths annually.2 Though doses above 10gms/day are associated with hepatotoxicity in adults3, cases of hepatotoxicity have also been reported near the recommended maximum doses (4gms/day).4,5 The proportion of acetaminophen-induced acute liver failure cases increased from 28% in 1998 to 51% in 2003.6 In January 2011, the FDA mandated that the manufacturers of APAP-containing combination-prescriptions limit the APAP-strength in these products to 325mgs.7
Although chronic APAP-use has been linked hepatotoxicity in case reports5, large-scale studies establishing this association are lacking. Studies regarding acetaminophen over-use lack data on acute and chronic APAP-use patterns in commercially insured populations.8-10 Using a commercial insurance claims database, we studied the proportion of adult acetaminophen users (>= 12 years of age) who potentially consumed more than the recommended APAP dose (4gms/day) or a toxic dose of 10gms/day. We also studied the proportion of children (< 12 years of age) who potentially used more than the recommended pediatric APAP dose (75mgs/kg). Furthermore, we explored the impact of substituting the maximum APAP-strength in combination-prescriptions to 325mg on potential APAP-overuse patterns.
METHODS
This was a retrospective cross-sectional study of a 10% random sample of the IMS LifeLink Health Plans data from 2001 to 2008. The data is representative of the commercially insured population in the US with respect to age, gender and region.
This study included enrollees continuously eligible for pharmacy benefits for 12 months during any calendar year from 2001-2008. ‘Acetaminophen-users’ were defined as those with at least one prescription for an acetaminophen-containing product based on the Generic Product Identifier (GPI) codes. Based on potential maximum daily dose (PMDD) and potential average daily dose (PADD) calculations adult users were classified as those with a potential use above and below/at the currently recommended dose (4gms/day) and a toxic dose of 10gms/day.
Pediatric users were classified as those with PMDD above and below/at the recommended dose of 75 mg/kg.11 Due to unavailability of patient weight in the data, U.S. average gender-age based weights from the National Health Statistics Report12 were used to determine the age and gender overuse thresholds. We repeated the analyses by substituting the maximum APAP-strength in combination-prescriptions to 325mgs.
Acetaminophen utilization measures
Potential maximum daily dose (PMDD): The highest potential APAP-dose on each day during a calendar year was calculated using the days-supplied, strength, and quantity fields in the data. Overlapping prescriptions were identified using fill dates and days-supplied and the daily doses were summed to obtain the potential maximum dose. We calculated the total days of potential overuse (sum of consecutive and non-consecutive days), maximum uninterrupted duration of potential overuse and number of scripts in each potential overuse interval.
Potential average daily dose (PADD): Dose obtained by summing up the APAP doses contained in all prescriptions and dividing by the total days-supply.
Potential annual cumulative dosage: The sum of acetaminophen dose (quantity*APAP-strength) from all acetaminophen-containing prescriptions during a calendar year.
ANALYSIS
Trends in potential APAP-use/overuse were tested using crude ordinary least squares regression models with linear-trend terms. This study was approved by the Institutional Review Board of the University of Arkansas for Medical Sciences.
RESULTS
Of the 2,656,161 unique eligible subjects across 2001-2008, 47.23% were women and the mean age was 30.41 years. 29.75% of these (790,188 individuals) used acetaminophen in one or more years. The annual proportion of APAP-users remained relatively constant; 15.90 % in 2001, and 17.76% in 2008 (p=0.0697).
Among the adult APAP-users from 2001 to 2008, the percentages of those with PMDD >4gm/day and those with PADD>4gm/day decreased significantly (p=0.0020 and p=0.0024 respectively) while there was no significant trend in the proportions of those with potential maximum or average daily doses >10gm/day (Table 1). If the maximum APAP-strength in combination-prescriptions was 325mg, the proportion of those with PMDD >4gms/day would be 14.08% and 13.67% in 2001 and 2008 respectively and the proportion of those with PMDD >10gms/day would be 0.21% and 2.30%. The average ‘total duration’ (interrupted and uninterrupted) of potential APAP-use > 4gms/day was 10.65 days across 49,836 PMDD-based APAP over-users in 2008. 24.44% of these users had a ‘total duration’ of potential over-use of more than 7 days. Among users with PMDD >4gm/day and those with PMDD >10gm/day, the proportions of individuals with more than 7 days of maximum consecutive potential overuse duration significantly increased from 2001-2008 (p = 0.0085 and p=0.0020 respectively) (Table 1). Among 6,820 users with potential maximum consecutive over-use >7 days in 2008, 40.58% were prescribed a single APAP-containing script while among 43,016 individuals with < 7 days of consecutive over-use, 24.92% used a single script.
Table 1.
Potential acetaminophen overuse among adult users: 2001-2008
| Year | Proportion* of APAP users with PMDD >4 gm | Proportion* of APAP users PMDD >10 gm | Proportion** of APAP overusers (PMDD >4gm) with uninterrupted duration of potential overuse >7 days | Proportion*** of APAP overusers (PMDD >10gm) with uninterrupted duration of potential overuse > 7 days | Proportion* of APAP users with PADD >4 gm | Proportion* of APAP users with PADD >10 gm |
|---|---|---|---|---|---|---|
| 2001 | 32.61% | 1.88% | 11.07% | 3.26% | 17.67% | 0.33% |
| 2002 | 32.90% | 2.11% | 10.60% | 3.57% | 18.12% | 0.31% |
| 2003 | 34.02% | 3.56% | 10.98% | 5.80% | 18.73% | 0.87% |
| 2004 | 30.06% | 3.01% | 11.54% | 5.44% | 15.23% | 0.96% |
| 2005 | 27.85% | 2.08% | 11.40% | 6.40% | 13.63% | 0.70% |
| 2006 | 27.18% | 1.51% | 11.58% | 9.42% | 12.82% | 0.40% |
| 2007 | 25.62% | 1.22% | 12.09% | 12.58% | 11.51% | 0.27% |
| 2008 | 26.84% | 3.17% | 13.68% | 19.57% | 12.93% | 2.24% |
denominator is the number of adult APAP users
denominator is the number of APAP users with a PMDD > 4gm/day
denominator is the number of APAP users with a PMDD > 10gm/day
APAP - Acetaminophen
PMDD – Potential Maximum Daily Dose
PADD – Potential Average Daily Dose
The mean potential annual cumulative acetaminophen dose for adult users increased from 55.29gms (95% CI: 54.22, 56.35) in 2001 to 81.94gms (95% CI: 80.56, 83.32) in 2008 (p=0.0039). Only 0.38% of the adult patients in 2008 potentially used more than equivalent of 4gms/day over an entire year. Narcotic–APAP combinations accounted for over 90% of all APAP-containing prescriptions among adults and about 50% of APAP-containing prescriptions among children.
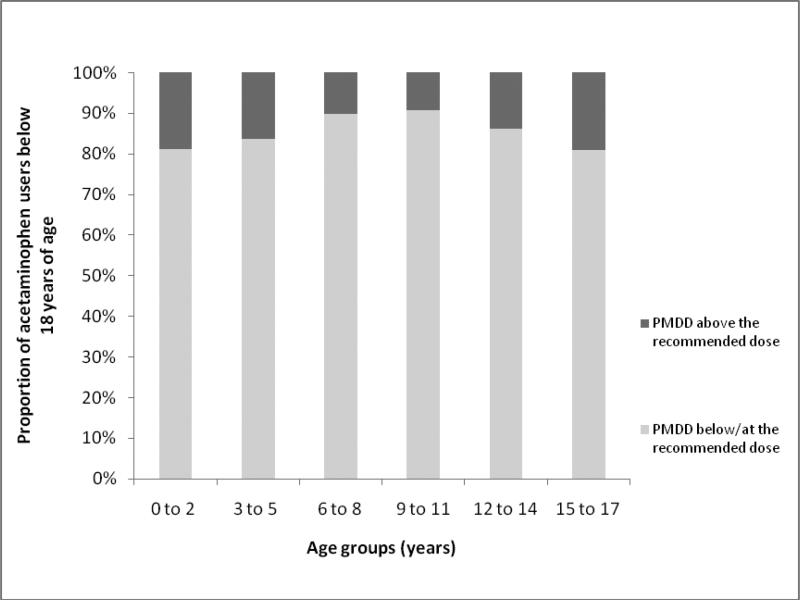
4.03% and 5.71% of the pediatric subjects consumed acetaminophen in 2001 and 2008 of which 12.90% and 13.95% had a PMDD above the recommended age-weight based dose. Among children below 18 years of age in 2008, the proportion likely to exceed the recommended dose was the highest in the 15-17 year old group (18.94%) followed by those 0-2 years (18.89%) with the smallest proportion in the 9-11 year olds (9.22%) (Figure 1).
Figure 1.
Annual proportion of acetaminophen users < 18 years of age with potential maximum daily dose above and below/at the recommended dose in the year 2008
DISCUSSION
It is concerning that over a quarter of the APAP-users have a potential peak APAP-consumption over 4gms/day based exclusively on prescription claims. Of particular alarm is the finding that nearly 3% of the APAP-users had a PMDD >10gms/day, a dose linked to hepatotoxicity.3 The proportion of all enrollees with a PMDD >4gms varied between 5.99% and 5.38% and is similar to the proportions reported for California9 (5.9%) and Oregon Medicaid8 (4.0%). These data suggest that APAP-overuse is not only a concern in vulnerable Medicaid populations, but it may also be an equal or greater concern in the commercially insured populations. Although smaller than the proportions based on PMDD, the proportions of potential over-users based on PADD are still concerning.
Approximately three in four APAP users with a PMDD >4gms for 1-3 consecutive days potentially used a single APAP-containing script and likely did so as a ‘take as needed’ pro-renata (PRN) prescription. An example from the dataset is a prescription for 40 tablets of Lortab (5mg Hydrocodone, 500mg APAP) with a 3-day supply. Assuming that a maximum of 12 tablets could be taken daily (2 tablets /4 hours in 24 hours), a pharmacist would likely enter a three or four day supply. Actual use of an acute PRN analgesic may be less than the calculated PMDD based on ‘days-supply’ and ‘quantity’ fields for a significant proportion of these patients. Nevertheless, physician and pharmacist cognizance of the maximum daily APAP dose should guide the instructions and counseling of those prescribed opioid/APAP combination products. In addition, such counseling should include clear and concise instructions on the maximum number of dosages (tablets, capsules, mls) that can be safely consumed within a 24 hour period. About a quarter of the APAP-users potentially exceeding the 4gms threshold for 1-3 days possibly used multiple overlapping prescriptions and these brief overlaps might represent ‘early-refills’, not actual overuse. Nonetheless, nearly half of the recipients had consecutive use potentially exceeding 4 days and nearly a quarter had consecutive use more than a week. These use patterns and the increasing trend in the proportion of APAP users with a PMDD > 4gm and >10gms/day for >7 consecutive days are more likely to represent actual use exceeding the limits and are cause for concern.
Excess acetaminophen use in adults may likely be caused by an increase in acetaminophen-narcotic combination-prescriptions for chronic non-cancer pain.13 If the maximum APAP-strength in combination-prescriptions was reduced to 325mg as per a recent FDA mandate, the PMDD-based proportion of APAP-users potentially exceeding the 4gms and 10gms threshold would drop by more than half thus lending support to the rationale behind implementing this policy. The proportion of pediatric recipients potentially exceeding the recommended dose was highest among the adolescents (15-17 years) followed by infants (0-2 years). Among children, confusion over APAP-strength in different products leads to overdose and regulatory changes to pediatric acetaminophen products may overcome this problem. Drug manufacturers now plan to standardize the APAP strength in pediatric liquid products to 160mg/5ml to avoid confusion14 and since infants have a high rate of excess acetaminophen use, this strategy of streamlining doses appears warranted.
Our results should be interpreted in light of the following limitations. Since OTC-use not billed as a prescription was not recorded in our data, this study reflects only a minimim risk of acetaminophen overuse for some subjects. The acetaminophen product market consists of 48% prescription and 52% OTC products15 and nearly 80% of acetaminophen doses may be OTC16, so we may observe only about 20-50% of all acetaminophen use with these data. Conversely, our PMDD calculation relies on ‘days-supplied’ and ‘fill-dates’ therefore the assumption of concurrent use of overlapping prescriptions may overstate actual use in certain cases, particularly among nearly 15% of the potential APAP over-users using multiple prescriptions with an overlap period of just 1-3 days (possibility of early refills) and in about 43% of the users potentially exceeding the 4gms threshold for 1-3 days from a single medication, which may often reflect ‘PRN’ usage. Finally the classification thresholds for children are based on average weights and this might have led to misclassification of some patients.
Based exclusively on prescription claims nearly 1 in every 4 acetaminophen recipients had a PMDD exceeding 4gms while approximately 3% had a PMDD > 10gms/day. PADD-based proportions, although smaller, show that if APAP is indeed used as prescribed, a small proportion of individuals may still have potential consumption above the recommended/toxic dose. Additional monitoring of opioid prescription-patterns, physician and pharmacist awareness in prescribing, acetaminophen-dose reduction strategies and regulatory changes in pediatric acetaminophen products should be considered to reduce potential acetaminophen overuse.
Key messages.
Between 2001 and 2008, the proportion of acetaminophen users in the population remained relatively stable.
About 1 in 4 adult acetaminophen users had a potential maximum daily dose over 4 gm/day while about 3% of the users potentially consumed more than 10gms/day of acetaminophen.
About 12% of the acetaminophen users had a potential average daily dose of over 4gm/day while about 2% of the users had a potential average daily dose more than 10gm/day. Though less than the proportions based potential maximum daily doses, these percentages are a cause of concern.
Among pediatric recipients, adolescent users had the highest rate of potential acetaminophen overuse followed by infants.
Opioid combinations with acetaminophen accounted for over 90% of acetaminophen prescription use among adults. If the maximum acetaminophen strength in combination products is limited to 325mg according to a recent FDA policy, the proportion of potential over-users would be cut by more than half.
ACKNOWLEDGEMENTS
The use of LifeLink Health Plans data was supported by the University of Arkansas Center for Clinical and Translational Research (NIH Grant # UL1RR029884). We are thankful for the helpful suggestions of two anonymous reviewers, Dr. John Farrar, and Dr. Paul O. Gubbins who carefully reviewed previous versions of this work.
Reference List
- 1.Krenzelok EP. The FDA Acetaminophen Advisory Committee Meeting - what is the future of acetaminophen in the United States? The perspective of a committee member. Clin Toxicol (Phila) 2009;47:784–789. doi: 10.1080/15563650903232345. [DOI] [PubMed] [Google Scholar]
- 2.Nourjah P, Ahmad SR, Karwoski C, Willy M. Estimates of acetaminophen (Paracetomal)-associated overdoses in the United States. Pharmacoepidemiol Drug Saf. 2006;15:398–405. doi: 10.1002/pds.1191. [DOI] [PubMed] [Google Scholar]
- 3.Chun LJ, Tong MJ, Busuttil RW, Hiatt JR. Acetaminophen hepatotoxicity and acute liver failure. J Clin Gastroenterol. 2009;43:342–349. doi: 10.1097/MCG.0b013e31818a3854. [DOI] [PubMed] [Google Scholar]
- 4.Kwan D, Bartle WR, Walker SE. Abnormal serum transaminases following therapeutic doses of acetaminophen in the absence of known risk factors. Dig Dis Sci. 1995;40:1951–1955. doi: 10.1007/BF02208663. [DOI] [PubMed] [Google Scholar]
- 5.Johnson GK, Tolman KG. Chronic liver disease and acetaminophen. Ann Intern Med. 1977;87:302–304. doi: 10.7326/0003-4819-87-3-302. [DOI] [PubMed] [Google Scholar]
- 6.Larson AM, Polson J, Fontana RJ, et al. Acetaminophen-induced acute liver failure: results of a United States multicenter, prospective study. Hepatology. 2005;42:1364–1372. doi: 10.1002/hep.20948. [DOI] [PubMed] [Google Scholar]
- 7.Acetaminophen information [16 February 2011]; http://www.fda.gov/Drugs/DrugSafety/InformationbyDrugClass/ucm165107.htm.
- 8.Drug Utilization Review: Acetaminophen overuse. Drug Use Research & Management Program. Oregon State University; [10 August 2010]. http://pharmacy.oregonstate.edu/drug_policy/pages/dur_board/evaluations/articles/APAP.pdf. [Google Scholar]
- 9.Albertson TE, Walker VM, Jr., Stebbins MR, Ashton EW, Owen KP, Sutter ME. A population study of the frequency of high-dose acetaminophen prescribing and dispensing. Ann Pharmacother. 2010;44:1191–1195. doi: 10.1345/aph.1P012. [DOI] [PubMed] [Google Scholar]
- 10.Heaton PC, Cluxton RJ, Jr., Moomaw CJ. Acetaminophen overuse in the Ohio Medicaid population. J Am Pharm Assoc (2003 ) 2003;43:680–684. doi: 10.1331/154434503322642606. [DOI] [PubMed] [Google Scholar]
- 11.Recommendations for FDA Interventions to Decrease the Occurrence of Acetaminophen Hepatotoxicity. Prepared by The Acetaminophen Hepatotoxicity Working Group, Center for Drug Evaluation and Research, Food and Drug Administration, Department of Human Health and Services; Feb 26, 2008. [1 September 2010]. www.cyberlewis.com/UCM164898-FDA%20458%20DeathsPerYear.pdf. [Google Scholar]
- 12. [1 July 2010];Anthropometric Reference Data for Children and Adults: United States, 2003–2006. National Health Statistics Reports, Number 10. www.cdc.gov/nchs/data/nhsr/nhsr010.pdf. [PubMed]
- 13.Sullivan MD, Edlund MJ, Fan MY, Devries A, Brennan BJ, Martin BC. Trends in use of opioids for non-cancer pain conditions 2000-2005 in commercial and Medicaid insurance plans: the TROUP study. Pain. 2008;138:440–449. doi: 10.1016/j.pain.2008.04.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. [1 July 2011];Drugmakers to Standardize Pediatric Liquid Acetaminophen Product Concentration. http://www.aafp.org/online/en/home/publications/news/news-now/health-of-the-public/20110516acetaminophendose.html.
- 15.Developing Guidance on Naming, Labeling, and Packaging Practices to Minimize Medication Errors; FDA Public Workshop; June 24, 2010; [12 October 2010]. http://www.fda.gov/downloads/Drugs/NewsEvents/UCM218768.pdf. [Google Scholar]
- 16. [12 October 2010];OTC and Rx Acetaminophen Market Overview Years 2004 – 2008. 2009 June 29; http://www.fda.gov/downloads/AdvisoryCommittees/CommitteesMeetingMaterials/Drugs/DrugSafetyandRiskManagementAdvisoryCommittee/UCM175767.pdf.