Abstract
Malignant gastrointestinal stromal tumors (GISTs) are rare non-epithelial, mesenchymal neoplasms of the gastrointestinal tract that metastasize or recur in 30% of patients who undergo surgical resection with curative intent. A 59-year-old man visited our hospital for an examination of a palpable mass in the left abdomen. Fourteen months prior to his visit, the patient underwent gastric wedge resection to remove a GIST of the gastric cardia. At the time of surgery, no evidence of metastatic disease was observed and the pathological interpretation was a high-risk GIST. A follow-up computed tomography scan of the abdomen revealed a partially necrotic solid mass (9.8 × 7.6 cm) and enhancing mass in the spleen (2.3 cm). On exploration, multiple masses were found in the liver, greater omentum, and mesentery. Here, we report a case of recurring GIST of the stomach that metastasized to the spleen. To the best of our knowledge, few reports of metastasis to the spleen exist.
Keywords: Gastrointestinal stromal tumors, Neoplasm metastasis, Spleen
INTRODUCTION
Gastrointestinal stromal tumors (GISTs) are thought to arise from the interstitial cells of Cajal (ICC), which function as gastrointestinal pacemaker cells [1]. GISTs exhibit a wide clinical spectrum, from benign to frankly malignant, and more than 30% of the patients with malignant tumors develop local recurrence and distant metastases who undergo resection with curative intent [2]. The most common metastatic site is the liver (65%) [3]. To the best of our knowledge, rare reports of metastasis to the spleen exist. Here, we document a case of recurring GIST of the stomach that metastasized to the spleen.
CASE REPORT
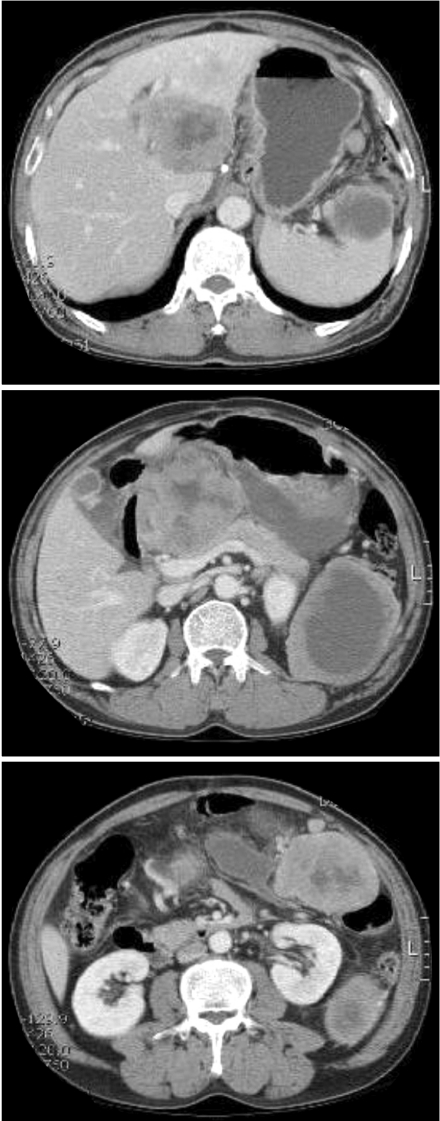
A 59-year-old man visited our hospital for an examination of a palpable mass in the left abdomen. Fourteen months prior to his visit, the patient underwent gastric wedge resection to remove a GIST of the gastric cardia (Fig. 1). At the time of surgery, no evidence of metastatic disease was observed and the pathological interpretation was a high-risk GIST. A follow-up computed tomography (CT) scan of the abdomen revealed a partially necrotic solid mass (9.8 × 7.6 cm) and enhancing mass in the spleen (2.3 cm) (Fig. 2). Two masses (8.3 × 5.5 cm, 10.7 × 8.5 cm) were also found in the liver. Laparoscopic examination indicated peritoneal seeding, and palliative surgery (completion gastrectomy, splenectomy, omentectomy, and left hepatectomy) was performed.
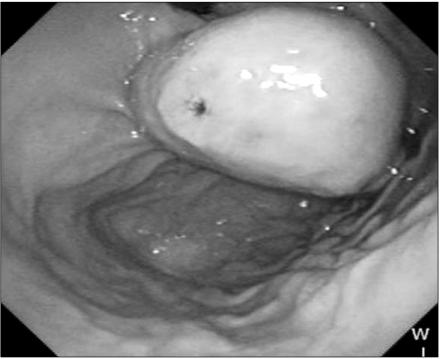
Fig. 1.
A 4 × 3.5 cm sized ovoid shaped mass covered with intact mucosa except small ulceration in the gastric cardia on upper gastrointestinal endoscopy.
Fig. 2.
Follow-up computed tomography shows metastasized mass in the spleen.
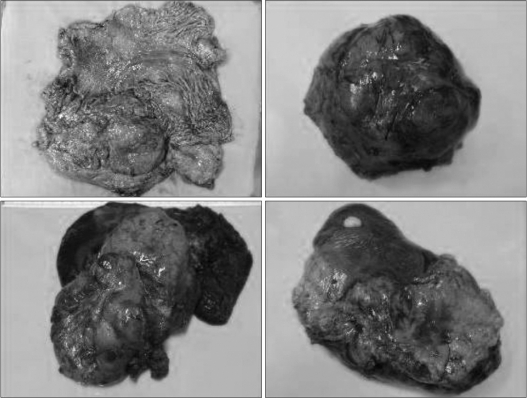
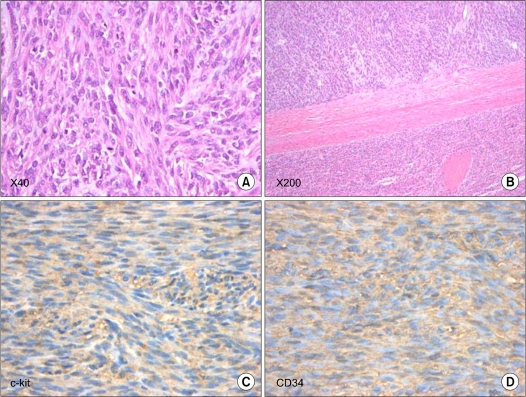
Upon macroscopic examination, a 0.8-cm tumor was found in the stomach of the cardia, 2.3 cm distal to the proximal margin, and two partially necrotic masses were detected in the liver and spleen (13.5 × 10.7 cm and 12.2 × 11 cm, respectively) (Fig. 3). A histopathological examination revealed that the stomach tumor was composed of spindle-shaped cells with numerous mitoses (>10 mitoses per 50 HPF) (Fig. 4). Almost all cells showed immunoreactivity for c-kit and CD34, but not for α-smooth muscle actin, S-100, or desmin. The histology of the masse in the spleen was similar to that of the gastric lesion. After surgery, he received chemotherapy with imatinib mesylate (a single daily oral dose of imatinib 400 mg) and showed a high response. There was no recurred mass on follow-up CT. He is alive at this time (6 years after 2nd operation).
Fig. 3.
Intraoperative photograph of mass in the stomach and metastasized masses in the spleen.
Fig. 4.
Tumor of the stomach was composed of spindle-shaped cells with numerous mitoses (more than 10 mitoses per 50 HPF) (A, B). Almost all of the cells showed immunoreactivity for c-kit and CD34 (C, D).
DISCUSSION
ICC, the intestinal pacemaker cells, may share the same nonneoplastic progenitor cells as GISTs; these tissues show ultrastructural similarities in terms of smooth muscle and neural differentiation [1,2,4]. Most GISTs (50 to 72%) show positive staining for CD34, but immunohistochemical staining for KIT (CD117) is a more specific diagnostic criterion [4,5]. About 5% of GISTs are so-called wildtype GISTs but still express the KIT receptor. These CD117-negative tumors often show other mutations in platelet-derived growth factor receptor-A, another tyrosine kinase [5].
GISTs may occur anywhere along the length of the digestive tract, from the esophagus to the anus. Approximately 60 to 70% of GISTs arise in the stomach, 20 to 30% in the small intestine, 5% in the colon and rectum [1]. Early-stage GISTs often do not present any symptoms. Because most GISTs show an exophytic growth pattern and arise within the muscularis propria of the stomach or intestinal wall, they present as dominant masses outside of the organ of origin [4]. True GISTs can be identified from other tumors by histological analysis and the expression of KIT (CD117) and CD34 [3,5]. Although GISTs are generally fragile and soft, creating a high possibility of bleeding, rupture, and tumor dissemination, preoperative histological confirmation is generally warranted for potentially resectable tumors whenever possible without major complications.
Among the various parameters used to predict clinical behavior, mitotic index and tumor size are considered to be the most reliable prognostic indicators [1,6]; tumor size, in particular, shows strong associations with survival and recurrence. According to Fletcher et al. [7], GISTs can be divided into categories based on size and mitoses: very low risk, low risk, intermediate risk, and high risk. Bucher et al. [8] proposed a revised classification of GISTs according to malignancy and other parameters discussed in the literature. This revised scale includes five minor criteria and two major criteria. GISTs exhibiting fewer than four minor criteria are considered of low malignant potential, whereas GISTs that show four or five minor criteria or one major criterion are classified as high malignant potential, for which the 5-year survival rate is less than 20%.
The principal treatment modality for patients with primary GIST is complete surgical resection. Lymphadenectomy is not routinely performed in patients with GIST [4,9]. Recurrent disease, however, is a serious problem, and the preferred treatment modality for recurrent tumors is to treat with drug. GIST patients also benefit from drug therapy using the specific molecular inhibitor, imatinib mesylate (Gleevec). This therapy is beneficial in unresectable diseases, as well as an adjuvant therapy after the resection of primary GIST [1,9,10]. Although complete recovery was not achieved, the patients showed a significant response with imatinib mesylate. In spite of clear surgical margins and no definite capsular tearing during the first operation, initial wedge resection was failed in this case. Capsular tearing and tumor seeding could be the causes of treatment failure during initial operation. So, surgeons should be careful not to occur capsular tearing and tumor seeding to prevent the recurrence.
Here, we report a case of recurring GIST of the stomach that metastasized to the spleen and the liver. To the best of our knowledge, rare reports of metastasis to the spleen exist.
Footnotes
No potential conflict of interest relevant to this article was reported.
References
- 1.Mochizuki Y, Kodera Y, Ito S, Yamamura Y, Kanemitsu Y, Shimizu Y, et al. Treatment and risk factors for recurrence after curative resection of gastrointestinal stromal tumors of the stomach. World J Surg. 2004;28:870–875. doi: 10.1007/s00268-004-7418-0. [DOI] [PubMed] [Google Scholar]
- 2.Krajinovic K, Germer CT, Agaimy A, Wünsch PH, Isbert C. Outcome after resection of one hundred gastrointestinal stromal tumors. Dig Surg. 2010;27:313–319. doi: 10.1159/000280022. [DOI] [PubMed] [Google Scholar]
- 3.Igwilo OC, Byrne MP, Nguyen KD, Atkinson J. Malignant gastric stromal tumor: unusual metastatic patterns. South Med J. 2003;96:512–515. doi: 10.1097/01.SMJ.0000047760.69924.AA. [DOI] [PubMed] [Google Scholar]
- 4.Kosmadakis N, Visvardis EE, Kartsaklis P, Tsimara M, Chatziantoniou A, Panopoulos I, et al. The role of surgery in the management of gastrointestinal stromal tumors (GISTs) in the era of imatinib mesylate effectiveness. Surg Oncol. 2005;14:75–84. doi: 10.1016/j.suronc.2005.05.002. [DOI] [PubMed] [Google Scholar]
- 5.Mazur MT, Clark HB. Gastric stromal tumors. Reappraisal of histogenesis. Am J Surg Pathol. 1983;7:507–519. doi: 10.1097/00000478-198309000-00001. [DOI] [PubMed] [Google Scholar]
- 6.Hsu KH, Yang TM, Shan YS, Lin PW. Tumor size is a major determinant of recurrence in patients with resectable gastrointestinal stromal tumor. Am J Surg. 2007;194:148–152. doi: 10.1016/j.amjsurg.2006.10.033. [DOI] [PubMed] [Google Scholar]
- 7.Fletcher CD, Berman JJ, Corless C, Gorstein F, Lasota J, Longley BJ, et al. Diagnosis of gastrointestinal stromal tumors: a consensus approach. Hum Pathol. 2002;33:459–465. doi: 10.1053/hupa.2002.123545. [DOI] [PubMed] [Google Scholar]
- 8.Bucher P, Taylor S, Villiger P, Morel P, Brundler MA. Are there any prognostic factors for small intestinal stromal tumors? Am J Surg. 2004;187:761–766. doi: 10.1016/j.amjsurg.2003.09.010. [DOI] [PubMed] [Google Scholar]
- 9.Silberhumer GR, Hufschmid M, Wrba F, Gyoeri G, Schoppmann S, Tribl B, et al. Surgery for gastrointestinal stromal tumors of the stomach. J Gastrointest Surg. 2009;13:1213–1219. doi: 10.1007/s11605-009-0872-0. [DOI] [PubMed] [Google Scholar]
- 10.Samelis GF, Ekmektzoglou KA, Zografos GC. Gastrointestinal stromal tumours: clinical overview, surgery and recent advances in imatinib mesylate therapy. Eur J Surg Oncol. 2007;33:942–950. doi: 10.1016/j.ejso.2006.11.025. [DOI] [PubMed] [Google Scholar]