Abstract
Acute mobile thrombus of the abdominal aorta after chemotherapy is a very unusual finding, which can be a potential source of arterial embolism. We report here on a case of an acute mobile aortic thrombus with renal infarction. We successfully treated the patient with hybrid operation-open surgical and endovascular approach. Our case shows that hybrid treatment using wire-directed balloon catheter thrombectomy is a feasible, minimally-invasive treatment for a mobile aortic thrombus.
Keywords: Abdominal, Angioplasty, Aorta, Thrombectomy, Thrombosis
INTRODUCTION
A mobile aortic thrombus in a non-atherosclerotic, non-aneurysmal aorta and is not a common finding [1]. A mobile aortic thrombus is a potential source of visceral, cerebral and peripheral embolism [1]. The management of a mobile aortic thrombus is not well established because of its rare occurrence [1]. Yet treatment is mandatory because of the potential risk for recurrent embolization. Management of a mobile aortic thrombus includes systemic anticoagulation, aortic thromboendarterectomy or covered stenting. Here we describe a case of acute mobile aortic thrombus with renal infarction, which was successfully treated with hybrid surgery using wire-directed balloon catheter thrombectomy.
CASE REPORT
A 44-year-old woman was diagnosed with advanced gastric cancer (T4N3) for which total gastrectomy was performed. Twelve days after the operation, adjuvant chemotherapy was started with cisplatin and 5-fluorouracil. She received cisplatin (92 mg/day for 4 days) and 5-fluorouracil (1,530 mg/day for 1 day). Two days after the 1st chemotherapy, the patient presented with a sudden onset of right flank pain and dizziness. Emergency computed tomography (CT) imaging of her abdomen and pelvis was performed. A large thrombus was identified in the abdominal aorta at the origin of the renal arteries, and it involved the right renal artery causing renal infarction (Fig. 1). The CT scan prior to the chemotherapy showed a normal aorta at the origin of the renal arteries. Chest X-rays showed no abnormality. The electrocardiography and echocardiogram excluded arrhythmia or intracardiac thrombi. Initial laboratory findings were within normal limits; prothrombin time international normalized ratio 1.01, activated partial thromboplastin time 33.7 seconds, protein C activity 125%, antithrombin III 108%, blood urea nitrogen 13.8 mg/dL, creatinine 0.74 mg/dL, except for protein S activity 56%. Only protein S activity was lower than the normal limit (71 to 103%). A therapeutic intravenous infusion of unfractionated heparin was commenced. To minimize surgical burden to the patient who was on her 17th day after major surgery, hybrid surgery was chosen. Hybrid treatment was performed under general anesthesia. A transbrachial aortic angiogram was performed, which revealed a large filling defect in the aorta just at the origin of the renal arteries and there was no flow to the right renal artery. A cut-down of the right common femoral artery and a puncture of the left common femoral artery were performed. After gaining percutaneous access, a 10 × 40 mm balloon (ev3 Inc., Plymouth, MN, USA) was placed to occlude the left iliac artery to prevent contralateral embolization. And then, a 5 × 20 mm balloon (Cordis Co., Miami Lakes, FL, USA) was used to occlude the left renal artery (Fig. 2). Thereby, we could avoid renal artery embolization and contralateral groin cut down. Wire-directed balloon catheter thrombectomy was performed using a 5F over-the-wire Fogarty catheter (LeMaitre Vascular Inc., Burlington, MA, USA) via the right femoral access. An angiogram after thrombectomy revealed a residual filling defect in the aorta. The aortic thrombus was macerated using a Trerotola mechanical thrombectomy device (Arrow International Inc., Reading, PA, USA). A wire-directed balloon catheter thrombectomy was then performed using a 27 mm Equalizer balloon catheter (Boston Scientific, Cork, Ireland, USA). The completion angiogram demonstrated no residual thrombus with no embolus in the left renal artery or in the bilateral lower extremity arterial systems (Fig. 3). Left renal balloon occlusion was maintained for 41 seconds and the left kidney function was preserved after reperfusion. We used about 150 mL of contrast media (VISIPAQUE Injection, 270 mgI/mL; Amersham health, Cork, Ireland) and the postoperative creatinine level was 0.98 mg/dL. Chemotherapy was stopped after thrombectomy and the patient received systemic heparinization for 1 week and then received antiplatelets including aspirin, cilostazol, and atorvastatin calcium, indefinitely. Follow-up CT scan 7 days after the procedure showed complete resolution of the thrombus (Fig. 4). Eighteen days after the procedure, chemotherapy was restarted with only 5-fluorouracil, because cisplatin has renal toxicity and can cause another thrombus. After 5-months follow-up, she is doing well without another aortic thrombus.
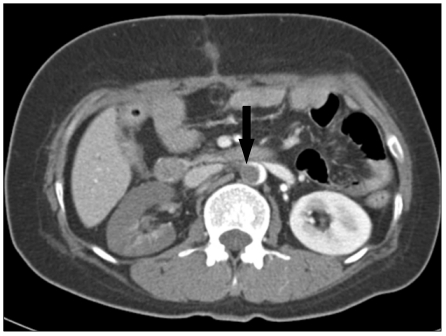
Fig. 1.
Abdominal computed tomography shows intraluminal aortic thrombus (arrow) with infarction of the right kidney.
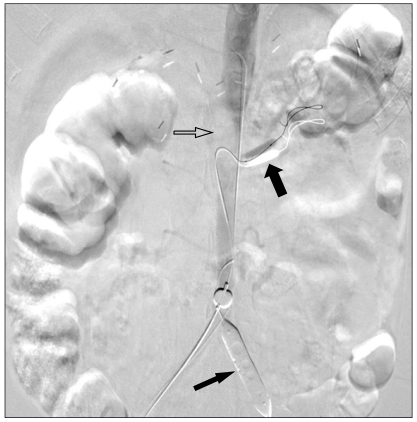
Fig. 2.
Angiogram shows occlusion balloons in the left iliac artery (thin arrow) and in the left renal artery (thick arrow). Abdominal aortic thrombus is shown as by intraluminal filling defect (open arrow).
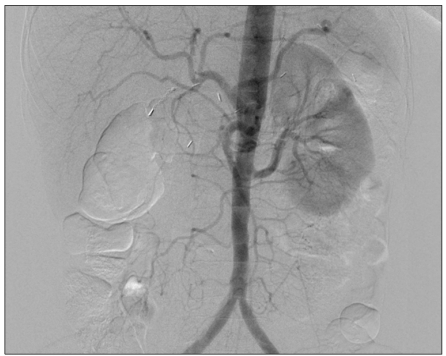
Fig. 3.
After balloon catheter thrombectomy, angiogram shows successful removal of the aortic thrombus.
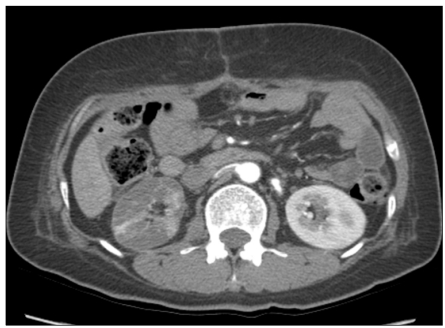
Fig. 4.
Follow-up computed tomography on 7 days after balloon catheter thrombectomy, shows complete resolution of the aortic thrombus. Right renal artery is partially recannalized.
DISCUSSION
Mobile aortic thrombus is a rare pathology and is an important source of arterial emboli. Mobile aortic thrombus mostly occurs in the distal aortic arch in the presence of severe diffuse atherosclerotic disease and has been reported in hypercoagulable patients [2]. In our case, however, the mobile aortic thrombus is thought to have developed due to the chemotherapy as there was no evidence of atherosclerotic disease, aneurysmal dilatation of the aorta. Because protein S activity was diminished, there is the possibility of protein S deficiency. But protein S deficiency usually manifests clinically as venous thromboembolism. Arterial thrombosis is not evident with protein S deficiency [3].
Chemotherapy agents have been documented as possible causes of acute arterial thrombosis Licciardello et al. [4] reported that cisplatin induced increase in the von Willebrand factor concentration, which increases the risk of arterial occlusive complications. So they emphasized monitoring the von Willebrand factor concentration before starting chemotherapy. Unfortunately, the von Willebrand factor concentrations in this patient were not investigated before chemotherapy.
While there are increasing number of reports on thoracoabdominal aortic thrombus as a cause of embolism, the optimal treatment is remains controversial [5]. Systemic anticoagulation, aortic thromboendarterectomy or placement of stent graft were reported as treatments of a mobile aortic thrombus [5-8]. Complete disappearance or a reduction in the size of the thrombus has been reported with the use of anticoagulation alone [6]. Kim et al. [5] suggested a strategy beginning with low molecular weight heparin as the first line of treatment, followed by surgical thrombus removal if the thrombus persists or recurrent embolism occurs during anticoagulation therapy. In contrast, other investigators have recommended an aggressive surgical approach using aortic thromboendarterectomy [1,7]. The management of mobile aortic thrombus is not well established because of its rarity, and there are few comparative studies. Recently, some cases were reported in the literatures that support the endovascular approach of aortic thrombus [8]. They attempted to manage mobile thrombus of the thoracic aorta with endovascular approach, specifically with stent graft, and showed successful results [8]. However, our case could not be treated with a covered stent because the aortic thrombus was in the aorta right at the origin of the renal arteries. So, we performed thrombectomy using a wire-directed balloon catheter with occlusion balloons. Another method to prevent distal embolization is using a filter [9], which was not available in our institution. In our case, a 5F Fogarty catheter was useless for thrombectomy. The aortic thrombus adhered to the wall firm enough to resist the thrombectomy balloon. Therefore, we used a mechanical thrombectomy device for maceration of the aortic thrombus; Trerotola device (Arrow International Inc.) with a 5F rotating nitinol basket fragmentation cage. We intended the wires of the basket directly contact the thrombus, not injuring the aortic wall, and we carefully performed maceration of the aortic thrombus. Yet, it has risks of peripheral embolization of the clot particles and vessel wall injury.
Because of the irreversible infarction of the right kidney at more than 29 hours, we did not perform wire-directed thrombectomy for the right renal thrombus. In our case, chemotherapy was discontinued after hybrid treatment and the patient took systemic heparinization for 1week, then oral antiplatelets. Antiplatelets have a proven efficacy in acute treatment and prevention of arterial thrombosis because a thrombus that forms in arteries are rich in platelets, as opposed to a fibrin-rich thrombus that forms in veins [10].
In conclusion, we report here on the successful treatment of a mobile abdominal aortic thrombus by hybrid treatment performing balloon catheter thrombectomy. Our case shows that this technique is a feasible, minimally invasive treatment for a symptomatic mobile abdominal aortic thrombus.
ACKNOWLEDGEMENTS
This work was supported by an Inha University Research Grant.
Footnotes
No potential conflict of interest relevant to this article was reported.
References
- 1.Kalangos A, Baldovinos A, Vuille C, Montessuit M, Faidutti B. Floating thrombus in the ascending aorta: a rare cause of peripheral emboli. J Vasc Surg. 1997;26:150–154. doi: 10.1016/s0741-5214(97)70161-7. [DOI] [PubMed] [Google Scholar]
- 2.Tunick PA, Lackner H, Katz ES, Culliford AT, Giangola G, Kronzon I. Multiple emboli from a large aortic arch thrombus in a patient with thrombotic diathesis. Am Heart J. 1992;124:239–241. doi: 10.1016/0002-8703(92)90955-u. [DOI] [PubMed] [Google Scholar]
- 3.ten Kate MK, van der Meer J. Protein S deficiency: a clinical perspective. Haemophilia. 2008;14:1222–1228. doi: 10.1111/j.1365-2516.2008.01775.x. [DOI] [PubMed] [Google Scholar]
- 4.Licciardello JT, Moake JL, Rudy CK, Karp DD, Hong WK. Elevated plasma von Willebrand factor levels and arterial occlusive complications associated with cisplatin-based chemotherapy. Oncology. 1985;42:296–300. doi: 10.1159/000226049. [DOI] [PubMed] [Google Scholar]
- 5.Kim SD, Hwang JK, Lee JH, Cho HJ, Sung GY, Moon IS, et al. Free floating thrombus of the aorta: an unusual cause of peripheral embolization. J Korean Surg Soc. 2011;80:204–211. [Google Scholar]
- 6.Choukroun EM, Labrousse LM, Madonna FP, Deville C. Mobile thrombus of the thoracic aorta: diagnosis and treatment in 9 cases. Ann Vasc Surg. 2002;16:714–722. doi: 10.1007/s10016-001-0314-2. [DOI] [PubMed] [Google Scholar]
- 7.Lozano P, Gomez FT, Julia J, M-Rimbau E, Garcia F. Recurrent embolism caused by floating thrombus in the thoracic aorta. Ann Vasc Surg. 1998;12:609–611. doi: 10.1007/s100169900209. [DOI] [PubMed] [Google Scholar]
- 8.Zhang WW, Abou-Zamzam AM, Hashisho M, Killeen JD, Bianchi C, Teruya TH. Staged endovascular stent grafts for concurrent mobile/ulcerated thrombi of thoracic and abdominal aorta causing recurrent spontaneous distal embolization. J Vasc Surg. 2008;47:193–196. doi: 10.1016/j.jvs.2007.07.050. [DOI] [PubMed] [Google Scholar]
- 9.Shammas NW, Dippel EJ, Coiner D, Shammas GA, Jerin M, Kumar A. Preventing lower extremity distal embolization using embolic filter protection: results of the PROTECT registry. J Endovasc Ther. 2008;15:270–276. doi: 10.1583/08-2397.1. [DOI] [PubMed] [Google Scholar]
- 10.Mackman N. Triggers, targets and treatments for thrombosis. Nature. 2008;451:914–918. doi: 10.1038/nature06797. [DOI] [PMC free article] [PubMed] [Google Scholar]