Abstract
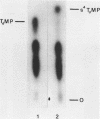
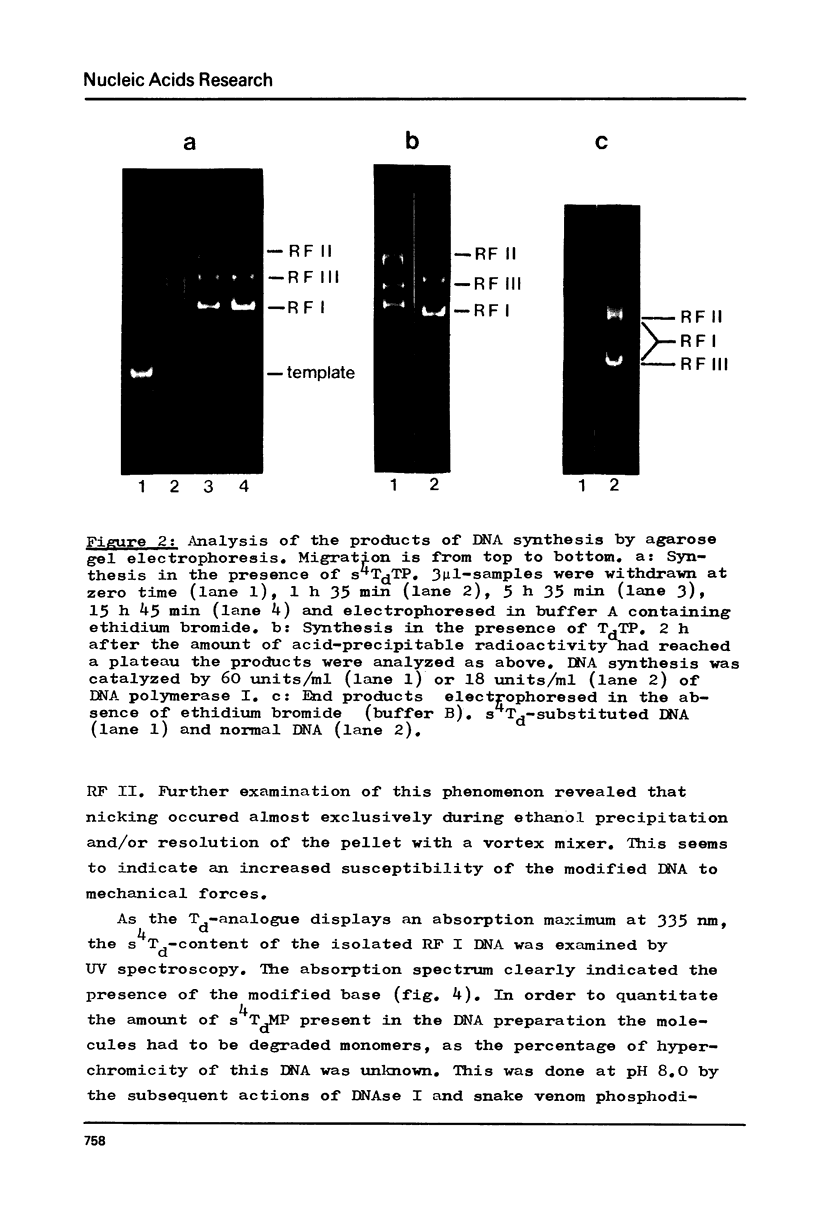
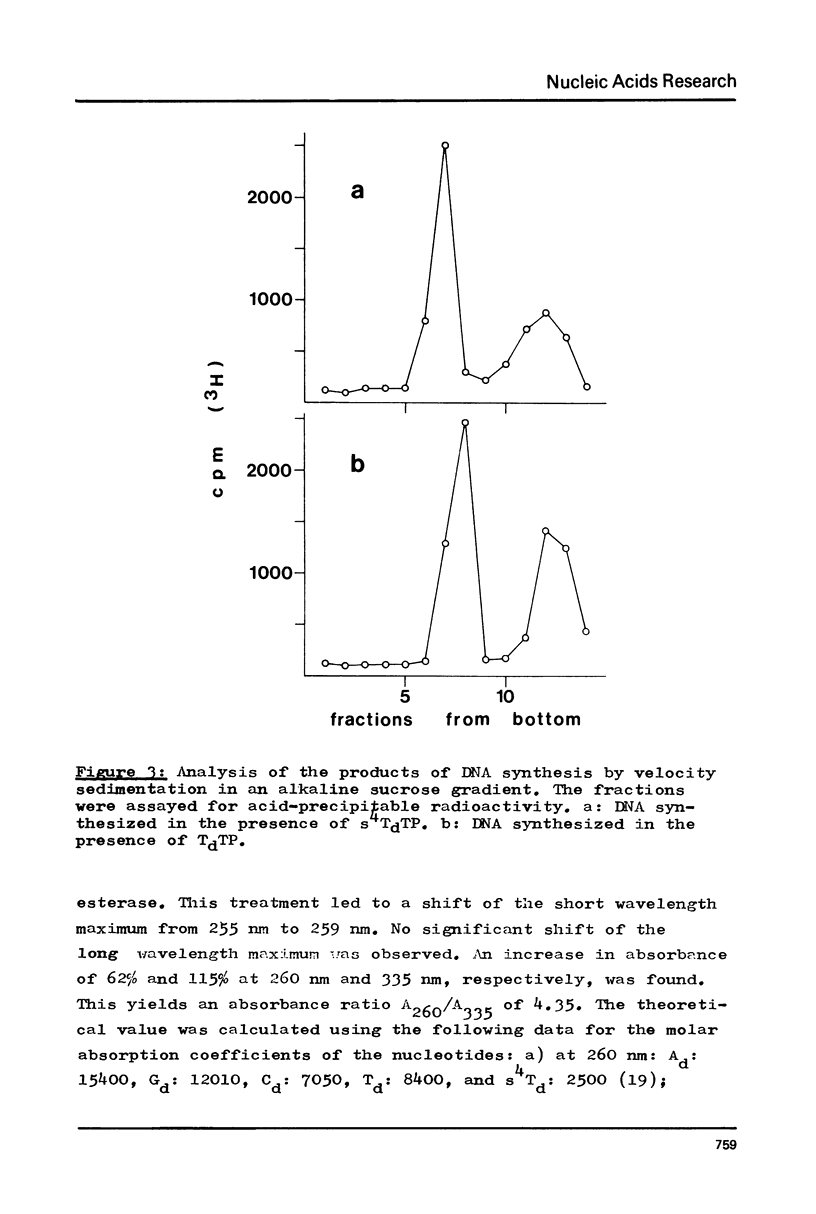
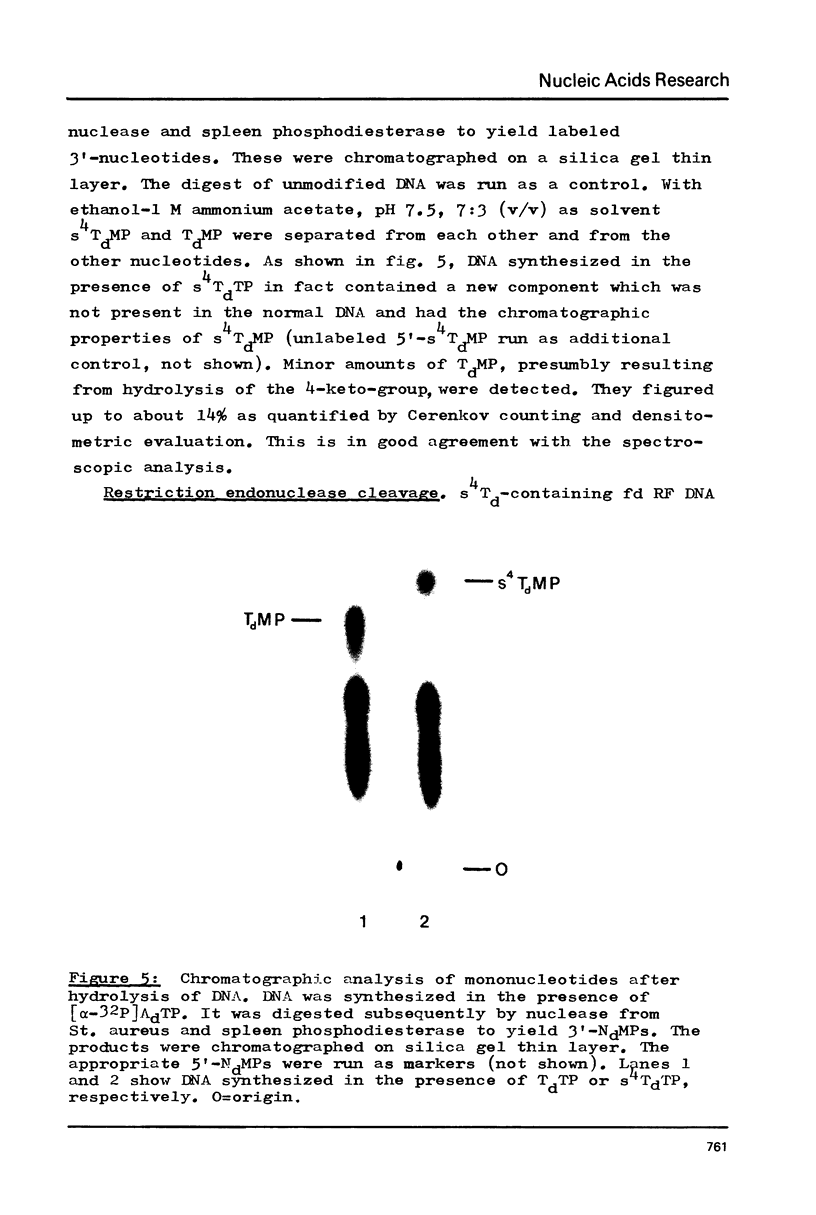
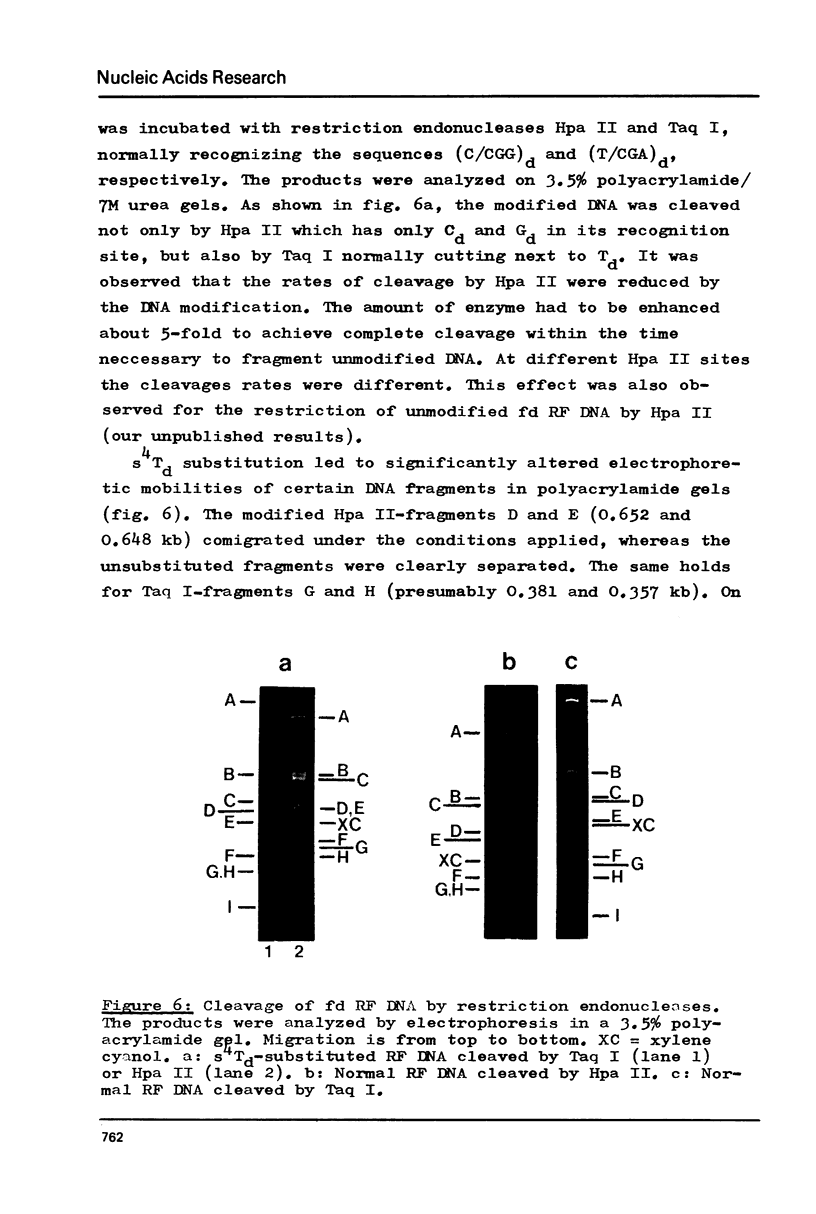
Phage fd RF I DNA1 about 90% substituted by deoxy-4-thiothymidine (s4Td) in the codogenic strand was synthesized by the simultaneous actions of DNA polymerase I and DNA ligase. While the rate of DNA synthesis was considerably reduced, the yield the rate of DNA synthesis was considerably reduced, the yield was not affected in the presence of s4TdTP. The conversion of RF II to RF I DNA by DNA ligase was even improved. This effect seems to be related with an altered ratio of affinity of polymerase and ligase for the s4Td-containing substrate. The presence of the base analogue in the DNA was verified independently by chromatographic and spectroscopic methods. The modified genome could be cleaved by restriction endonucleases Hpa II (C/CGG)d and Taq I (T/CGA)d. A number of the fragments produced showed altered mobilities under the conditions of polyacrylamide gel electrophoresis.
Full text
PDF










Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beck E., Sommer R., Auerswald E. A., Kurz C., Zink B., Osterburg G., Schaller H., Sugimoto K., Sugisaki H., Okamoto T. Nucleotide sequence of bacteriophage fd DNA. Nucleic Acids Res. 1978 Dec;5(12):4495–4503. doi: 10.1093/nar/5.12.4495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CHAMBERLIN M., BALDWIN R. L., BERG P. AN ENZYMICALLY SYNTHESIZED RNA OF ALTERNATING BASE SEQUENCE: PHYSICAL AND CHEMICAL CHARACTERIZATION. J Mol Biol. 1963 Oct;7:334–349. doi: 10.1016/s0022-2836(63)80028-5. [DOI] [PubMed] [Google Scholar]
- Cullen B. R., Bick M. D. Thermal denaturation of DNA from bromodeoxyuridine substituted cells. Nucleic Acids Res. 1976 Jan;3(1):49–62. doi: 10.1093/nar/3.1.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Depew D. E., Wang J. C. Conformational fluctuations of DNA helix. Proc Natl Acad Sci U S A. 1975 Nov;72(11):4275–4279. doi: 10.1073/pnas.72.11.4275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donohue J. On N-H--S hydrogen bonds. J Mol Biol. 1969 Oct 28;45(2):231–235. doi: 10.1016/0022-2836(69)90102-8. [DOI] [PubMed] [Google Scholar]
- Fasy T. M., Cullen B. R., Luk D., Bick M. D. Studies on the enhanced interaction of halodeoxyuridine-substituted DNAs with H1 histones and other polypeptides. J Biol Chem. 1980 Feb 25;255(4):1380–1387. [PubMed] [Google Scholar]
- Favre A., Yaniv M., Michelson A. M. The photochemistry of 4-thiouridine in Escherichia coli t-RNA Vał1. Biochem Biophys Res Commun. 1969 Oct 8;37(2):266–271. doi: 10.1016/0006-291x(69)90729-3. [DOI] [PubMed] [Google Scholar]
- Flavell R. A., Sabo D. L., Bandle E. F., Weissmann C. Site-directed mutagenesis: generation of an extracistronic mutation in bacteriophage Q beta RNA. J Mol Biol. 1974 Oct 25;89(2):255–272. doi: 10.1016/0022-2836(74)90517-8. [DOI] [PubMed] [Google Scholar]
- Goeddel D. V., Yansura D. G., Caruthers M. H. How lac repressor recognizes lac operator. Proc Natl Acad Sci U S A. 1978 Aug;75(8):3578–3582. doi: 10.1073/pnas.75.8.3578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray C. P., Sommer R., Polke C., Beck E., Schaller H. Structure of the orgin of DNA replication of bacteriophage fd. Proc Natl Acad Sci U S A. 1978 Jan;75(1):50–53. doi: 10.1073/pnas.75.1.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofer B., Köster H. Elimination of promoter function by base modification of DNA. FEBS Lett. 1979 Jun 1;102(1):87–90. doi: 10.1016/0014-5793(79)80934-5. [DOI] [PubMed] [Google Scholar]
- INMAN R. B., BALDWIN R. L. Helix-random coil transitions in synthetic DNAs of alternating sequence. J Mol Biol. 1962 Aug;5:172–184. doi: 10.1016/s0022-2836(62)80082-5. [DOI] [PubMed] [Google Scholar]
- Iida S., Chung K. C., Hayatsu H. The reaction of hydroxylamine with 4-thiouridine. Biochim Biophys Acta. 1973 May 10;308(2):198–204. doi: 10.1016/0005-2787(73)90149-4. [DOI] [PubMed] [Google Scholar]
- JOSSE J., KAISER A. D., KORNBERG A. Enzymatic synthesis of deoxyribonucleic acid. VIII. Frequencies of nearest neighbor base sequences in deoxyribonucleic acid. J Biol Chem. 1961 Mar;236:864–875. [PubMed] [Google Scholar]
- Jansz H. S., Pouwels P. H., Schiphorst J. Preparation of double-stranded DNA (replicative form) of bacteriophage phi-X174: a simplified method. Biochim Biophys Acta. 1966 Sep;123(3):626–627. doi: 10.1016/0005-2787(66)90233-4. [DOI] [PubMed] [Google Scholar]
- Kallos J., Fasy T. M., Hollander V. P., Bick M. D. Estrogen receptor can distinguish among various halodeoxyuridine-substituted DNAs. FEBS Lett. 1979 Feb 15;98(2):347–350. doi: 10.1016/0014-5793(79)80214-8. [DOI] [PubMed] [Google Scholar]
- Lezium A. G., Rath U. Synthesis of poly (d(A-s 4 T)-d(A-s 4 T)) by Bacillus subtilis DNA polymerase. Eur J Biochem. 1971 Dec 22;24(1):163–167. doi: 10.1111/j.1432-1033.1971.tb19667.x. [DOI] [PubMed] [Google Scholar]
- Lezius A. G., Scheit K. H. Enzymatic synthesis of DNA with 4-thio-thymidine triphosphate as substitute for dTTP. Eur J Biochem. 1967 Dec;3(1):85–94. doi: 10.1111/j.1432-1033.1967.tb19501.x. [DOI] [PubMed] [Google Scholar]
- Lezius A. G. Sequenzrestriktionen der E.-coli-DNA-Polymerase mit den basenanalogen Substraten 4-Thiothymidin- und Desoxyinosintriphosphat. Hoppe Seylers Z Physiol Chem. 1971 Mar;352(3):491–500. [PubMed] [Google Scholar]
- Lin S., Lin D., Riggs A. D. Histones bind more tightly to bromodeoxyuridine-substituted DNA than to normal DNA. Nucleic Acids Res. 1976 Sep;3(9):2183–2191. doi: 10.1093/nar/3.9.2183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MAHLER H. R., KLINE B., MEHROTRA B. D. SOME OBSERVATIONS ON THE HYPOCHROMISM OF DNA. J Mol Biol. 1964 Sep;9:801–811. doi: 10.1016/s0022-2836(64)80186-8. [DOI] [PubMed] [Google Scholar]
- Marchionni M. A., Roufa R. J. Digestion of 5-bromodeoxyuridine-substituted lambda-DNA by restriction endonucleases. J Biol Chem. 1978 Dec 25;253(24):9075–9081. [PubMed] [Google Scholar]
- Modrich P., Lehman I. R. Enzymatic joining of polynucleotides. IX. A simple and rapid assay of polynucleotide joining (ligase) activity by measurement of circle formation from linear deoxyadenylate-deoxythymidylate copolymer. J Biol Chem. 1970 Jul 25;245(14):3626–3631. [PubMed] [Google Scholar]
- Müller W., Weber H., Meyer F., Weissmann C. Site-directed mutagenesis in DNA: generation of point mutations in cloned beta globin complementary dna at the positions corresponding to amino acids 121 to 123. J Mol Biol. 1978 Sep 15;124(2):343–358. doi: 10.1016/0022-2836(78)90303-0. [DOI] [PubMed] [Google Scholar]
- Olivera B. M., Lehman I. R. Linkage of polynucleotides through phosphodiester bonds by an enzyme from Escherichia coli. Proc Natl Acad Sci U S A. 1967 May;57(5):1426–1433. doi: 10.1073/pnas.57.5.1426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pulleyblank D. E., Shure M., Tang D., Vinograd J., Vosberg H. P. Action of nicking-closing enzyme on supercoiled and nonsupercoiled closed circular DNA: formation of a Boltzmann distribution of topological isomers. Proc Natl Acad Sci U S A. 1975 Nov;72(11):4280–4284. doi: 10.1073/pnas.72.11.4280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheit K. G. Die Synthese von 4-Thiouridin-5'-diphosphat, 4-Thiouridin-5'-triphosphat und Desoxy-4-thiothymidin-5'-triphosphat. Chem Ber. 1968;101(4):1141–1147. doi: 10.1002/cber.19681010402. [DOI] [PubMed] [Google Scholar]
- Simpson R. B. Contacts between Escherichia coli RNA polymerase and thymines in the lac UV5 promoter. Proc Natl Acad Sci U S A. 1979 Jul;76(7):3233–3237. doi: 10.1073/pnas.76.7.3233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stahl S. J., Chamberlin M. J. Transcription of T7 DNA containing modified nucleotides by bacteriophage T7 specific RNA polymerase. J Biol Chem. 1978 Jul 25;253(14):4951–4959. [PubMed] [Google Scholar]
- Sági J., Brahms S., Brahms J., Otvös L. Effect of 5-alkyl substitution of uracil on the thermal stability of poly [d(A-r5U)] copolymers. Nucleic Acids Res. 1979 Jun 25;6(8):2839–2848. doi: 10.1093/nar/6.8.2839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wheeler F. C., Fishel R. A., Warner R. C. Agarose gel electrophoresis of circular DNA of replicative form of bacteriophage G4. Anal Biochem. 1977 Mar;78(1):260–275. doi: 10.1016/0003-2697(77)90031-8. [DOI] [PubMed] [Google Scholar]
- Ziff E. B., Fresco J. R. A method for locating 4-thiouridylate in the primary structure of transfer ribonucleic acids. Biochemistry. 1969 Aug;8(8):3242–3248. doi: 10.1021/bi00836a016. [DOI] [PubMed] [Google Scholar]
- Zimmerman S. B., Oshinsky C. K. Enzymatic joining of deoxyribonucleic acid strands. 3. Further purification of the deoxyribonucleic acid ligase from Escherichia coli and multiple forms of the purified enzyme. J Biol Chem. 1969 Sep 10;244(17):4689–4695. [PubMed] [Google Scholar]
- Zipper P. A small-angle- x-ray scattering study on poly (d(A-t)-d(A-T)), poly(d(A-s4T)-d(A-s4T)) and poly (d(A-s4U)-d(A-s4U)). Eur J Biochem. 1973 Nov 15;39(2):493–498. doi: 10.1111/j.1432-1033.1973.tb03148.x. [DOI] [PubMed] [Google Scholar]