Abstract
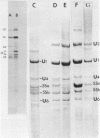
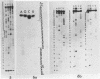
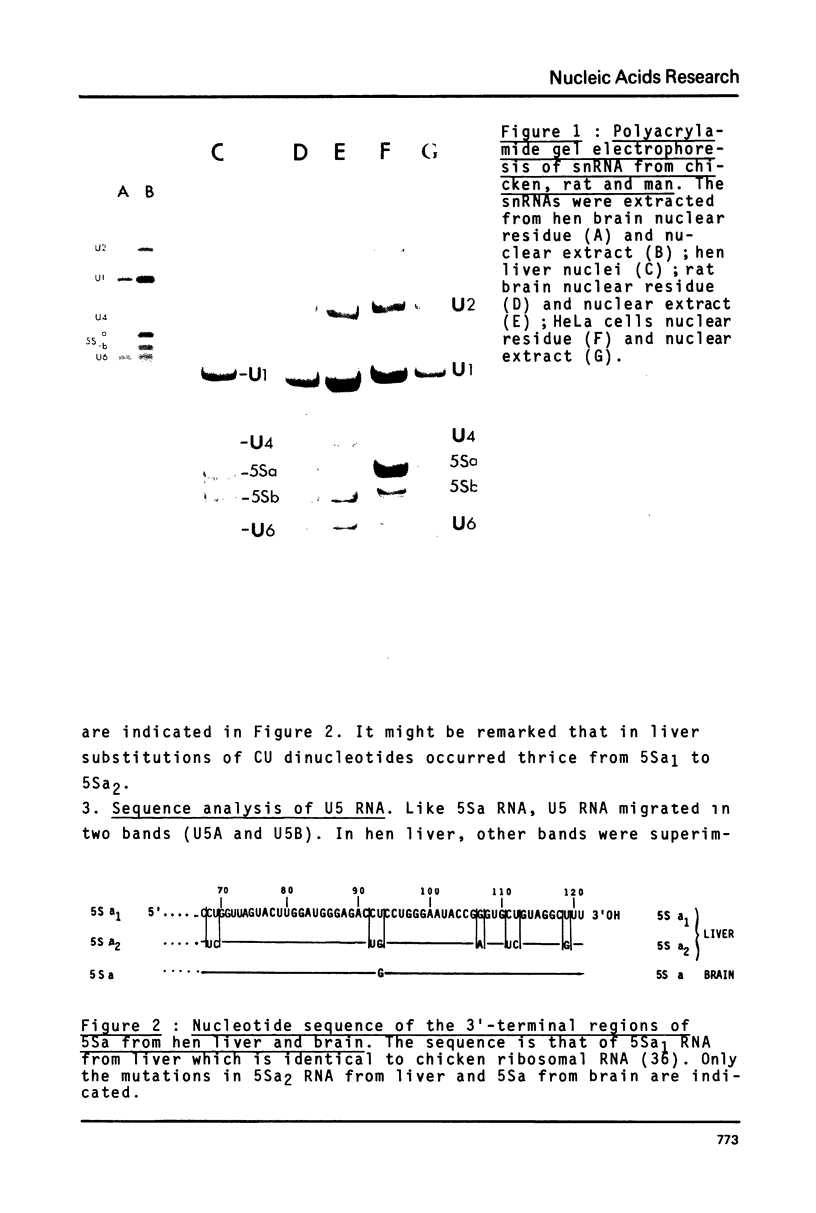
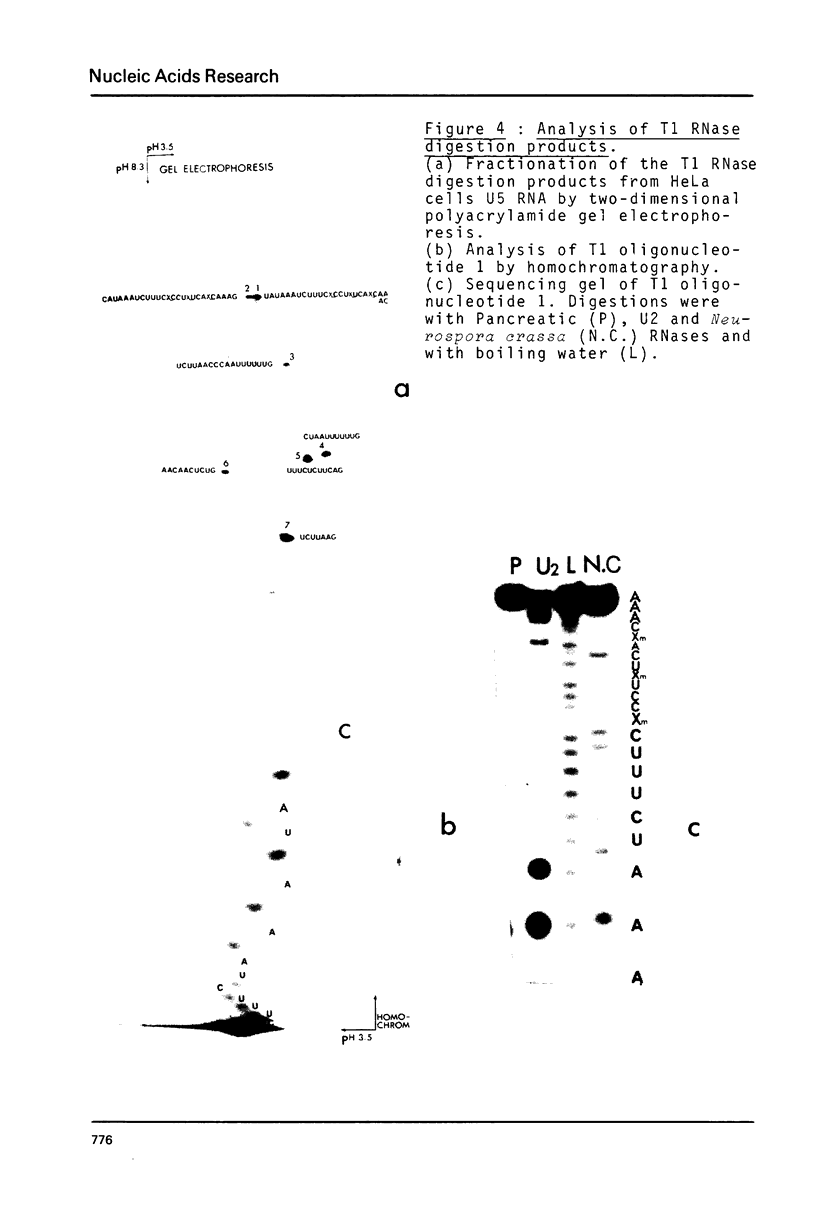
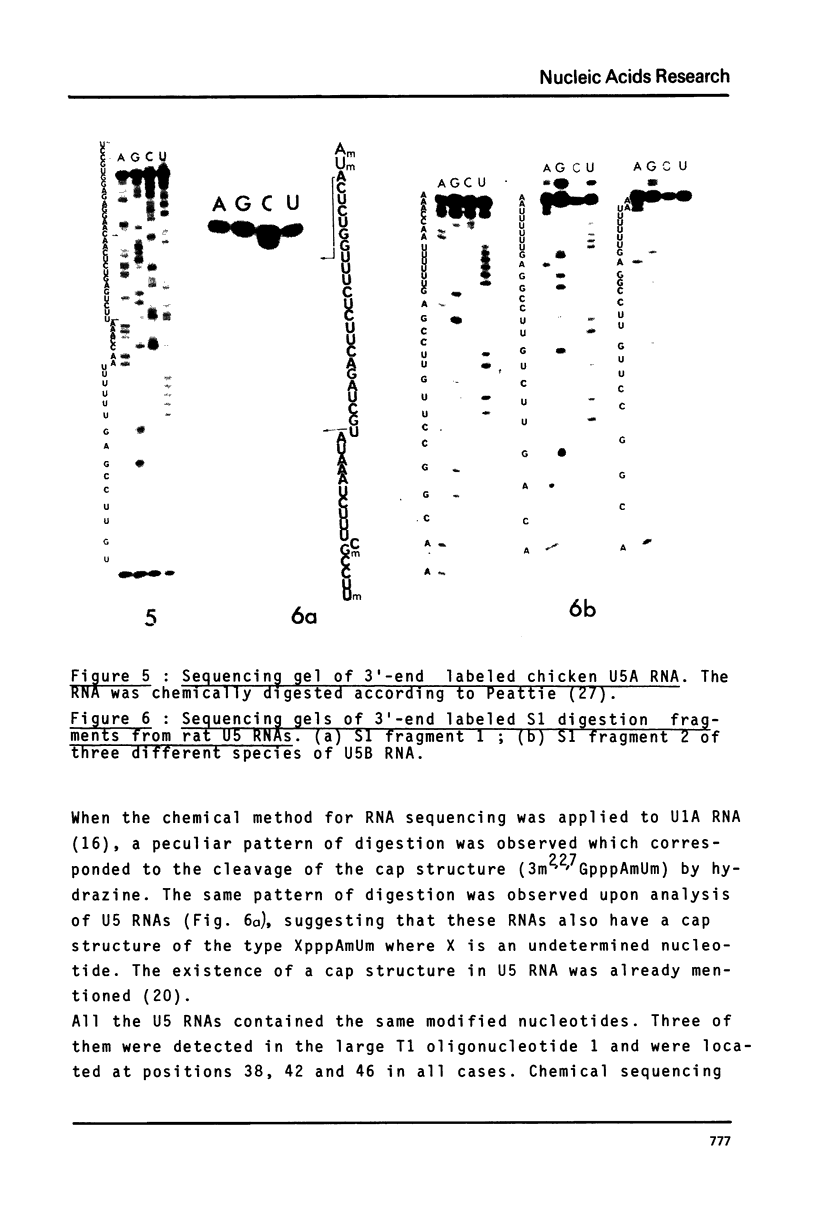
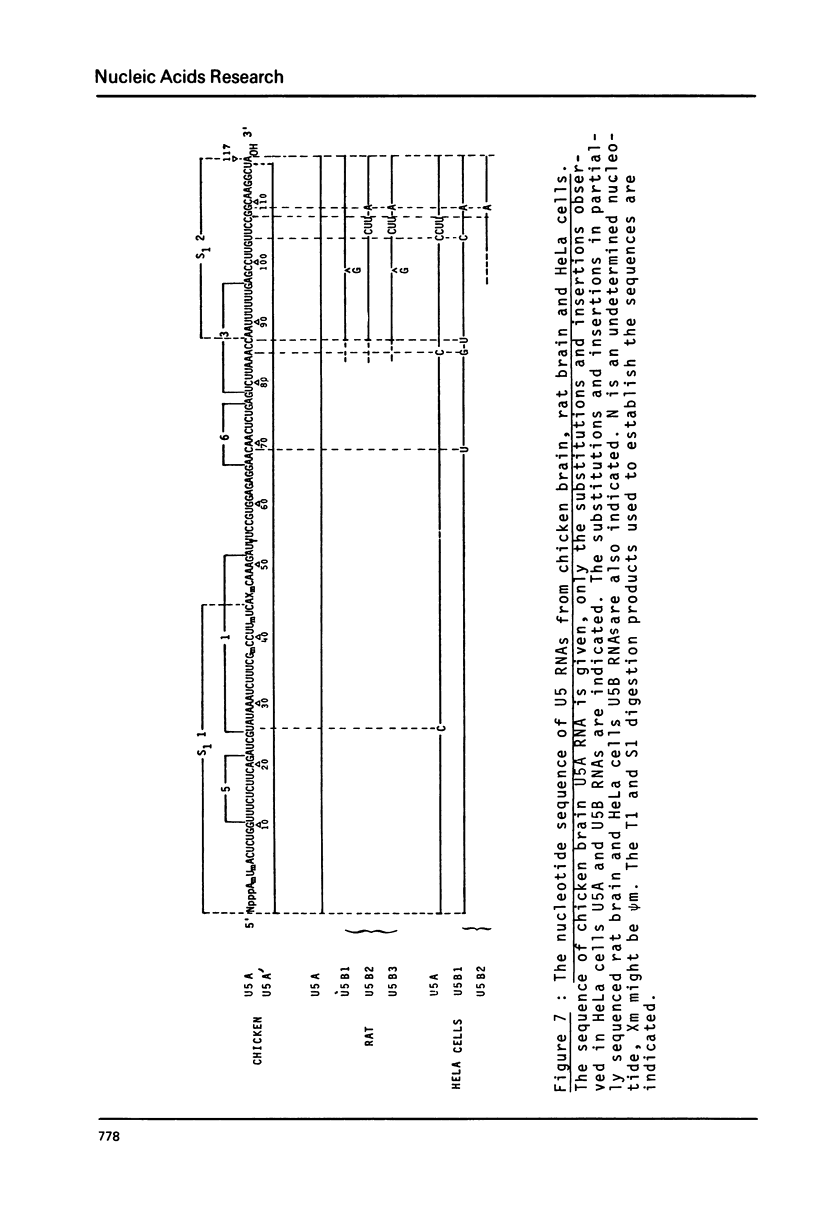
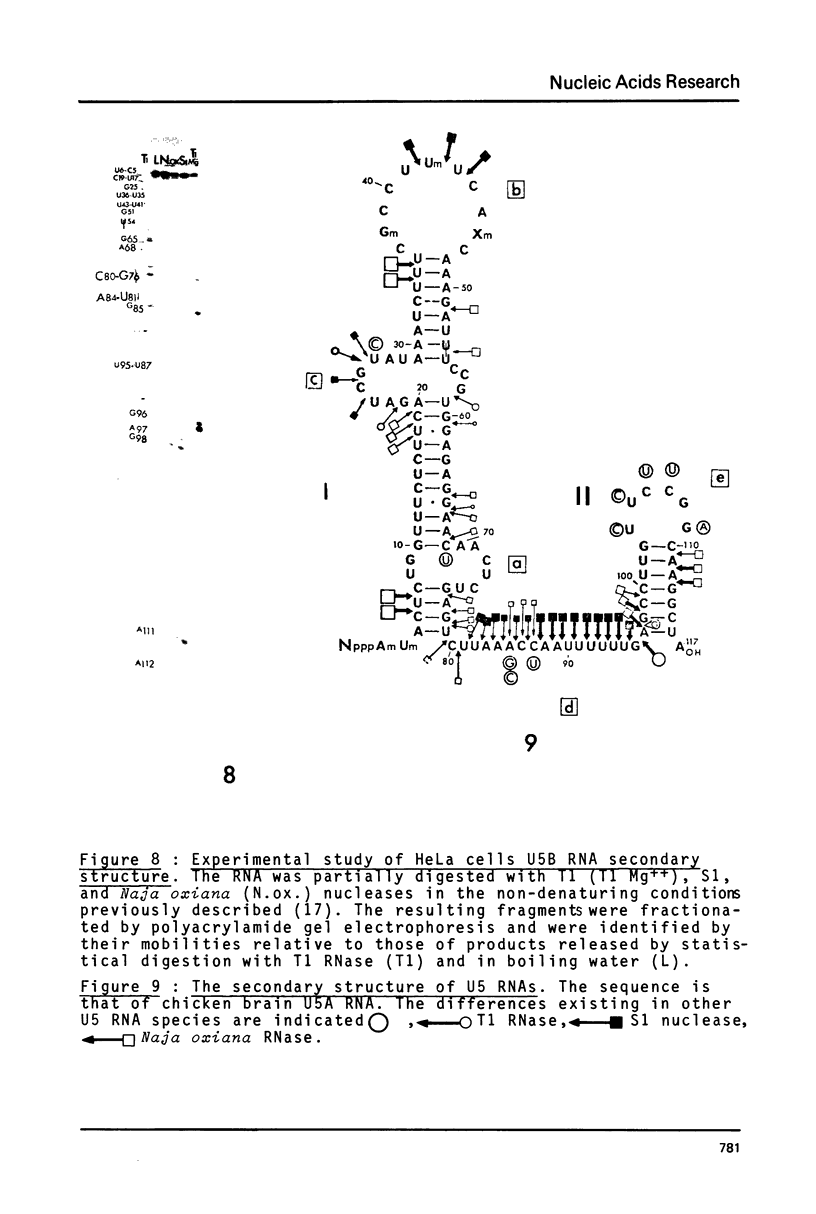
Preparations of chicken, rat and human nuclear 5S RNA contain two sets of molecules. The set with the lowest electrophoretic mobility (5Sa) contains RNAs identical or closely related to ribosomal 5S RNA from the corresponding animal species. In HeLa cells and rat brain, we only detected an RNA identical to the ribosomal 5S RNA. In hen brain and liver, we found other species differing by a limited number of substitutions. The results suggest that mutated 5S genes may be expressed differently according to the cell type. The set with the highest mobility corresponds to U5 RNA. In both rat brain and HeLa cells, U5 RNA was found to be composed of 4 and 5 different molecules respectively (U5A, U5B1-4) differing by a small number of substitutions or insertions. In hen brain, no U5B was detected but U5A' differing from U5A by the absence of the 3'-terminal adenosine. All the U5 RNAs contain the same set of modified nucleotides. They also have the same secondary structure which consists of two hairpins joined together by a 17 nucleotide long single-stranded region. The 3' half of the molecule has a compact conformation. Together, the results suggest that U5 RNAs are transcribed from a multigene family and that mutated genes may be expressed as far as secondary structure is conserved. The conformation of U5 RNA is likely to be related to its function and it is of interest to mention that several similarities of structure are found between U5 and U1A RNA.
Full text
PDF













Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Branlant C., Krol A., Ebel J. P., Lazar E., Gallinaro H., Jacob M., Sri-Widada J., Jeanteur P. Nucleotide sequences of nuclear U1A RNAs from chicken, rat and man. Nucleic Acids Res. 1980 Sep 25;8(18):4143–4154. doi: 10.1093/nar/8.18.4143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Branlant C., Krol A., Machatt M. A., Ebel J. P. Structural study of ribosomal 23 S RNA from Escherichia coli. FEBS Lett. 1979 Nov 1;107(1):177–181. doi: 10.1016/0014-5793(79)80490-1. [DOI] [PubMed] [Google Scholar]
- Brown R. S., Rubin J. R., Rhodes D., Guilley H., Simoncsits A., Brownlee G. G. The nucleoside sequence of tyrosine tRNA from Bacillus stearothermophilus. Nucleic Acids Res. 1978 Jan;5(1):23–36. doi: 10.1093/nar/5.1.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brownlee G. G., Cartwright E. M. The nucleotide sequence of the 5S RNA of chicken embryo fibroblasts. Nucleic Acids Res. 1975 Dec;2(12):2279–2288. doi: 10.1093/nar/2.12.2279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brownlee G. G., Sanger F. Chromatography of 32P-labelled oligonucleotides on thin layers of DEAE-cellulose. Eur J Biochem. 1969 Dec;11(2):395–399. doi: 10.1111/j.1432-1033.1969.tb00786.x. [DOI] [PubMed] [Google Scholar]
- Busch H. The function of the 5' cap of mRNA and nuclear RNA species. Perspect Biol Med. 1976 Summer;19(4):549–567. doi: 10.1353/pbm.1976.0064. [DOI] [PubMed] [Google Scholar]
- Deimel B., Louis C. H., Sekeris C. E. The presence of small molecular weight RNAs in nuclear ribonucleoprotein particles carrying HnRNA. FEBS Lett. 1977 Jan 15;73(1):80–84. [PubMed] [Google Scholar]
- England T. E., Uhlenbeck O. C. 3'-terminal labelling of RNA with T4 RNA ligase. Nature. 1978 Oct 12;275(5680):560–561. doi: 10.1038/275560a0. [DOI] [PubMed] [Google Scholar]
- Epstein P., Reddy R., Henning D., Busch H. The nucleotide sequence of nuclear U6 (4.7 S) RNA. J Biol Chem. 1980 Sep 25;255(18):8901–8906. [PubMed] [Google Scholar]
- Flytzanis C., Alonso A., Louis C., Krieg L., Sekeris C. E. Association of small nuclear RNA with HnRNA isolated from nuclear RNP complexes carrying HnRNA. FEBS Lett. 1978 Dec 1;96(1):201–206. doi: 10.1016/0014-5793(78)81094-1. [DOI] [PubMed] [Google Scholar]
- Forget B. G., Weissman S. M. Low molecular weight RNA components from KB cells. Nature. 1967 Mar 4;213(5079):878–882. doi: 10.1038/213878a0. [DOI] [PubMed] [Google Scholar]
- Gallinaro-Matringe H., Stevenin J., Jacob M. Salt dissociation of nuclear particles containing DNA-like RNA. Distribution of phosphorylated and nonphosphorylated species. Biochemistry. 1975 Jun 3;14(11):2547–2554. doi: 10.1021/bi00682a039. [DOI] [PubMed] [Google Scholar]
- Gallinaro H., Jacob M. An evaluation of small nuclear RNA in hnRNP. FEBS Lett. 1979 Aug 1;104(1):176–182. doi: 10.1016/0014-5793(79)81110-2. [DOI] [PubMed] [Google Scholar]
- Gattoni R., Stevenin J., Jacob M. Comparison of the nuclear ribonucleoproteins containing the transcripts of adenovirus-2 and HeLa cell dna. Eur J Biochem. 1980;108(1):203–211. doi: 10.1111/j.1432-1033.1980.tb04713.x. [DOI] [PubMed] [Google Scholar]
- Guimont-Ducamp C., Sri-Widada J., Jeanteur P. Occurrence of small molecular weight RNAs in Hela nuclear ribonucleoprotein particles containing HnRNA. Biochimie. 1977;59(8-9):755–758. doi: 10.1016/s0300-9084(77)80259-9. [DOI] [PubMed] [Google Scholar]
- Harada F., Kato N., Nishimura S. The nucleotide sequence of nuclear 4.8S RNA of mouse cells. Biochem Biophys Res Commun. 1980 Aug 14;95(3):1332–1340. doi: 10.1016/0006-291x(80)91620-4. [DOI] [PubMed] [Google Scholar]
- Hatlen L. E., Amaldi F., Attardi G. Oligonucleotide pattern after pancreatic ribonuclease digestion and the 3' and 5' termini of 5S ribonucleic acid from HeLa cells. Biochemistry. 1969 Dec;8(12):4989–5005. doi: 10.1021/bi00840a048. [DOI] [PubMed] [Google Scholar]
- Jelinek W., Leinwand L. Low molecular weight RNAs hydrogen-bonded to nuclear and cytoplasmic poly(A)-terminated RNA from cultured Chinese hamster ovary cells. Cell. 1978 Sep;15(1):205–214. doi: 10.1016/0092-8674(78)90095-8. [DOI] [PubMed] [Google Scholar]
- Korn L. J., Brown D. D. Nucleotide sequence of Xenopus borealis oocyte 5S DNA: comparison of sequences that flank several related eucaryotic genes. Cell. 1978 Dec;15(4):1145–1156. doi: 10.1016/0092-8674(78)90042-9. [DOI] [PubMed] [Google Scholar]
- Krupp G., Gross H. J. Rapid RNA sequencing: nucleases from Staphylococcus aureus and Neurospora crassa discriminate between uridine and cytidine. Nucleic Acids Res. 1979 Aug 10;6(11):3481–3490. doi: 10.1093/nar/6.11.3481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larsen C. J., Galibert F., Lelong J. C., Boiron M. Caractérisation dans la fraction nucléaire de cellules KB d'un ARN métaboliquement stable de faible poids moléculaire. C R Acad Sci Hebd Seances Acad Sci D. 1967 Mar 13;264(11):1523–1526. [PubMed] [Google Scholar]
- Lerner M. R., Boyle J. A., Mount S. M., Wolin S. L., Steitz J. A. Are snRNPs involved in splicing? Nature. 1980 Jan 10;283(5743):220–224. doi: 10.1038/283220a0. [DOI] [PubMed] [Google Scholar]
- Lerner M. R., Steitz J. A. Antibodies to small nuclear RNAs complexed with proteins are produced by patients with systemic lupus erythematosus. Proc Natl Acad Sci U S A. 1979 Nov;76(11):5495–5499. doi: 10.1073/pnas.76.11.5495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Northemann W., Scheurlen M., Gross V., Heinrich P. C. Circular dichroism of ribonucleoprotein complexes from rat liver nuclei. Biochem Biophys Res Commun. 1977 Jun 20;76(4):1130–1137. doi: 10.1016/0006-291x(77)90973-1. [DOI] [PubMed] [Google Scholar]
- Peattie D. A. Direct chemical method for sequencing RNA. Proc Natl Acad Sci U S A. 1979 Apr;76(4):1760–1764. doi: 10.1073/pnas.76.4.1760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prestayko A. W., Busch H. Low molecular weight RNA of the chromatin fraction from Novikoff hepatoma and rat liver nuclei. Biochim Biophys Acta. 1968 Dec 17;169(2):327–337. doi: 10.1016/0005-2787(68)90041-5. [DOI] [PubMed] [Google Scholar]
- Reddy R., Henning D., Busch H. Nucleotide sequence of nucleolar U3B RNA. J Biol Chem. 1979 Nov 10;254(21):11097–11105. [PubMed] [Google Scholar]
- Reddy R., Henning D., Busch H. Substitutions, insertions, and deletions in two highly conserved U3 RNA species. J Biol Chem. 1980 Jul 25;255(14):7029–7033. [PubMed] [Google Scholar]
- Ro-Choi T. S., Reddy R., Henning D., Busch H. 5S RNA 3 , a new nucleus-specific 5S RNA. Biochem Biophys Res Commun. 1971 Aug 20;44(4):963–972. doi: 10.1016/0006-291x(71)90806-0. [DOI] [PubMed] [Google Scholar]
- Rogers J., Wall R. A mechanism for RNA splicing. Proc Natl Acad Sci U S A. 1980 Apr;77(4):1877–1879. doi: 10.1073/pnas.77.4.1877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger F., Coulson A. R. The use of thin acrylamide gels for DNA sequencing. FEBS Lett. 1978 Mar 1;87(1):107–110. doi: 10.1016/0014-5793(78)80145-8. [DOI] [PubMed] [Google Scholar]
- Silberklang M., Gillum A. M., RajBhandary U. L. Use of in vitro 32P labeling in the sequence analysis of nonradioactive tRNAs. Methods Enzymol. 1979;59:58–109. doi: 10.1016/0076-6879(79)59072-7. [DOI] [PubMed] [Google Scholar]
- Stevenin J., Gallinaro-Matringe H., Gattoni R., Jacob M. Complexity of the structure of particles containing heterogeneous nuclear RNA as demonstrated by ribonuclease treatment. Eur J Biochem. 1977 Apr 15;74(3):589–602. doi: 10.1111/j.1432-1033.1977.tb11428.x. [DOI] [PubMed] [Google Scholar]
- Tinoco I., Jr, Borer P. N., Dengler B., Levin M. D., Uhlenbeck O. C., Crothers D. M., Bralla J. Improved estimation of secondary structure in ribonucleic acids. Nat New Biol. 1973 Nov 14;246(150):40–41. doi: 10.1038/newbio246040a0. [DOI] [PubMed] [Google Scholar]
- Tschudi C., Pirrotta V. Sequence and heterogeneity in the 5S RNA gene cluster of Drosophila melanogaster. Nucleic Acids Res. 1980 Feb 11;8(3):441–451. doi: 10.1093/nar/8.3.441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinberg R. A., Penman S. Small molecular weight monodisperse nuclear RNA. J Mol Biol. 1968 Dec;38(3):289–304. doi: 10.1016/0022-2836(68)90387-2. [DOI] [PubMed] [Google Scholar]
- Weinberg R. A., Penman S. Small molecular weight monodisperse nuclear RNA. J Mol Biol. 1968 Dec;38(3):289–304. doi: 10.1016/0022-2836(68)90387-2. [DOI] [PubMed] [Google Scholar]