Abstract
In intergroup competition and conflict, humans benefit from coalitions with strong partners who help them to protect their in-group and prevail over competing out-groups. Here, we link oxytocin, a neuropeptide produced in the hypothalamus, to ally selection in intergroup competition. In a double-blind placebo-controlled experiment, males self-administered oxytocin or placebo, and made selection decisions about six high-threat and six low-threat targets as potential allies in intergroup competition. Males given oxytocin rather than placebo viewed high-threat targets as more useful allies and more frequently selected them into their team than low-threat targets.
Keywords: hormones, coalition formation, person perception, decision-making
1. Introduction
In intergroup competition and conflict, both humans and non-human primates engage allies to defend and promote group interests [1–4]. Group interests are served by inviting strong partners with high threat potential, who help defend the in-group and promote its chances of prevailing in intergroup competition and conflict [5–7]. As such, coalescing with strong allies who can ward off rivalling outsiders may be part of a general inclination to defend and promote one's own group, which may be driven by the same neurobiological circuitries as other forms of in-group cooperation, such as parochial altruism, social attachment formation and parental care [8].
Here, we examine this possibility, and focus on oxytocin, a neuropeptide that is produced in the hypothalamus and functions as hormone and neurotransmitter. Oxytocin has a well-established role in reproduction and pair-bond formation [9,10], reduces psychological and physiological stress responses, and modulates brain areas and neural circuitries involved in the processing of fear-related information [11,12]. For example, when given an intranasal dose of oxytocin (versus placebo), humans had reduced activation of the amygdala, and attenuated coupling of the amygdala to brainstem centres responsible for autonomic and behavioural components of fear [13].
Because fearful, anxiety-provoking stimuli and situations typically motivate an immediate and automatic fight-or-flight response [14,15], and because oxytocin has anxiolytic effects, oxytocin may allow the individual to consider a broader variety of behavioural strategies than immediate fight-or-fly responding [16,17]. Indeed, individuals given oxytocin rather than placebo respond less fearfully to angry faces [18], couples given intranasal oxytocin rather than placebo manage their conflicts more constructively [19] and individuals given oxytocin rather than placebo display reduced betrayal aversion [20,21]. Interestingly, these effects emerge especially when protagonists are familiar or categorized as belonging to one's in-group, and not when protagonists are unfamiliar or belonging to out-groups. Intranasal administration of oxytocin (versus placebo) increases cooperation only when participants familiarized themselves with their interaction partner [22], when protagonists were described as trustworthy [23], or when protagonists belonged to one's in-group [24]. Furthermore, in free-living meerkats, peripheral administration of oxytocin rather than placebo increased an array of cooperative behaviours, including digging, associating with pups and, most relevant here, time spent on guard [8]. Finally, in intergroup competition, individuals given oxytocin rather than placebo were less cooperative towards the out-group, especially when the out-group represented a threat to in-group outcomes [25] (see also [26]).
These emerging insights together suggest that oxytocin shifts the individual from being focused on self-interest towards tending for the interests of the in-group and its members. As noted, one way to protect and promote in-group interests is to coalesce with strong allies that have high rather than low threat potential, and are thus particularly instrumental in allowing the in-group to prevail in intergroup competition and conflict. Accordingly, we predicted that individuals given oxytocin rather than placebo would be more likely to select allies with high rather than low threat potential.
2. Material and methods
Seventy-two males received €10 (USD 13) to participate in a study on medication and decision-making. Exclusion criteria were medical or psychiatric illness, medication, smoking, and drug or alcohol abuse. Participants came in groups of six, and were seated in soundproof cubicles, randomly assigned to the oxytocin or placebo group (double-blind, placebo-controlled study design) and tested individually.
Participants self-administered, under experimenter supervision, a single intranasal dose of 24 IU oxytocin (Syntocinon-Spray Novartis; three puffs per nostril) or placebo. To avoid any subjective effects (e.g. olfactory effects), other than those caused by oxytocin, the placebo contained all the active ingredients except for the neuropeptide. The placebo was manufactured by Stichting Apothekers Haarlemse Ziekenhuizen in coordination with the pharmacy at the Amsterdam Medical Centre, adhering to the European Union guidelines on Good Manufacturing Practice and Good Clinical Practice. The placebo was produced using the exact recipes and procedures used by Novartis Inc. to produce the carrier of Syntocinon—the synthetic analogue of oxytocin. Placebos were delivered in the same bottles as Syntocinon. In short, the only difference between the placebo and the treatment was the absence versus presence of the active neuropeptide.
The experimenter left and participants completed computer-guided unrelated tests. Because effects of oxytocin plateau 40 min after administration [20], the computer switched to the instructions for the main tasks after 30 min. To prime intergroup competition, we followed the procedures and tasks used in an earlier study (exp. 3 of [25]). Participants were organized into two three-person groups and informed that they would engage in decision-making affecting the financial earnings of their own and the other group. Specifically, each member of each group would make a decision that affected their personal income, that of their fellow in-group members and that of the out-group.
The decision situation was structured as a prisoner's dilemma [27,28]. Each participant had to choose between A (reflecting cooperation) and B (reflecting non-cooperation), and was informed that when he and the out-group representative chose A (joint cooperation), each individual in each group would earn €3; unilateral cooperation (A by participant, B by out-group) would result in €0 to each in-group member and €4 to each out-group member; unilateral non-cooperation (B by participant, A by out-group) would result in €4 to each in-group member and €0 to each out-group member; and joint non-cooperation (B by participant, B by out-group) resulted in €1 to each individual in each group. Thus, a cooperative choice would benefit the out-group at the expense of the in-group when the out-group would choose non-cooperatively, and benefit in-group and out-group equally when the out-group would (also) make a cooperative choice. A non-cooperative choice would protect the in-group against non-cooperation by the out-group, and benefit the in-group most in case the out-group would choose cooperatively. Accordingly, to serve one's in-group best, participants should make a non-cooperative rather than cooperative choice [25,27].
Participants made three confidential decisions without feedback, with one decision being randomly chosen for pay-out to oneself, one's fellow in-group members and the out-group. On average, the instructions and decision-making took approximately 10 min. Confirming earlier findings [25] across the three decisions, males given oxytocin were less cooperative (M = 2.39) towards the out-group than males given placebo (M = 2.88; directional t(70) = 1.76, p < 0.042; range 0 = non-cooperative to 3 = fully cooperative).
Following decision-making, participants read that in many competitions, players can choose who is in their team. Then they were shown 12 pictures, each on a new screen and randomized per participant. Participants indicated for each picture whether they would select the target for their team (0 = no; 1 = yes), as well as how dangerous and how useful the target was (both 1 = not at all; 5 = very much). We used six different pictures of faces morphed into low and high threat by Oosterhof & Todorov [29] (www.facegen.com; figure 1). Although people rely on a multitude of cues when perceiving and interpreting faces [30], Oosterhof & Todorov [29] identified trustworthiness and dominance as the two orthogonal dimensions that are sufficient to describe face evaluation. Although face-trustworthiness is more sensitive to features signalling whether the person should be avoided or approached, dominance evaluation is more sensitive to features signalling physical strength/weakness. Threatening faces—the focus of the current experiment—should be both untrustworthy (signalling that the person may have harmful intentions) and dominant (signalling that the person is capable of causing harm). Although these computer faces are somewhat artificial, the advantage is that other features of the face (e.g. symmetry) can be kept constant, thus creating optimal conditions for a clean hypothesis test [29,31]. Indeed, participants saw high-threat targets as more dangerous (M = 2.75, s.d. = 0.67) than low-threat targets (M = 2.01, s.d. = 0.62; F1,70 = 92.88, p < 0.001). The lack of effects involving treatment (all F < 1) confirms earlier research showing that oxytocin does not modulate the human ability to perceive emotional expressions [32]. The total selection task lasted approximately 7 min. Upon completion of the experiment, participants were paid and debriefed.
Figure 1.
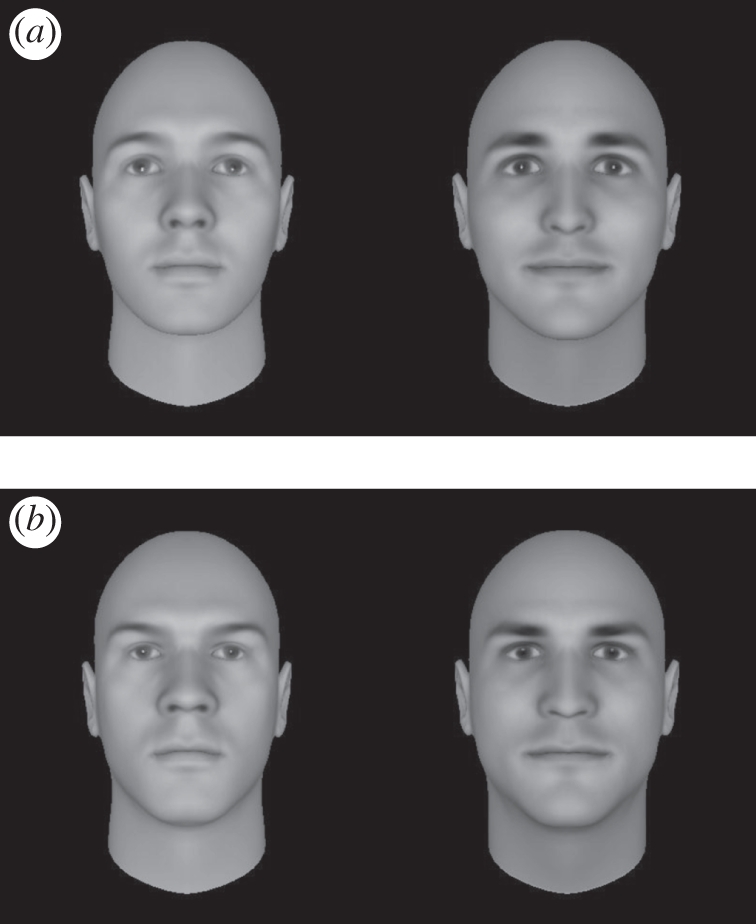
Examples of (a) low- and (b) high-threat facial morphs used as targets (adapted from [29]).
3. Analyses and results
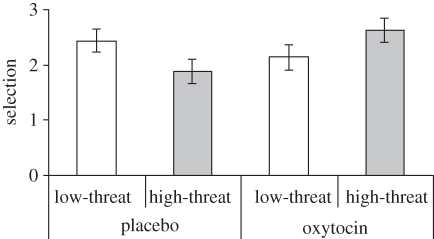
We averaged responses per measure across the six low-threat and six high-threat targets, and submitted indices to 2(treatment: oxytocin/placebo) × 2(target: high/low threat) ANOVA with treatment as between-subjects factor and target as within-subjects factor. For selection, we only found a treatment × target interaction (F1,70 = 13.17, p < 0.001; figure 2). Levene's test showed no heterogeneity of variance (F1,70 = 0.030, p < 0.862). Simple effects using the overall error term showed that males given oxytocin preferred high-threat targets more than males given placebo (F1,70 = 5.28, p < 0.025), and paired t-tests showed that they preferred high-threat over low-threat targets as in-group allies (t(34) = 2.69, p < 0.011). Males given placebo showed a reversed pattern: they preferred low-threat targets more than males given oxytocin (F1,70 = 8.13, p < 0.006), and paired t-tests showed that they preferred low-threat over high-threat targets as in-group allies (t(36) = −2.56, p < 0.015).
Figure 2.
Under oxytocin, high (low)-threat targets are more (less) often selected as in-group ally, range 0–6; displayed ±s.e.
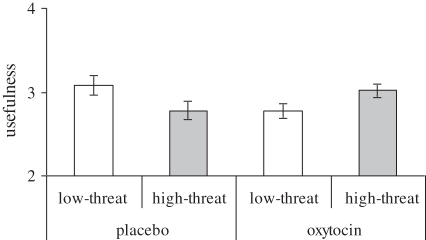
Consistent with our expectation that high-threat allies were selected because they are seen as more useful, we found a treatment × target interaction on usefulness ratings (F1,70 = 13.11, p < 0.001; figure 3). Levene's test for heterogeneity of variance was not significant (F1,70 = 0.056, p < 0.745). Simple main effects showed that individuals given oxytocin saw high-threat targets as more useful allies than males given placebo (F1,70 = 5.27, p < 0.027); paired t-tests showed that males given oxytocin rated high-threat targets as more useful allies than low-threat targets (t(34) = 2.82, p < 0.008). Under placebo, this pattern was reversed: males given placebo rated low-threat targets as more useful than those given oxytocin (F1,70 = 8.08, p < 0.005) and they saw low-threat allies as more useful than high-threat allies (t(36) = –2.49, p < 0.017). These effects remained unchanged when we took into account whether targets were selected or not. This analysis only revealed an additional main effect for selection decision (F1,70 = 52.06, p < 0.001), showing that selected targets were rated as more useful (M = 3.11) than targets not selected (M = 2.73).
Figure 3.
Under oxytocin, high (low)-threat targets are perceived as more (less) useful as in-group ally, range 1–5; displayed ±s.e.
To directly test for the possibility that selection decisions were due to perceived target usefulness, we tested the effects of treatment on selection decisions while controlling for usefulness. We calculated a difference score for selection (high-threat selections – low-threat selections) and for usefulness (high-threat usefulness – low-threat usefulness), and used the bootstrapping method for simple mediation [33]. This analysis showed that: (i) oxytocin motivated the selection of high-threat allies (B = 1.02, t = 3.63, p < 0.005); (ii) oxytocin increased usefulness ratings of high-threat allies (B = 0.536, t = 3.621, p < 0.001); (iii) usefulness ratings positively related to selection of high-threat targets (B = 0.99, t = 5.128, p < 0.001); and (iv) controlling for usefulness rendered the effect of treatment on ally selection non-significant (B = 0.487, t = 1.83, p < 0.10; bias and accelerated 95% CI: d =0.69/1.06). From these results, it follows that effects of oxytocin on ally selection emerge because of the effects of oxytocin on perceived target usefulness.
4. Conclusions and discussion
After being primed with intergroup competition, males given oxytocin rather than placebo perceived potential high-threat allies as more useful and more often decided to select them into their team than potential low-threat allies. Together, these findings indicate that oxytocin's functions include in-group protection [8,24–26]. In intergroup competition, oxytocin motivates humans to select allies that have high threat potential and appear aggressive rather than friendly, presumably to make their in-group a stronger and more threatening competitor to rival out-groups.
Oxytocin's anxiolytic effects [17] have been linked to reduced betrayal aversion [20,21], and may have been involved in the presently found tendency to select high-threat allies. However, oxytocin did not influence ratings of target dangerousness, ruling out that oxytocin altered danger perceptions, which in turn might have driven selection decisions. Furthermore, if reduced fear is the only explanation, we should have found no differences in selection decisions and usefulness judgements between high- and low-threat allies among participants given oxytocin. Clearly, additional processes are involved, and we conjecture that these relate to the motivation to protect in-group members against outside dangers, including those posed by competing out-groups. New research is needed to identify the neural circuitries involved in oxytocin-modulated in-group protection. In addition, because in the current study all participants were primed with intergroup competition prior to the ally selection task, new research is needed to examine whether current findings pertain to intergroup competition and conflict only, or generalize to ally selection in the context of other threats to in-group functioning and survival, including those posed by impending non-social catastrophes.
A limitation of the current study is that selection decisions were costless and facial morphs were approximations of human faces. While this may have lowered mundane realism, the advantage is that we have a clean test of the hypothesis that facially communicated threat drives our results [29]. One possible concern is, however, that threat covaries with facial attractiveness [29]. It may be difficult to see why oxytocin motivates people to prefer unattractive over attractive allies, but at present it cannot be excluded that perceived attractiveness explains some of the variance in selection decisions and usefulness ratings. New research is needed to conclusively settle this issue. Another question awaiting future research is whether the selection of high-threat allies is contingent upon personal interests at stake. For example, in intergroup competition and conflict, high-threat allies may help in-group protection and heighten the probability of winning the conflict, yet high-threat allies may also alter within-group status hierarchies and claim resources [6]. It would be interesting to examine whether (i) high-threat allies are more likely to be selected to the extent that within-group status hierarchies are stable rather than unstable, and (ii) oxytocin modulates such trade-offs between personal and group interests in ally selection. Given that oxytocin shifts the focus from personal to group interest, it may be that oxytocin renders people more tolerant of dominant newcomers claiming group resources. But if such claiming behaviour by dominant newcomers is perceived as a threat to in-group functioning and viability, it may well be that oxytocin actually motivates resistance and defensive aggression.
Oxytocin has been portrayed as ‘trust elixir’ [34], as the ‘peptide of love’ [35] and as the ‘moral molecule’ [36]. Whereas such labels suggest that oxytocin in humans promotes indiscriminate pro-sociality, generosity and honesty, current findings do not fit such an interpretation. Individuals given oxytocin did not select more allies—they were not generally more inclusive than participants given placebo. Also, those given oxytocin did not see potential allies as less dangerous than those given placebo, and they did not see others as generally more useful. Put differently, we obtained no evidence that oxytocin indiscriminately alters social cognition and judgement, or tendencies towards social inclusion. Instead, we found that under oxytocin, humans perceive high-threat allies as more useful than low-threat allies, and therefore more often select high-threat than low-threat allies as partners. Current results better fit the emerging insight that the functions of oxytocin are adaptive [37], and its effects contingent upon context [38]. We conjecture that oxytocin's functions extend beyond reproduction and pair-bond formation to include the motivation to tend and defend group life, including the willingness to ally with partners that increase the in-group's threat potential.
Acknowledgements
Financial support was granted by The Netherlands Science Foundation (400-06-098). The authors declare no competing interests.
References
- 1.Duffy K. G., Wrangham R. W., Silk J. B. 2007. Male chimpanzees exchange political support for mating opportunities. Curr. Biol. 17, 586–587 10.1016/j.cub.2007.06.001 (doi:10.1016/j.cub.2007.06.001) [DOI] [PubMed] [Google Scholar]
- 2.Harcourt A. H., De Waal F. B. M. 1992. Coalitions and alliances in humans and other animals. Oxford, UK: Oxford University Press [Google Scholar]
- 3.Johnstone R. A., Dugatkin L. A. 2000. Coalition formation in animals and the nature of winner and loser effects. Proc. R. Soc. Lond. B 267, 17–21 10.1098/rspb.2000.0960 (doi:10.1098/rspb.2000.0960) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Watts D. P. 1998. Coalitionary mate guarding by male chimpanzees at Ngogo, Kibale National Park, Uganda. Behav. Ecol. Sociobiol. 44, 43–55 10.1007/s002650050513 (doi:10.1007/s002650050513) [DOI] [Google Scholar]
- 5.Kurzban R., Tooby J., Cosmides L. 2001. Can race be erased? Coalitional computation and social categorization. Proc. Natl Acad. Sci. USA 98, 15 387–15 392 10.1073/pnas.251541498 (doi:10.1073/pnas.251541498) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Benenson J. F., Markovits H., Thompson M. E., Wrangham R. W. 2009. Strength determines coalitional strategies in humans. Proc. R. Soc. B 276, 2589–2595 10.1098/rspb.2009.0314 (doi:10.1098/rspb.2009.0314) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sorokin G. L. 1994. Arms, alliances, and security tradeoffs in enduring rivalries. Int. Stud. Q. 38, 421–446 10.2307/2600740 (doi:10.2307/2600740) [DOI] [Google Scholar]
- 8.Madden J. R., Clutton-Brock T. H. 2011. Experimental peripheral administration of oxytocin elevates a suite of cooperative behaviours in a wild social animal. Proc. R. Soc. B 278, 1189–1194 10.1098/rspb.2010.1675 (doi:10.1098/rspb.2010.1675) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Carter C. S., Grippo A. J., Pournajafi-Nazarloo H., Ruscio M. G., Porges S. W. 2008. Oxytocin, vasopressin and sociality. Progress Brain Res. 170, 331–336 10.1016/S0079-6123(08)00427-5 (doi:10.1016/S0079-6123(08)00427-5) [DOI] [PubMed] [Google Scholar]
- 10.Donaldson Z., Young L. J. 2008. Oxytocin, vasopressin and the neurogenetics of sociality. Science 322, 900–905 10.1126/science.1158668 (doi:10.1126/science.1158668) [DOI] [PubMed] [Google Scholar]
- 11.Heinrichs M., Baumgartner T., Kirschbaum C., Ehlert U. 2003. Social support and oxytocin interact to suppress cortisol and subjective responses to psychological stress. Biol. Psychiatry 54, 1389–1398 10.1016/S0006-3223(03)00465-7 (doi:10.1016/S0006-3223(03)00465-7) [DOI] [PubMed] [Google Scholar]
- 12.Bos P. A., Panksepp J., Bluthe R.-M., Van Honk J. In press Acute effects of steroid hormones and neuropeptides on human social-emotional behavior: a review of single administration studies. Front. Neuroendocrinol. 10.1016/j.yfrne.2011.01.002 (doi:10.1016/j.yfrne.2011.01.002) [DOI] [PubMed] [Google Scholar]
- 13.Kirsch P., et al. 2005. Oxytocin modulates neural circuitry for social cognition and fear in humans. J. Neurosci. 25, 11 489–11 493 10.1523/JNEUROSCI.3984-05.2005 (doi:10.1523/JNEUROSCI.3984-05.2005) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.LeDoux J. E. 2000. Emotion circuits in the brain. Annu. Rev. Neurosci. 23, 155–184 10.1146/annurev.neuro.23.1.155 (doi:10.1146/annurev.neuro.23.1.155) [DOI] [PubMed] [Google Scholar]
- 15.Phelps E. A. 2006. Emotion and cognition: insights from studies of the human amygdala. Annu. Rev. Psychol. 57, 27–53 10.1146/annurev.psych.56.091103.070234 (doi:10.1146/annurev.psych.56.091103.070234) [DOI] [PubMed] [Google Scholar]
- 16.Ross H. E., Young L. J. 2009. Oxytocin and the neural mechanisms regulating social cognition and affiliative behavior. Front. Neuroendocrinol. 30, 534–547 10.1016/j.yfrne.2009.05.004 (doi:10.1016/j.yfrne.2009.05.004) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Heinrichs M., von Dawans B., Domes G. 2009. Oxytocin, vasopressin, and human social behavior. Front. Neuroendocrinol. 30, 548–557 10.1016/j.yfrne.2009.05.005 (doi:10.1016/j.yfrne.2009.05.005) [DOI] [PubMed] [Google Scholar]
- 18.Evans S., Shergill S. S., Averbeck B. B. 2010. Oxytocin decreases aversion to angry faces in an associative learning task. Neuropsychopharmacology 35, 2502–2509 10.1038/npp.2010.110 (doi:10.1038/npp.2010.110) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ditzen B., Schaer M., Gabriel B., Bodenmann G., Ehlert U., Heinrichs M. 2009. Intranasal oxytocin increases positive communication and reduces cortisol levels during couple conflict. Biol. Psychiatry 65, 728–731 10.1016/j.biopsych.2008.10.011 (doi:10.1016/j.biopsych.2008.10.011) [DOI] [PubMed] [Google Scholar]
- 20.Baumgartner T., Heinrichs M., Vonlanthen A., Fischbacher U., Fehr E. 2008. Oxytocin shapes the neural circuitry of trust in humans. Neuron 58, 639–650 10.1016/j.neuron.2008.04.009 (doi:10.1016/j.neuron.2008.04.009) [DOI] [PubMed] [Google Scholar]
- 21.Kosfeld M., Heinrichs M., Zak P. J., Fischbacher U., Fehr E. 2005. Oxytocin increases trust in humans. Nature 435, 673–676 10.1038/nature03701 (doi:10.1038/nature03701) [DOI] [PubMed] [Google Scholar]
- 22.Declerck C. H., Boone C., Kiyonari T. 2010. Oxytocin and cooperation under conditions of uncertainty: the modulating role of incentives and social information. Horm. Behav. 57, 368. 10.1016/j.yhbeh.2010.01.006 (doi:10.1016/j.yhbeh.2010.01.006) [DOI] [PubMed] [Google Scholar]
- 23.Mikolajczak M., Gross J. J., Lane A., Corneille O., de Timary P. h., Luminet O. 2010. Oxytocin makes people trusting, not gullible. Psychol. Sci. 21, 1072–1075 10.1177/0956797610377343 (doi:10.1177/0956797610377343) [DOI] [PubMed] [Google Scholar]
- 24.De Dreu C. K. W., Greer L. L., Van Kleef G. A., Shalvi S., Handgraaf M. J. J. 2011. Oxytocin promotes human ethnocentrism. Proc. Natl Acad. Sci. USA 108, 1262–1266 10.1073/pnas.1015316108 (doi:10.1073/pnas.1015316108) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.De Dreu C. K. W., Greer L. L., Handgraaf M. J. J., Shalvi S., Van Kleef G. A., Baas M., Ten Velden F. S., Van Dijk E., Feith S. W. W. 2010. The neuropeptide oxytocin regulates parochial altruism in intergroup conflict among humans. Science 328, 1408–1411 10.1126/science.1189047 (doi:10.1126/science.1189047) [DOI] [PubMed] [Google Scholar]
- 26.Bosch O. J., Meddle S. L., Beiderbeck D. L., Douglas A. J., Neumann I. D. 2005. Brain oxytocin correlates with maternal aggression. J. Neurosci. 25, 6807–6815 10.1523/JNEUROSCI.1342-05.2005 (doi:10.1523/JNEUROSCI.1342-05.2005) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bornstein G. 2003. Intergroup conflict: individual, group and collective interests. Pers. Soc. Psychol. Rev. 7, 129–145 10.1207/S15327957PSPR0702_129-145 (doi:10.1207/S15327957PSPR0702_129-145) [DOI] [PubMed] [Google Scholar]
- 28.Komorita S. S., Parks C. D. 1995. Interpersonal relations: mixed-motive interaction. Annu. Rev. Psychol. 46, 183–207 10.1146/annurev.ps.46.020195.001151 (doi:10.1146/annurev.ps.46.020195.001151) [DOI] [Google Scholar]
- 29.Oosterhof N. N., Todorov A. 2008. The functional basis of face evaluation. Proc. Natl Acad. Sci. USA 105, 11 087–11 092 10.1073/pnas.0805664105 (doi:10.1073/pnas.0805664105) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Atkinson A., Adolphs R. 2011. The neuropsychology of face perception: beyond simple dissociations and functional selectivity. Phil. Trans. R. Soc. B 366, 1726–1738 10.1098/rstb.2010.0349 (doi:10.1098/rstb.2010.0349) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Said C. P., Haxby J. V., Todorov A. 2011. Brain systems for assessing the affective value of faces. Phil. Trans. R. Soc. B 366, 1660–1670 10.1098/rstb.2010.0351 (doi:10.1098/rstb.2010.0351) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Guastella A. J., Carson D., Dadds M. R., Mitchel P. h. B., Cox R. E. 2009. Does oxytocin influence the early detection of happy and angry faces? Psychoneuroendocrinology 34, 220–225 10.1016/j.psyneuen.2008.09.001 (doi:10.1016/j.psyneuen.2008.09.001) [DOI] [PubMed] [Google Scholar]
- 33.Preacher K. J., Hayes A. F. 2004. SPSS and SAS procedures for estimating indirect effects in simple mediation models. Behav. Res. Methods Instr. Comput. 36, 717–731 10.3758/BF03206553 (doi:10.3758/BF03206553) [DOI] [PubMed] [Google Scholar]
- 34.Miller G. 2010. The prickly side of oxytocin. Science 328, 1343. 10.1126/science.328.5984.1343-a (doi:10.1126/science.328.5984.1343-a) [DOI] [PubMed] [Google Scholar]
- 35.Carter C. S. 1998. Neuroendocrine perspectives on social attachment and love. Psychoneuroendocrinology 23, 779–818 10.1016/S0306-4530(98)00055-9 (doi:10.1016/S0306-4530(98)00055-9) [DOI] [PubMed] [Google Scholar]
- 36.Zak P. J. 2011. The physiology of moral sentiments. J. Econ. Behav. Organ 77, 212–233 10.1016/j.jebo.2010.09.004 (doi:10.1016/j.jebo.2010.09.004) [DOI] [Google Scholar]
- 37.Van Honk J., Terburg D., Bos P. A. 2011. Further notes on testosterone as social hormone. Trends Cogn. Sci. 15, 291–292 10.1016/j.tics.2011.05.003 (doi:10.1016/j.tics.2011.05.003) [DOI] [PubMed] [Google Scholar]
- 38.Bartz J. A., Zaki J., Bolger N., Ochsner K. N. 2011. Social effects of oxytocin in humans: context and person matter. Trends Cogn. Sci. 15, 301–309 10.1016/j.tics.2011.05.002 (doi:10.1016/j.tics.2011.05.002) [DOI] [PubMed] [Google Scholar]