Abstract
Competitive ability is a major determinant of fitness, but why individuals vary so much in their competitiveness remains only partially understood. One increasingly prevalent view is that realized competitive ability varies because it represents alternative strategies that arise because of the costs associated with competitiveness. Here we use a population of great tits (Parus major) to explore whether individual differences in competitive ability when foraging can be explained by two traits that have previously been linked to alternative behavioural strategies: the personality trait ‘exploration behaviour’ and a simple cognitive trait, ‘innovative problem-solving performance’. We assayed these traits under standardized conditions in captivity and then measured competitive ability at feeders with restricted access in the wild. Competitive ability was repeatable within individual males across days and correlated positively with exploration behaviour, representing the first such demonstration of a link between a personality trait and both competitive ability and food intake in the wild. Competitive ability was also simultaneously negatively correlated with problem-solving performance; individuals who were poor competitors were good at problem-solving. Rather than being the result of variation in ‘individual quality’, our results support the hypothesis that individual variation in competitive ability can be explained by alternative behavioural strategies.
Keywords: competitive ability, innovative problem-solving, exploration behaviour, personality, Parus major, alternative strategies
1. Introduction
Competition for limited resources is a fundamental ecological process in most natural systems [1] and competitive ability can have major consequences for individual fitness [2–4]. Although empirical studies show that individual state is an important determinant of competitive ability—for example, sex, age or body size (reviewed by Piper [5])—much individual variation over and above these state-dependent effects has yet to be explained (e.g. [6]). Traditionally, competitive ability has been assumed to primarily reflect either ‘individual quality’ or random error. This contrasts with an increasingly prevalent view that variation in competitive ability is adaptive and arises because the benefits of competitiveness can be outweighed by associated costs [7–12]. Commonly, these costs might include fighting [13], higher exposure to stress hormones [14] and elevated metabolic rate leading to increased food demands [15,16]. Consequently, under some circumstances, relatively poor competitors can paradoxically outperform good competitors [17,18], suggesting that variation in competitive ability may reflect alternative behavioural strategies. The extent to which alternative strategies might explain variation in competitive ability in natural systems, however, remains largely untested.
In this paper, we test whether variation in competitive ability can be explained simultaneously by links with two sources of behavioural variation that reflect alternative behavioural strategies. The first of these is referred to as the proactive–reactive axis, and represents perhaps the best-studied personality axis in non-human animals [19]. In contrast to individuals at the ‘reactive’ end of the continuum, ‘proactives’ are bolder, more aggressive and more risk-prone (reviewed by Sih et al. [7]). Potential explanations for how behavioural correlations are maintained within populations are diverse (reviewed by Dingemanse & Wolf [20] and Wolf & Weissing [21]). One theory with increasing empirical evidence links variation in proactivity to life-history variation, suggesting that proactives tend to sustain high productivity but at a potential cost to survival, while reactives do the opposite [8–10]. Proactives should therefore have higher realized competitive ability in order to meet the demands of their faster pace of life [9,10]. Indeed, a positive relationship between the proactive–reactive behavioural axis and both social dominance and competitive ability has been demonstrated in rodents [22,23], fish [24,25] and birds [26–28]. However, several studies have found that proactive individuals take longer to recover from social defeat than reactive individuals, and under some conditions are poorer competitors [29,30]. The only study to date that has examined links between the reactive–proactive axis and competitive ability in the wild found contrasting correlations within the same population, suggesting that this relationship may be dependent on the physical and social environment [31].
In addition to the reactive–proactive axis, competitive ability has also been linked to another source of behavioural variation: the tendency to innovate or problem-solve [32–34]. The ‘necessity drives innovation’ hypothesis predicts that good competitors monopolize access to limiting resources, forcing poor competitors to innovate [32,34,35]. Although it is often assumed that the observed variation in innovativeness is temporary (i.e. not consistent over time) and arises through phenotypic plasticity, it may equally represent more permanent, inherent differences between individuals. Irrespective of whether these differences are temporary or permanent, innovative foraging can be viewed as an alternative foraging strategy that helps some individuals avoid costs associated with intense competition [34,36], a hypothesis that is largely supported by empirical evidence from primates, fish and birds [32–34,37,38]. Individual consistency in innovativeness has rarely been assessed, however, and the extent to which inherent, long-term individual differences in innovativeness may contribute to variation in competitive ability remains unclear.
In this paper, we explore whether individual variation in competitive ability when foraging is linked simultaneously to the reactive–proactive behavioural axis, as assayed by ‘exploration behaviour in a novel environment’, and innovative problem-solving performance using a wild population of great tits (Parus major). Exploration behaviour is heritable, and correlated with a range of ecologically significant behaviours in several different populations, including ours [39–44]. Great tits are a generalist species [45], and anecdotal evidence suggests that they frequently investigate novel objects in the wild [46] and are capable of considerable innovative foraging behaviour [47–49]. Food-related problem-solving is therefore thought to be an ecologically relevant trait for this species, which we hypothesized would influence competition between individuals. Problem-solving performance is also repeatable and independent of exploration behaviour in our population [50].
To date, most studies that have tested for links between competitive ability and other behavioural traits in the same individuals simultaneously did so under laboratory conditions (but see [31,51]). Furthermore, few studies measure competitive ability directly (i.e. through food intake), which is important because subdominant individuals could potentially compensate by increasing foraging efficiency [52]. Great tits live in flocks during the winter, and competition for clumped, but limited food resources is intense [45]. Using observations of competitive interactions and food intake in the wild, we measured both social dominance and competitive ability for birds that had undergone behavioural assays in captivity. We then tested whether competitive ability could simultaneously be predicted by proactivity and problem-solving performance.
2. Methods
(a). Study site, catching procedure and housing
All behavioural assays were carried out on great tits in the context of a long-term study population at Wytham Woods, Oxfordshire, UK (51°46′ N, 1°20′ W). All birds were caught between September and March, from 2005 to 2009 (see the electronic supplementary material for details of trapping procedure). Unringed individuals were fitted with a unique BTO (British Trust for Ornithology) metal leg-ring, and were aged (adult or juvenile if less than 1 year old) and sexed based on plumage [53]. Biometrics were also taken (wing length, millimetres, and body mass, grams). Birds caught in the 2007–2008 and 2008–2009 winters, and in the 2007 and 2008 breeding seasons, were also fitted with a unique passive integrated transponder (PIT), attached to plastic leg ring, which was automatically detectable at feeders using antennae (see below). Birds were then taken into captivity (under a Natural England licence), where exploration behaviour and problem-solving performance were assayed under standardized conditions. All individuals were subsequently released back into the wild, where dominance and competitive ability were measured at eight experimental sites.
(b). Captive behavioural assays
(i). Exploration behaviour
Here, we use an assay of ‘exploration behaviour in a novel environment’ (hereafter exploration behaviour) as our measure of proactivity. In great tits, exploration behaviour is thought to reflect general activity levels, and has been shown to correlate with boldness, aggressiveness and risk-taking under captive conditions [26,54,55]. On the morning after capture, all birds underwent exploration behaviour assays. The assay room was based on the design used by Verbeek et al. [54], and consisted of a rectangular room containing five artificial trees. An event recorder was used to record the number of hops, the number and duration of flights, and the areas of the room used, for 8 min. These data were then entered into a principal component analysis, and the square-root of the first component (all major loadings referred to increased activity) used in a general linear model (GLM) to produce an exploration behaviour score for each bird from the parameter estimates derived from using individual as a fixed effect, controlling for time of year (see [41] for further details).
(ii). Problem-solving performance
Birds caught between November 2007 and March 2009 were presented with a problem task on the afternoon they were housed. The device used consisted of a vertical transparent Perspex tube containing a platform, which was supported by a horizontal lever. Four waxworms (Galleria mellonella) were placed on the platform to bait the device. To solve the task, birds had to remove the lever, causing the platform to drop and the waxworms to fall into a feeding dish (full methods in [50]). Forty-four per cent of birds solved the task. In contrast to other studies (e.g. [56]), solving performance was unrelated to exploration behaviour, neophobia towards the problem-solving device, motivation to feed from freely available food and residual body mass as a measure of body condition [50]. Solving propensity was repeatable over periods of up to a year and across different types of foraging-related tasks, suggesting our assay measures a biologically significant trait that we assume reflects a tendency to innovate, specifically to solve food-related problems [50].
(c). Field observations: competitive ability and dominance
Competitive ability and related traits were measured at eight feeders distributed throughout the woods during January to March 2009. On the morning of observation, the multiple-access-point peanut feeder used to attract birds to the catching site was replaced with a single-access-point feeder containing peanut granules (approx. 2 mm3; Jacobi Jayne Ltd, Herne Bay, UK). Tits generally prefer to take food away from feeders to protective cover before handling [57]. We assumed that by using smaller food items, we forced birds to either stay on the feeder for prolonged periods or visit more often in order to feed effectively, thus increasing the competition over access to the food. The feeder was also fitted with a PIT tag reader (Francis Scientific Instruments, Cambridge, UK) at the feeding hole, which logged the time and ID of visits by tagged birds. The tag reader has previously been demonstrated to detect the vast majority of visits by tagged individuals [58]. Birds were given 30 min to acclimatize to this change, and then their behaviour at the feeder was monitored for approximately 150 min using a Handycam DCR-SR33E camcorder (Sony Corporation, Tokyo, Japan). Monitoring was always carried out at the same time of day, beginning at approximately 9.00 h and ending by 12.00 h. The following day the procedure was repeated at the same site, allowing us to measure the repeatability of competitiveness in our sample. We note that this only allowed us to assess the repeatability of behaviour of birds that visited the feeder on both days, which we assume did not lead to bias. For an unrelated experiment, the feeder was positioned either near protective cover (a thicket or bushy area) or in the open on the first day, and in the opposite position on the second day. This was randomized across days and controlled for in all models.
The video footage and the PIT tag data were then analysed to establish the number and duration of all visits, as well as all competitive interactions (displacements and challenges) involving focal individuals (see the electronic supplementary material for details). Competitive ability was estimated as the amount of time that each individual had sole access to the food resource. To test whether this was a good predictor of the amount of food consumed, we also recorded the number of food items consumed on 100 randomly selected visits.
We used information on competitive interactions to establish whether our measure of competitive ability correlated with dominance status. Male great tits are invariably dominant over females [59], which was confirmed by our data: only 2 per cent of all displacement events involved a female displacing a male (n = 301), even though 42 per cent of all individuals visiting the feeders were female. We initially intended to construct sex-specific dominance hierarchies for all individuals, but only detected sufficient interactions between known birds to produce a male hierarchy for one of the eight sites (containing nine individuals, based on 41 aggressive interactions, for six of which we also collected data on visit duration). We were therefore unable to test the link between the total amount of time spent feeding and dominance for females, and consequently restricted our analyses to males (although inclusion of data, where available, for both sexes did not significantly change the results). Note also that the general lack of aggressive interactions is expected since physical fights are rare in great tits, and instead the outcome of contests is generally determined by physical assessment and posturing [45]; moreover, the dominance hierarchies of the flocks had probably already been established before we began our observations, reducing the probability of contests [45].
Dominance ranks were derived for the nine individuals for whom we had sufficient interaction data using David's scores [60,61] (see the electronic supplementary material). Scores were calculated using methodology from Gammell et al. [62]: the proportion of wins (Pij) by individual i in his interactions with another individual j is the number of times (αij) that i defeats j divided by the total number of interactions (nij) between i and j; thus Pij = αij/nij. The proportion of losses by i in interactions with j is Pji = 1 − Pij. The David's score for each member i of a group is calculated with the formula
where w = ∑Pij, w2 = ∑w (weighted by the appropriate Pij values of those individuals with which i interacted), l = ∑Pji and l2 = ∑l (weighted by the appropriate Pji values of those individuals with which i interacted).
(d). Statistical analysis
We initially tested whether the total time spent on the feeder (TTF) was an appropriate proxy for competitive ability in a feeding context by testing whether TTF predicted (i) the amount of food an individual consumed using a linear regression, and (ii) dominance measures using two-tailed Pearson's correlations and linear mixed models (LMMs). An LMM was also used to rule out the possibility that TTF merely reflected the order in which birds arrived at the feeder. TTF repeatability was estimated using Pearson's correlations. We tested whether TTF was influenced by both exploration behaviour and problem-solving performance using an LMM, with the following fixed effects: ‘age’ (juvenile/adult), ‘natal origin’ (Wytham-born/immigrant), ‘treatment’, ‘wing length’, ‘exploration behaviour’ and ‘problem-solving performance’ (solve/not solve). Effect significance was tested by dropping terms individually from the full model, and non-significant terms were removed via backwards elimination.
A previous study on great tits found that the dominance–exploration behaviour relationship was context-dependent [31]: fast-exploring territorial males had relatively high dominance ranks, but the relationship was reversed in non-territorial males. We tested this by assuming a bird was ‘territorial’ if it bred in Wytham, either in 2008 or 2009 (LMM, controlling for ‘age’ and ‘treatment’). The same study also found that the distance between a territorial male's nest-box and the feeder predicted social dominance; we tested this using straight-line distance from the breeding nest-box. The random terms ‘bird ID’ and ‘location’ were included in all mixed models, and all tests were two-tailed.
3. Results
(a). Competitive ability
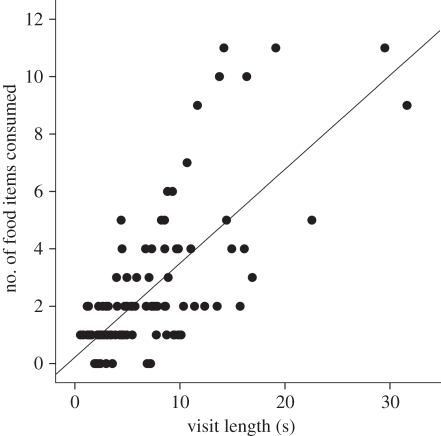
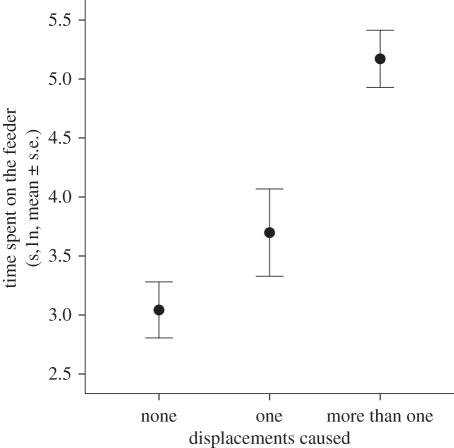
TTF predicted the number of food items consumed by individuals (Linear regression: F1,98 = 101.5, p < 0.001; figure 1) and was significantly repeatable across days (Pearson's correlation: r = 0.455, p = 0.014, n = 27). Dominance score was positively correlated with both TTF (Pearson's correlation: r = 0.892, p = 0.017, n = 6) and the total number of times an individual displaced another male from the feeder (Pearson's correlation: r = 0.930, p = 0.007, n = 6). TTF was also correlated with the total number of same-sex displacements caused by males (considering all birds; LMM: F1,84 = 15.35, p < 0.001; figure 2). TTF was unrelated to the order in which birds first visited the feeder during trials (LMM: F1,82 = 0.51, p = 0.476). Together, these results suggest that the total amount of time spent at the feeder is an appropriate proxy for competitive ability.
Figure 1.
Relationship between the number of food items consumed by great tits and visit length (n = 100).
Figure 2.
Relationship between the amount of time individual great tits spent feeding and the number of times they displaced another male from the feeder. Where individuals were recorded on more than 1 day, their feeding times and number of displacements were averaged across days (n = 62).
(b). Competitive ability, exploration behaviour and problem-solving
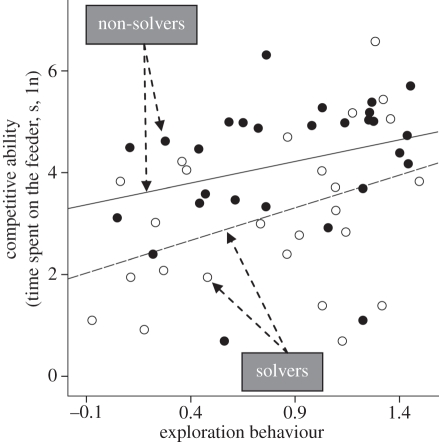
Fifty-three males of known exploration behaviour and problem-solving performance visited the feeders during our trial (exploration behaviour, mean ± s.e. = 0.829 ± 0.062; solving rate, 25 solvers and 28 non-solvers). TTF was higher in juveniles than in adults (LMM: F1,41 = 7.65, p = 0.008; table 1), but there was no difference between Wytham-born and immigrant birds, nor did body size (as estimated by wing length) predict TTF (table 1). TTF correlated positively with exploration behaviour (LMM: F1,39 = 6.45, p = 0.015; figure 3 and table 1), but correlated negatively with problem-solving performance (LMM: F1,43 = 4.53, p = 0.039; figure 3 and table 1); that is, fast explorers and non-solvers had significantly more access to the feeder than slow explorers and solvers. Finally, the relationship between exploration behaviour and TTF was independent of territoriality (LMM: interaction term: F1,40 = 1.34, p = 0.254) and distance from the breeding territory (LMM: F1,38 = 0.89, p = 0.352).
Table 1.
Factors contributing to variation in the amount of time individuals had access to the feeder. TTF was log-transformed to achieve normality. p-values derived from dropping individual terms from a linear mixed model. This minimum model was derived via backward elimination, removing non-significant terms. All terms shown were also significant in the full model (with n.s. terms: immigration status, wing length and treatment). The model also included random terms location (variance ± s.e. = 0.330 ± 0.304; likelihood ratio test: χ2 = 4.49, d.f. = 1, p = 0.026) and bird ID (variance ± s.e. = 0.189 ± 0.368; likelihood ratio test: χ2 = 0.260, d.f. = 1, p = 0.610). n = 80 records for 53 individuals.
| fixed terms | estimate ± s.e | F | p |
|---|---|---|---|
| constant | 4.400 ± 0.335 | n.a. | n.a. |
| agea | −0.889 ± 0.322 | 7.65 | 0.008 |
| exploration behaviour | 0.894 ± 0.352 | 6.45 | 0.015 |
| problem-solving performanceb | −0.685 ± 0.322 | 4.53 | 0.039 |
aJuvenile set to 0.
bNon-solver set to 0.
Figure 3.
Relationship between competitive ability (TTF) and exploration behaviour for solvers and non-solvers. Data plotted are the feeding times for each individual (averaged across days for birds observed more than once) and the lines are fitted regression lines (n = 53).
4. Discussion
Our results suggest that individual differences in competitive ability may be explained by two sources of behavioural variation: a heritable personality trait (exploration behaviour) and a repeatable cognitive trait (innovative problem-solving). In males, competitive ability was itself repeatable and correlated positively with both dominance rank and exploration behaviour, but negatively with problem-solving performance. The repeatability of competitive ability was assessed over a short time period, and only among those individuals that visited the feeder on consecutive days. The stability of competitive ability over longer time periods and in different social or physical conditions is unknown, though we note that exploration behaviour and problem-solving performance are repeatable in our population over years [41,50], suggesting that competitive ability may also be stable over longer time periods. To our knowledge, this is the first demonstration that a repeatable measure of competitive ability correlates in a contrasting manner with multiple behavioural traits that are often believed to represent independent, alternative behavioural strategies. It is also one of only a small number of studies to examine correlations between multiple behavioural traits of any kind in the same individuals simultaneously in the wild (but see [51,63]).
During winter, great tits compete for clumped but limited food resources, and suffer significant mortality from starvation, which is especially pronounced among subordinate individuals [64]. The amount of time an individual has access to a limited food resource is therefore likely to be a good indicator of competitive ability. Indeed, we found that time spent on feeders strongly predicted the amount of food consumed by an individual. Direct antagonistic interactions were relatively uncommon at the feeders in our experiment, probably because dominance hierarchies are also partly determined by physical assessment and posturing, neither of which are easy to detect [45]. Nevertheless, based on the interactions that did occur we found that the number of times individual male great tits displaced other males was a strong predictor of total time spent on the feeder (TTF). Our feeders represented a clumped food resource, which we assumed mimicked patterns of variation in the distribution of natural winter foods, such as beech mast (seed crop from Fagus sylvatica). Although we cannot rule out other sources of uncontrolled variation, together the effects observed support previous findings linking dominance status to feeding success in free-living great tits [65].
Proactivity, as measured by exploration behaviour, was a predictor of competitive ability when foraging. Several studies have demonstrated a positive relationship between the proactive–reactive axis and competitive ability in captivity [22,23,25–28], but the present study is one of the first to demonstrate this effect under largely natural conditions (but see [31]), and the first to show a direct link with the ability to acquire resources in the wild at the level of the individual. Most models describing adaptive personality variation explain the behavioural differences on the basis of differences in state [21]. Recent theories have viewed life-history strategy as the key state variable, suggesting that proactive individuals adopt a behavioural strategy that maximizes productivity, whereas reactives maximize survival [8–10]. Our findings are consistent with this idea, indicating that different personality types may reflect alternative life-history strategies [9,10]. Despite this link between competitive ability and proactivity, previously we reported no survival selection on exploration behaviour in our population [41], contrasting with the variable survival selection on exploration behaviour reported for another population of great tits [66]. However, the selective consequences of behavioural correlations are probably moderated by other ecological factors (e.g. the severity of weather conditions, food shortages or population density). They are also likely to be influenced by correlations with other traits that have not been measured, which themselves may be population-specific [67,68]. Understanding selection on any personality axis can therefore only be achieved by measuring partial selection gradients on all correlated, functionally significant traits simultaneously.
Individuals who performed poorly in a problem-solving task in captivity spent more time monopolizing the feeder compared with those who performed well. This agrees with the ‘necessity drives innovation hypothesis’, which predicts that subordinate individuals adopt alternative foraging strategies to avoid the costs associated with intense competition. Female great tits were no better at solving problems than males [50], to whom they are invariably subordinate [69], suggesting a restricted individual, rather than a general, effect of necessity on innovativeness. The ‘necessity drives innovation hypothesis’ implies a causal link between competitive ability and innovativeness, but nevertheless consistent individual differences in innovative foraging instead may lead to differences in realized competitive ability. Previously, we have shown that problem-solving performance is consistent over long time periods among individuals in our population [50], suggesting that the link between competitive ability and innovativeness may be the result of genetic or permanent environmental factors, rather than more transient environmental effects. Inherent individual differences in innovative problem-solving are therefore likely to contribute to variation in competitive ability, but it remains to be seen whether the phenotypic correlation observed occurs at the genetic level.
In contrast to the traditional idea that conspecifics are ecologically equivalent, many generalist species are composed of relatively specialized individuals that vary in their preference for, and productivity when using, different foraging methods [70]. In great tits, older individuals are thought to use a wider variety of food sources than younger birds [45]. Indeed, we found that first-year males spent more time on the feeder than adults, even though they are normally subordinate [71]. This supports previous work on our population showing that, even when competition at artificial feeders was relaxed, first-years used feeders more than adults [58]. Variation in competitive ability for a specific resource may therefore arise because the value of the resource differs between individuals. In this way, both adults with better knowledge of other food sources and innovative foragers that use an alternative foraging strategy will have a lower realized competitive ability as they rely more on sources of food other than the one subject to intense competition. This argument implies a causal link between problem-solving performance and competitive ability, which we are unable to establish in this study. If the phenotypic correlation proves to have a genetic basis, this would have important evolutionary implications because it would mean that individuals within the same population would be subject to different selection pressures owing to resource-specific ecological interactions [70,72].
An important facet of evolution is that natural selection is rarely likely to act on single traits in isolation owing to correlations between traits [73,74]. Few attempts have been made to establish the extent to which consistent variation in multiple behavioural traits measured under standardized conditions in captivity is correlated with functionally significant behaviour in nature (but see [51]), let alone examine the genetic basis [75,76] or selective consequences [12,51] of these associations. The behavioural correlations shown here suggest how individuals with different competitive abilities may be able to coexist; the evolutionary significance of these patterns now needs investigation.
Acknowledgements
The protocols outlined in this paper were all subject to ethical review by the Department of Zoology (Oxford) ethical committee.
We thank B. Sheldon and A. Kacelnik for useful discussion, and J. Morand-Ferron commenting on the manuscript. We also thank C. O'Luanaigh and J. Bates for help with data collection in the field, D. Wilson for bird husbandry, J. Dunn, S. Bouwhuis, S. Patrick, M. Wood and A. Gosler for help catching and assaying birds, and to B. Sheldon for supporting this project. This work was supported by a Biotechnological and Biological Sciences Research Council (BBSRC) studentship to E.F.C. and a Royal Society research grant to J.L.Q.
References
- 1.Milinski M., Parker G. A. 1991. Competition for resources. In Behavioural ecology: an evolutionary approach (eds Krebs J. R., Davies N. B.). Oxford, UK: Blackwell [Google Scholar]
- 2.Piper W. H., Wiley R. H. 1990. The relationship between social dominance, subcutaneous fat, and annual survival in wintering white-throated sparrows (Zonotrichia albicollis). Behav. Ecol. Sociobiol. 26, 201–208 10.1007/BF00172087 (doi:10.1007/BF00172087) [DOI] [Google Scholar]
- 3.Packer C., Collins D. A., Sindimwo A., Goodall J. 1995. Reproductive constraints on aggressive competition in female baboons. Nature 373, 60–63 10.1038/373060a0 (doi:10.1038/373060a0) [DOI] [PubMed] [Google Scholar]
- 4.Frank L. G. 1986. Social organization of the spotted hyena (Crocuta crocuta). II. Dominance and reproduction. Anim. Behav. 34, 1510–1527 10.1016/S0003-3472(86)80221-4 (doi:10.1016/S0003-3472(86)80221-4) [DOI] [Google Scholar]
- 5.Piper W. H. 1997. Social dominance in birds: early findings and new horizons. Curr. Ornithol. 14, 125–187 [Google Scholar]
- 6.Piper W. H., Haven Wiley R. 1989. Correlates of dominance in wintering white-throated sparrows: age, sex and location. Anim. Behav. 37, 298–310 10.1016/0003-3472(89)90119-X (doi:10.1016/0003-3472(89)90119-X) [DOI] [Google Scholar]
- 7.Sih A., Bell A., Johnson J. C. 2004. Behavioural syndromes: an ecological and evolutionary overview. Trends Ecol. Evol. 19, 372–378 10.1016/j.tree.2004.04.009 (doi:10.1016/j.tree.2004.04.009) [DOI] [PubMed] [Google Scholar]
- 8.Wolf M., Doorn G. S., van Leimar O., Weissing F. J. 2007. Life-history trade-offs favour the evolution of animal personalities. Nature 447, 581–584 10.1038/nature05835 (doi:10.1038/nature05835) [DOI] [PubMed] [Google Scholar]
- 9.Biro P. A., Stamps J. A. 2008. Are animal personality traits linked to life-history productivity? Trends Ecol. Evol. 23, 361–368 10.1016/j.tree.2008.04.003 (doi:10.1016/j.tree.2008.04.003) [DOI] [PubMed] [Google Scholar]
- 10.Réale D., Garant D., Humphries M. M., Bergeron P., Careau V., Montiglio O. 2010. Personality and the emergence of the pace-of-life syndrome concept at the population level. Phil. Trans. R. Soc. B 365, 4051–4063 10.1098/rstb.2010.0208 (doi:10.1098/rstb.2010.0208) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dall S. R. X., Houston A. I., McNamara J. M. 2004. The behavioural ecology of personality: consistent individual differences from an adaptive perspective. Ecol. Lett. 7, 734–739 10.1111/j.1461-0248.2004.00618.x (doi:10.1111/j.1461-0248.2004.00618.x) [DOI] [Google Scholar]
- 12.Réale D., Reader S. M., Sol D., McDougall P. T., Dingemanse N. J. 2007. Integrating animal temperament within ecology and evolution. Biol. Rev. 82, 291–318 10.1111/j.1469-185X.2007.00010.x (doi:10.1111/j.1469-185X.2007.00010.x) [DOI] [PubMed] [Google Scholar]
- 13.Rohwer S., Ewald P. W. 1981. The cost of dominance and advantage of subordination in a badge signalling system. Evolution 35, 441–454 10.2307/2408193 (doi:10.2307/2408193) [DOI] [PubMed] [Google Scholar]
- 14.Creel S. 2001. Social dominance and stress hormones. Trends Ecol. Evol. 16, 491–497 10.1016/S0169-5347(01)02227-3 (doi:10.1016/S0169-5347(01)02227-3) [DOI] [Google Scholar]
- 15.Røskaft E., Järvi T., Bakken M., Bech C., Reinertsen R. E. 1986. The relationship between social status and resting metabolic rate in great tits (Parus major) and pied flycatchers (Ficedula hypoleuca). Anim. Behav. 34, 838–842 10.1016/S0003-3472(86)80069-0 (doi:10.1016/S0003-3472(86)80069-0) [DOI] [Google Scholar]
- 16.Hogstad O. 1987. It is expensive to be dominant. Auk 104, 333–336 [Google Scholar]
- 17.Höjesjö J., Johnsson J., Bohlin T. 2004. Habitat complexity reduces the growth of aggressive and dominant brown trout (Salmo trutta) relative to subordinates. Behav. Ecol. Sociobiol. 56, 286–289 10.1007/S00265-004-0784-7 (doi:10.1007/S00265-004-0784-7) [DOI] [Google Scholar]
- 18.Metcalfe N. B. 1986. Intraspecific variation in competitive ability and food intake in salmonids: consequences for energy budgets and growth rates. J. Fish Biol. 28, 525–531 10.1111/j.1095-8649.1986.tb05190.x (doi:10.1111/j.1095-8649.1986.tb05190.x) [DOI] [Google Scholar]
- 19.Koolhaas J. M., Korte S. M., De Boer S. F., Van Der Vegt B. J., Van Reenen C. G., Hopster H., De Jong I. C., Ruis M. A. W., Blokhuis H. J. 1999. Coping styles in animals: current status in behaviour and stress-physiology. Neurosci. Biobehav. Rev. 23, 925–935 10.1016/S0149-7634(99)00026-3 (doi:10.1016/S0149-7634(99)00026-3) [DOI] [PubMed] [Google Scholar]
- 20.Dingemanse N., Wolf M. 2010. Recent models for adaptive personality differences: a review. Phil. Trans. R. Soc. B 365, 3947–3958 10.1098/rstb.2010.0221 (doi:10.1098/rstb.2010.0221) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wolf M., Weissing F. 2010. An explanatory framework for adaptive personality differences. Phil. Trans. R. Soc. B 365, 3959–3968 10.1098/rstb.2010.0215 (doi:10.1098/rstb.2010.0215) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fokkema D. S. 1985. Social behaviour and blood pressure: a study of rats. PhD thesis, University of Groningen, The Netherlands [Google Scholar]
- 23.Oortmerssen G. A., van Benus R. F., Dijk D. J. 1985. Studies in wild house mice: genotype environment interactions for attack latency. Netherlands J. Zool. 35, 155–169 10.1163/002829685X00118 (doi:10.1163/002829685X00118) [DOI] [Google Scholar]
- 24.Ward A. J. W., Thomas P., Hart P. J. B., Krause J. 2004. Correlates of boldness in three-spined sticklebacks (Gasterosteus aculeatus). Behav. Ecol. Sociobiol. 55, 561–568 10.1007/s00265-003-0751-8 (doi:10.1007/s00265-003-0751-8) [DOI] [Google Scholar]
- 25.Riebli T., Avgan B., Bottini A. M., Duc C., Taborsky M., Heg D. 2011. Behavioural type affects dominance and growth in staged encounters of cooperatively breeding cichlids. Anim. Behav. 81, 313–323 10.1016/j.anbehav.2010.11.001 (doi:10.1016/j.anbehav.2010.11.001) [DOI] [Google Scholar]
- 26.Verbeek M. E. M., Boon A., Drent P. J. 1996. Exploration, aggressive behaviour and dominance in pair-wise confrontations of juvenile male great tits. Behaviour 133, 945–963 10.1163/156853996X00314 (doi:10.1163/156853996X00314) [DOI] [Google Scholar]
- 27.Drent P. J., Marchetti C. 1999. Individuality, exploration and foraging in hand raised juvenile great tits. In Proc. 22nd Int. Ornithological Congress (eds Adams N. J., Slotow R. H.), pp. 896–914 Durban, South Africa: Johannesburg [Google Scholar]
- 28.David M., Auclair Y., Cézilly F. 2011. Personality predicts social dominance in female zebra finches, Taeniopygia guttata, in a feeding context. Anim. Behav. 81, 219–224 10.1016/j.anbehav.2010.10.008 (doi:10.1016/j.anbehav.2010.10.008) [DOI] [Google Scholar]
- 29.Verbeek M. E. M., Goede P. D., Drent P. J., Wiepkema P. R. 1999. Individual behavioural characteristics and dominance in aviary groups of great tits. Behaviour 136, 23–48 [Google Scholar]
- 30.Fox R., Ladage L. D., Roth T. C., Pravosudov V. V. 2009. Behavioural profile predicts dominance status in mountain chickadees. Anim. Behav. 77, 1441–1448 10.1016/j.anbehav.2009.02.022 (doi:10.1016/j.anbehav.2009.02.022) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dingemanse N. J., de Goede P. 2004. The relation between dominance and exploratory behaviour is context-dependent in wild great tits. Behav. Ecol. 15, 1023–1030 10.1093/beheco/arh115 (doi:10.1093/beheco/arh115) [DOI] [Google Scholar]
- 32.Laland K. N., Reader S. M. 1999. Foraging innovation is inversely related to competitive ability in male but not in female guppies. Behav. Ecol. 10, 270–274 10.1093/beheco/10.3.270 (doi:10.1093/beheco/10.3.270) [DOI] [Google Scholar]
- 33.Reader S., Laland K. 2001. Primate innovation: sex, age and social rank differences. Int. J. Primatol. 22, 787–805 10.1023/A:1012069500899 (doi:10.1023/A:1012069500899) [DOI] [Google Scholar]
- 34.Reader S. M., Laland K. N. 2003. Animal innovation. Oxford, UK: Oxford University Press [Google Scholar]
- 35.Laland K., Reader S. 1999. Foraging innovation in the guppy. Anim. Behav. 57, 331–340 10.1006/anbe.1998.0967 (doi:10.1006/anbe.1998.0967) [DOI] [PubMed] [Google Scholar]
- 36.Bolnick D. I., Svanbäck R., Fordyce J. A., Yang L. H., Davis J. M., Hulsey C. D., Forister M. L. 2003. The ecology of individuals: incidence and implications of individual specialization. Am. Nat. 161, 1–28 10.1086/343878 (doi:10.1086/343878) [DOI] [PubMed] [Google Scholar]
- 37.Bunnell B., Perkins M. 1980. Performance correlates of social behaviour and organization: social rank and complex problem solving in crab-eating macaques (M. fascicularis). Primates 21, 515–523 10.1007/BF02373840 (doi:10.1007/BF02373840) [DOI] [Google Scholar]
- 38.Katzir G. 1983. Relationships between social structure and response to novelty in captive jackdaws, (Corvus monedula) L. II. Response to novel palatable food. Behaviour 87, 183–208 10.1163/156853983X00426 (doi:10.1163/156853983X00426) [DOI] [Google Scholar]
- 39.Oers K., van Drent P. J., Dingemanse N. J., Kempenaers B. 2008. Personality is associated with extrapair paternity in great tits, Parus major. Anim. Behav. 76, 555–563 10.1016/j.anbehav.2008.03.011 (doi:10.1016/j.anbehav.2008.03.011) [DOI] [Google Scholar]
- 40.Quinn J. L., Cole E. F., Patrick S., Sheldon B. C. 2011. Scale and state-dependence of the relationship between personality and dispersal in a great tit population. J. Anim. Ecol. 80, 918–928 10.1111/j.1365-2656.2011.01835.x (doi:10.1111/j.1365-2656.2011.01835.x) [DOI] [PubMed] [Google Scholar]
- 41.Quinn J. L., Patrick S. C., Bouwhuis S., Wilkin T., Sheldon B. C. 2009. Heterogeneous selection on a heritable temperament trait in a variable environment. J. Anim. Ecol. 78, 1203–1215 10.1111/j.1365-2656.2009.01585.x (doi:10.1111/j.1365-2656.2009.01585.x) [DOI] [PubMed] [Google Scholar]
- 42.Dingemanse N. J., Both C., Noordwijk A. J., van Rutten A. L., Drent P. J. 2003. Natal dispersal and personalities in great tits (Parus major). Proc. R. Soc. Lond. B 270, 741–747 10.1098/rspb.2002.2300 (doi:10.1098/rspb.2002.2300) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hollander F. A., Van Overveld T., Tokka I., Matthysen E. 2008. Personality and nest defence in the great tit (Parus major). Ethology 114, 405–412 10.1111/j.1439-0310.2008.01488.x (doi:10.1111/j.1439-0310.2008.01488.x) [DOI] [Google Scholar]
- 44.Groothuis T. G. G., Carere C. 2005. Avian personalities: characterization and epigenesis. Neurosci. Biobehav. Rev. 29, 137–150 10.1016/j.neubiorev.2004.06.010 (doi:10.1016/j.neubiorev.2004.06.010) [DOI] [PubMed] [Google Scholar]
- 45.Gosler A. 1993. The great tit. London, UK: Hamlyn Press [Google Scholar]
- 46.Gibb J. 1957. Food requirements and other observations on captive tits. Bird Study 4, 207. 10.1080/00063655709475892 (doi:10.1080/00063655709475892) [DOI] [Google Scholar]
- 47.Ennion H. E. 1962. Notes on birds seen in Aden and the western Aden Protectorate. Ibis 104, 560–562 10.1111/j.1474-919X.1962.tb08685.x (doi:10.1111/j.1474-919X.1962.tb08685.x) [DOI] [Google Scholar]
- 48.Fisher J., Hinde R. A. 1949. The opening of milk bottles in birds. Br. Birds 42, 347–357 [Google Scholar]
- 49.Estók P., Zsebok S., Siemers B. M. 2010. Great tits search for, capture, kill and eat hibernating bats. Biol. Lett. 6, 59–62 10.1098/rsbl.2009.0611 (doi:10.1098/rsbl.2009.0611) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Cole E. F., Cram D. L., Quinn J. L. 2011. Individual variation in spontaneous problem-solving performance among wild great tits. Anim. Behav. 81, 491–498 10.1016/j.anbehav.2010.11.025 (doi:10.1016/j.anbehav.2010.11.025) [DOI] [Google Scholar]
- 51.Duckworth R. A. 2006. Behavioural correlations across breeding contexts provide a mechanism for a cost of aggression. Behav. Ecol. 17, 1011–1019 10.1093/beheco/arl035 (doi:10.1093/beheco/arl035) [DOI] [Google Scholar]
- 52.Caldow R. W. G., Goss-Custard J. D., Stillman R. A., Durell S. E. A. L. V. D., Swinfen R., Bregnballe T. 1999. Individual variation in the competitive ability of interference-prone foragers: the relative importance of foraging efficiency and susceptibility to interference. J. Anim. Ecol. 68, 869–878 10.1046/j.1365-2656.1999.00334.x (doi:10.1046/j.1365-2656.1999.00334.x) [DOI] [Google Scholar]
- 53.Svensson L. 1992. Identification guide to European passerines. Thetford, UK: British Trust for Ornithology [Google Scholar]
- 54.Verbeek M. E. M., Drent P. J., Wiepkema P. R. 1994. Consistent individual differences in early exploratory behaviour of male great tits. Anim. Behav. 48, 1113–1121 10.1006/anbe.1994.1344 (doi:10.1006/anbe.1994.1344) [DOI] [Google Scholar]
- 55.Oers K., van Drent P. J., de Goede P., van Noordwijk A. J. 2004. Realized heritability and repeatability of risk-taking behaviour in relation to avian personalities. Proc. R. Soc. Lond. B 271, 65–73 10.1098/rspb.2003.2518 (doi:10.1098/rspb.2003.2518) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sol D., Griffin A. S., Bartomeus I., Boyce H. 2011. Exploring or avoiding novel food resources? The novelty conflict in an invasive bird. PLoS ONE 6, e19535. 10.1371/journal.pone.0019535 (doi:10.1371/journal.pone.0019535) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hogstad O. 1988. Social rank and antipredator behaviour of Willow Tits (Parus montanus) in winter flocks. Ibis 130, 45–56 10.1111/j.1474-919X.1988.tb00954.x (doi:10.1111/j.1474-919X.1988.tb00954.x) [DOI] [Google Scholar]
- 58.Morand-Ferron J., Cole E. F., Quinn J. L. 2011. Who are the innovators? A field experiment with 2 passerine species. Behav. Ecol. (doi:10.1093/beheco/arr120) [Google Scholar]
- 59.Saitou T. 1979. Ecological study of social organization in the great tit Parus major LIII. Home range of the basic flocks and dominance relationship of the members in a basic flock. J. Yamashina Inst. Ornithol. 111, 149–171 10.3312/jyio1952.11.3_149 (doi:10.3312/jyio1952.11.3_149) [DOI] [Google Scholar]
- 60.David H. A. 1987. Ranking from unbalanced paired-comparison data. Biometrika 74, 432–436 10.1093/biomet/74.2.432 (doi:10.1093/biomet/74.2.432) [DOI] [Google Scholar]
- 61.David H. A. 1988. The method of paired comparisons. London, UK: Charles Griffin [Google Scholar]
- 62.Gammell M. P., de Vries H., Jennings D. J., Carlin C. M., Hayden T. J. 2003. David's score: a more appropriate dominance ranking method than Clutton-Brock et al.'s index. Anim. Behav. 66, 601–605 10.1006/anbe.2003.2226 (doi:10.1006/anbe.2003.2226) [DOI] [Google Scholar]
- 63.Herborn K. A., Macleod R., Miles W. T. S., Schofield A. N. B., Alexander L., Arnold K. E. 2010. Personality in captivity reflects personality in the wild. Anim. Behav. 79, 835–843 10.1016/j.anbehav.2009.12.026 (doi:10.1016/j.anbehav.2009.12.026) [DOI] [Google Scholar]
- 64.Gosler A. G. 1996. Environmental and social determinants of winter fat storage in the great tit Parus major. J. Anim. Ecol. 65, 1–17 [Google Scholar]
- 65.Poeysae H. 1988. Feeding consequences of the dominance status in great tit (Parus major) groups. Ornis Fennica 65, 69–75 [Google Scholar]
- 66.Dingemanse N. J., Both C., Drent P. J., Tinbergen J. M. 2004. Fitness consequences of avian personalities in a fluctuating environment. Proc. R. Soc. Lond. B 271, 847–852 10.1098/rspb.2004.2680 (doi:10.1098/rspb.2004.2680) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Bell A. M. 2005. Behavioural differences between individuals and two populations of stickleback. (Gasterosteus aculeatus). J. Evol. Biol. 18, 464–473 10.1111/j.1420-9101.2004.00817.x (doi:10.1111/j.1420-9101.2004.00817.x) [DOI] [PubMed] [Google Scholar]
- 68.Dingemanse N. J., Wright J., Kazem A. J. N., Thomas D. K., Hickling R., Dawnay N. 2007. Behavioural syndromes differ predictably between 12 populations of three-spined stickleback. J. Anim. Ecol. 76, 1128–1138 10.1111/j.1365-2656.2007.01284.x (doi:10.1111/j.1365-2656.2007.01284.x) [DOI] [PubMed] [Google Scholar]
- 69.Drent P. J. 1983. The functional ethology of the great tit Parus major. Groningen, The Netherlands: University of Groningen [Google Scholar]
- 70.Svanbäck R., Bolnick D. I. 2007. Intraspecific competition drives increased resource use diversity within a natural population. Proc. R. Soc. B 274, 839–844 10.1098/rspb.2006.0198 (doi:10.1098/rspb.2006.0198) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Sandell M., Smith H. 1991. Dominance, prior occupancy, and winter residency in the great tit (Parus major). Behav. Ecol. Sociobiol. 29, 147–152 10.1007/BF00166490 (doi:10.1007/BF00166490) [DOI] [Google Scholar]
- 72.Annett C. A., Pierotti R. 1999. Long-term reproductive output in western gulls: consequences of alternate tactics in diet choice. Ecology 80, 288–297 10.1890/0012-9658(1999)080[0288:LTROIW]2.0.CO;2 (doi:10.1890/0012-9658(1999)080[0288:LTROIW]2.0.CO;2) [DOI] [Google Scholar]
- 73.Roff D. A. 1997. Evolutionary quantitative genetics. New York, NY: Chapman and Hall [Google Scholar]
- 74.Price T., Langen T. 1992. Evolution of correlated characters. Trends Ecol. Evol. 7, 307–310 10.1016/0169-5347(92)90229-5 (doi:10.1016/0169-5347(92)90229-5) [DOI] [PubMed] [Google Scholar]
- 75.Mery F., Kawecki T. J. 2003. A fitness cost of learning ability in Drosophila melanogaster. Proc. R. Soc. Lond. B 270, 2465–2469 10.1098/rspb.2003.2548 (doi:10.1098/rspb.2003.2548) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Duckworth R. A., Kruuk L. E. B. 2009. Evolution of genetic integration between dispersal and colonization ability in a bird. Evolution 63, 968–977 10.1111/j.1558-5646.2009.00625.x (doi:10.1111/j.1558-5646.2009.00625.x) [DOI] [PubMed] [Google Scholar]