Abstract
Parental effort is usually associated with high metabolism that could lead to an increase in the production of reactive oxidative species giving rise to oxidative stress. Since many antioxidants involved in the resistance to oxidative stress can also enhance immune function, an increase in parental effort may diminish the level of antioxidants otherwise involved in parasite resistance. In the present study, we performed brood size manipulation in a population of great tits (Parus major) to create different levels of parental effort. We measured resistance to oxidative stress and used a newly developed quantitative PCR assay to quantify malarial parasitaemia. We found that males with an enlarged brood had significantly higher level of malarial parasites and lower red blood cell resistance to free radicals than males rearing control and reduced broods. Brood size manipulation did not affect female parasitaemia, although females with an enlarged brood had lower red blood cell resistance than females with control and reduced broods. However, for both sexes, there was no relationship between the level of parasitaemia and resistance to oxidative stress, suggesting a twofold cost of reproduction. Our results thus suggest the presence of two proximate and independent mechanisms for the well-documented trade-off between current reproductive effort and parental survival.
Keywords: Plasmodium, qPCR, oxidative stress, antioxidants, Parus major, great tit
1. Introduction
The foundations of life-history theory are based on the assumption that allocation of energy or resource into one trait is made at the expense of another trait. This gives rise to resource allocation trade-offs [1] that may explain why there is a cost of reproduction [2]. However, some studies have challenged this classical interpretation for the cost of reproduction [3]. To understand why investment in current reproduction reduces future reproduction and survival, it has been thus advocated that one has to identify the proximate mechanisms involved in the deterioration of organisms [3,4].
Brood size manipulation in experimental bird studies is commonly used to manipulate current investment in reproduction [5,6]. As period of rearing nestlings represent high levels of energy expenditure, especially for parents with an enlarged brood, metabolic rates increase dramatically during this phase [5]. A by-product of metabolism is the formation of oxygen free radicals and one could thus expect different mobilization of antioxidant defences in relation to brood size manipulation. As several antioxidants can enhance immune function, an increased parental effort may diminish the level of antioxidants otherwise involved in parasite resistance or in sexual signalling [7,8]. This mechanism has been suggested in captive zebra finches (Taeniopygia guttata) where non-breeding males and females had an overall higher antioxidant capacity of the blood than reproductive birds [8] and where the activity of two major antioxidant enzymes decreased with increasing brood size [9]. Alonso-Alvarez et al. [8] showed experimentally that reproduction is associated with an increased susceptibility to oxidative stress and proposed the hypothesis that this mechanism could explain breeding immunosuppression. Oxidative stress has thus recently gained considerable interest as a mediator of life-history trade-offs [10–13].
A negative relationship between reproductive effort and immune defences or parasite susceptibilities has been shown in both mammals [14,15] and birds [6,16,17], and has been mainly interpreted as the results of immunosupression during the reproductive period. Immunosuppression would be either the result of an energetic trade-off between immune defences and physical workload or an adaptive response to avoid physiological disorders such as oxidative stress or immunopathology during this intense period, ultimately impairing future survival [18,19]. Immunosuppression caused by reproductive effort may thus be an important mechanism mediating the life-history cost of reproduction [18,20], but the physiological mechanisms involved in this process are far from clear.
On the other hand, avian malaria parasites have emerged as a promising field of investigation for understanding life-history trade-offs [6,17,21–24]. A recent experimental study has demonstrated that even low levels of Plasmodium parasitaemia can have fitness consequences in breeding blue tits (Cyanistes cyanistes) [25]. Once infected with malaria parasites, cell-mediated immune response is activated and may lead to the clearance of parasites but also to some negative effects for the host owing to inflammation [26,27]. Pathogen-induced inflammation is usually associated with an increase in reactive oxygen species, and thus activation of the inflammatory response depletes antioxidants and exposes the host to increased risk of oxidative stress [28,29]. These lines of evidence suggest that parental effort reduces the level of antioxidants, downregulating the immune system and leading to an increased parasitaemia. To our knowledge, the potential link between susceptibility to parasites and resistance to oxidative stress has never been investigated in field conditions.
As emphasized by Costantini [11], ecological studies on the costs of reproduction have mainly focused on the stress response mediated by glucocorticoids, whereas the links between oxidative stress and life-history traits have been largely ignored [10,11,30] (but see [13,31]). In the present study performed in natural conditions, we experimentally investigated the effect of parental investment on both the capacity of blood cell resistance against a free-radical attack and on blood parasite susceptibilities. Moreover, we investigated the possible link between these two variables. Studies on avian malaria have revealed that prevalence and intensities of blood parasites were often underestimated because microscopical examination of blood smears did not permit the detection of low parasitaemia. It was thus suggested to reconsider with molecular-based methods the previous studies based on prevalence of malarial parasites [25,32–34]. We therefore developed a quantitative real-time PCR (qPCR) assay to detect and quantify intensities of blood parasites.
2. Material and methods
(a). Study sites
The study was carried out in 2006 and 2007 in two populations of great tits (Parus major) breeding in nest-boxes located near the University of Lausanne (Dorigny Forest) and 15 km apart in the Marais des Monods, Switzerland.
(b). Brood manipulation experiment, blood sampling and morphological measures
Brood size manipulations were performed on the day of hatching by removing two chicks from a brood (reduced group; n = 42) and adding them to a brood with the same hatching date (enlarged brood; n = 42). Control broods were visited on the day of hatching but were left unmanipulated (n = 38). In Dorigny, 20 broods were enlarged, 12 were used as control and 20 were reduced in 2006, whereas in the Marais des Monods three broods were enlarged, six were left unmanipulated as control and three were reduced. In 2007, 14 were enlarged, 12 were used as control and 14 were reduced in Dorigny, and five were enlarged, eight were used as control and five were reduced in the Marais des Monods. Before manipulation, clutch size was similar between the different groups (mean ± s.e.: reduced 8.4 ± 0.2; control 8.3 ± 0.3; enlarged 8.3 ± 0.2; F = 0.07, p = 0.927).
After brood size manipulation, the number of nestlings reared in the three experimental groups was significantly different (mean ± s.e.: reduced 6.3 ± 0.4; control 7.8 ± 0.4; enlarged 9.3 ± 0.3; F = 20.5, p < 0.001).
At day 14, parents were captured. Adults and chicks were ringed with an individually numbered aluminium ring, weighed with a precision of 0.1 g using an electronic balance (Pesola MS500, Baar, Switzerland), and tarsus length was measured with a digital caliper to the nearest 0.01 mm. In adults, a blood sample was taken from the brachial vein to assess malarial parasites (in 2006), antioxidant defences (in 2006 and 2007) and haematocrit value (in 2006 and 2007), which is the proportion of total blood volume occupied by erythrocytes after centrifugation (10 min at 13 000g).
Because ectoparasites may strongly modify the amount of parental effort [35,36], all nests were treated twice with a microwave appliance in order to kill all ectoparasites inhabiting the nest (see [37]).
(c). Resistance to oxidative stress
Resistance to oxidative stress was assessed by measuring the time needed to haemolyse 50 per cent of red blood cells exposed to a controlled free-radical attack. We used the Kit Radicaux Libres (KRL) test adapted to bird parameters (Brevet spiral V02023, Couternon, France; [38–42]). The principle of the test is to submit whole blood to a thermo-controlled free-radical aggression by a chemical reagent: the 2,2′-azobis-(aminodinopropane) hydrochloride (AAPH) [43]. All families of free-radical scavengers present in the whole blood are used to fight off the oxidant attack [44]. Twenty microlitres of blood from the brachial vein were immediately diluted in 730 µl of KRL buffer. Samples were stored at 4°C before analysis, which occurred within 10 h. Ninety microlitres of KRL-diluted blood was incubated at 37°C with 153 µl of a 150 mM solution of AAPH. The lysis of red blood cells was assessed with a microplate reader device, which measures the decrease of optical density at a wavelength of 620 nm.
(d). Molecular analyses
DNA was extracted from adult's blood samples using the DNeasy tissue extraction kit (QIAGEN, Germany). Concentration of genomic DNA was measured with a Nanodrop-1000 Spectrophotometer (Fisher Scientific) and samples were subsequently diluted to a working concentration of 10 ng µl–1 DNA.
Haemosporidian parasites prevalence was detected using a nested PCR method developed by Waldenström et al. [45]. Pre-amplification of part of the mitochondrial cytochrome b (cyt b) gene was performed in a volume of 25 µl including 1X Buffer (QIAGEN), 2.5 mM MgCl2, 2 µl of genomic DNA (10 ng µl–1), 400 µM dNTPs, 0.625 U of Taq (QIAGEN), 0.6 µM HAEMNF and HAEMNR2 primers at the following conditions: 95°C for 2 min followed by 25 cycles of 95°C for 30 s, 54°C for 30 s and 72°C for 45 s, and with a final extension at 72°C for 7 min. The final PCR amplification was performed with 1 µl of initial PCR product and 0.6 µM primers HEAMF and HAEMR2 following these conditions: 95°C for 2 min followed by 45 cycles of 95°C for 30 s, 54°C for 30 s and 72°C for 45 s, and with a final extension at 72°C for 7 min.
For the quantification of Plasmodium sp. and Haemoproteus sp. parasitaemia, we developed a TaqMan qPCR approach. We designed new specific primers L4050Plasmo (5′-GCTTTATGTATTGTATTTATAC-3′) and H4121Plasmo (5′-GACTTAAAAGATTTGGATAG-3′) targeting the parasite's cyt b gene. In addition, we designed a pair of primers amplifying a portion of the 18S rRNA gene of P. major: Plasmo18S-f (5′-GGCAGCTTTGGTGACTCTAGA-3′) and Plasmo18S-r (5′-AGTTGATAGGGCAGACATTCG-3′). Host DNA was amplified in order to check for DNA quality and as an internal standard (see below). The parasite cyt b TaqMan probe (CY3-CYTb-BHQ2: 5′-AACCTCGAGCCGATCGCACG-3′) was labelled with indocarbocyanine (CY3) as a reporter at the 5′ end and Black Hole Quencher Amidites (BHQ2) at the 3′ end. Host 18S probe (FAM-18S-BHQ1: 5′-CCTTTAGGGTATGATACAGC-3′) was labelled with 6-carboxy-fluorescein (FAM) as a reporter and BHQ1 as a quencher. Each probe was designed in order to be highly specific to its target sequence and no homology with other parts of the genome was found after a Blast search in GenBank.
Before starting the analyses of the whole dataset, we conducted preliminary tests for six samples for cyt b gene and three samples for 18S rRNA gene. To establish standard curves, we performed serial twofold dilutions (starting with a concentration of 20 ng µl–1 genomic DNA) of parasites and host DNA. All test samples were amplified twice in the same run. For a PCR efficiency of 100 per cent, the slope of the standard curve corresponds to −3.32, which means that the amount of DNA amplified double after each cycle. Our qPCR procedure was validated only after the calibration samples showed a slope comprised between −3.8 and −3.2.
Subsequently, qPCR was used to quantify parasite infection. Reactions were run for both genes in a final volume of 40 µl; including 20 µl of qPCR MasterMix Low Rox (Eurogentec), 2 µl of genomic DNA (10 ng µl–1), 0.9 µM of each primer, 0.2 µM of probe, and 2 µl of nanopure water. qPCR was performed in a 7500 real-time PCR System (Applied Biosystem) with the following thermal profile: 2 min at 50°C, 10 min at 95°C, followed by 48 cycles of 15 s at 95°C and 1 min at 54°C (annealing temperature). In each run of the qPCR, four calibration standard samples and template blood samples were analysed twice for reproducibility. Ct value was estimated as the mean of the two replicates, and taken into account only if the difference between the two values was less than 1 Ct value.
Relative parasitaemia (Rp) was quantified as relative to host 18S rRNA gene quantity, expected to be present in two copies per cell, following the relation 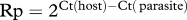 [46]. This correction was used to compensate for Ct value differences among cyt b products other than actual parasite infection.
[46]. This correction was used to compensate for Ct value differences among cyt b products other than actual parasite infection.
(e). Statistical analyses
The effect of brood size manipulation on nestling body mass was tested with a general linear model (GLM) with year and locality as fixed effects and nest as the random term.
The effect of brood size manipulation on resistance to oxidative stress was analysed with a mixed model using the function lme with the maximum-likelihood method in R library lme4 [47].
Treatment, locality, sex, years and body condition, calculated as the residuals of the regression between body weight and tarsus length, were entered as fixed effects, and individual identity was entered as a random term to account for the fact that some individuals were measured in two different years. The starting model included fixed effects plus all first-order interactions, and we selected the best final model by sequentially removing interactions and factors with p > 0.10. Model comparison and simplification was performed using the function ANOVA and a log-likelihood ratio test in R package [47].
The effect of brood size manipulation on the level of parasitaemia was tested with a GLM with a quasi-Poisson error structure to control for overdispersion in the data using the function glm in R package [47]. Treatment, locality, sex, initial clutch size and body condition were entered as fixed effects. There was only one measure of parasitaemia per individual (i.e. parasitaemia was measured only in 2006), and therefore, we did not include individual identity as a random term when analysing parasitaemia. Model simplification was performed by sequentially removing interactions and factors with p > 0.10 using the function ANOVA and an F-test in R package [47]. Only the final models are presented.
We used JMP v. 7.0.0 software and R [47] for all analyses and the level of significance was set at p < 0.05. Values are presented as mean ± s.e.
3. Results
(a). Effect of brood size manipulation on nestlings
Nestling from enlarged broods had lower body mass than nestlings from control and reduced groups (F = 92.63, p < 0.001, enlarged: 14.2 ± 0.22 g; control 16.2 ± 0.23 g, reduced 16.4 ± 0.22 g). Nestlings born in 2007 were heavier (15.9 ± 0.22 g) than in 2006 (15.3 ± 0.20 g, F = 14.06, p < 0.001) and there was also an effect of the locality, with nestlings located in the Marais des Monods being heavier than those from Dorigny (F = 13.77, p < 0.001, 16.2 ± 0.28 g versus 15.0 ± 0.19 g). Finally, there was a significant interaction between year and locality, with nestlings born in 2006 in Dorigny being the lighter (interaction year × locality: F = 54.67, p < 0.001).
(b). Resistance to oxidative stress
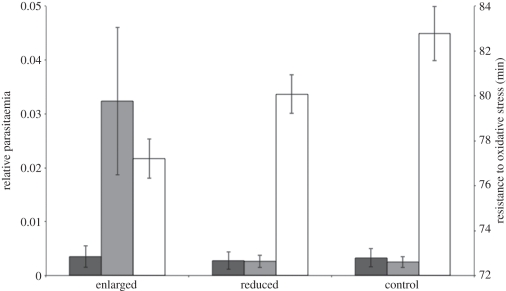
The brood size manipulation significantly affected the resistance to oxidative stress, the enlarged broods having the lowest resistance (brood size manipulation: F2,114 = 9.62, p = 0.001; figure 1 and table 1). Males and females had similar levels of resistance to oxidative stress (sex: F1,114 = 2.08, p = 0.153), whereas birds located in the Marais des Monods had higher levels than those breeding in the forest of Dorigny (locality F1,114 = 14.50, p < 0.001). Finally, there was a year effect on the resistance to oxidative stress (2006: 78.4 ± 0.7; 2007: 83.5 ± 0.7; F1,114 = 43.93, p < 0.001) and a non-significant interaction between locality and sex: F1,114 = 3.57, p = 0.061).
Figure 1.
Relative parasitaemia in females and males (dark and light grey bars, respectively), and resistance to oxidative stress (white bars) in relation to brood size manipulation. Males with enlarged broods had higher level of parasitaemia than males with reduced and control broods, whereas the level of resistance to oxidative stress was lower for both sexes. Error bars represented s.e.
Table 1.
Estimate effects and standard errors of terms retained from a mixed model of the resistance to oxidative stress as a function of brood size manipulation, locality (M denotes Monods) and sex (M denotes males). (Individuals were entered as a random term in the model.)
| factors | estimate | s.e. |
|---|---|---|
| intercept | 72.625 | 1.088 |
| year 2007 | 5.104 | 0.984 |
| control group | 4.022 | 1.049 |
| reduced group | 2.607 | 1.159 |
| locality M | 6.397 | 1.534 |
| sex M | 2.708 | 1.218 |
| locality: sex M | −4.309 | 2.281 |
(c). Prevalence and parasitaemia
The number of birds infected with Plasmodium was extremely high and did not differ between the experimental groups. All males and females from the enlarged group, 93 per cent of males and 87.5 per cent of females of the control group and all males and 94 per cent of females of the reduced group were infected as revealed by the nested PCR assay.
Preliminary analyses showed a significant interaction between treatment and sex (F = 3.25, p = 0.044). Therefore, we analysed males and females separately in further analyses. For males, experimental manipulation of brood size strongly affected parasitaemia. Individuals with an enlarged brood had significantly higher intensities than control and reduced broods (F2,50 = 22.12, p < 0.001; figure 1 and table 2). Moreover, individuals located in the Marais des Monods also had a higher level of parasitaemia than birds breeding in the forest of Dorigny (F1,51 = 12.93, p < 0.001; table 2), and males from the reduced group had higher body condition than males from control and enlarged groups (F1,51 = 30.41, p < 0.001). However, the interaction between treatment and body condition was not statistically significant (F2,51 = 2.66, p = 0.083). Non-significant first-order interactions dropped from the final model were the following: treatment × locality: F = 0.78, p = 0.466; treatment × clutch size: F = 0.07, p = 0.495; body condition × locality: F = 0.65, p = 0.426; body condition × clutch size: F = 0.56, p = 0.460.
Table 2.
Estimate effects and standard errors of terms retained from a GLM of parasitaemia as a function of brood size manipulation, locality (M denotes Monods) and body condition (BC denotes body condition).
| males |
females |
|||
|---|---|---|---|---|
| factors | estimate | s.e. | estimate | s.e. |
| intercept | −4.157 | 0.289 | −5.778 | 0.5618 |
| control group | −2.782 | 1.049 | −0.079 | 0.8650 |
| reduced group | −2.472 | 0.988 | −0.189 | 0.8306 |
| locality M | 1.339 | 0.356 | 0.4803 | 0.8149 |
| BC | 2.096 | 0.464 | 0.1133 | 0.3600 |
| control group: BC | −2.695 | 1.264 | — | — |
| reduced group: BC | −1.239 | 1.025 | — | — |
Female parasitaemia was neither affected by brood size manipulation (F2,50 = 0.09, p = 0.971) nor by locality (F1,50 = 0.32, p = 0.573), nor by body condition (F1,50 = 0.09, p = 0.757; figure 1 and table 2). Non-significant first-order interactions dropped from the final model were the following: treatment × locality: F = 0.45, p = 0.643; treatment × clutch size: F = 0.48, p = 0.623; body condition × locality: F = 0.09, p = 0.755; body condition × clutch size: F = 0.81, p = 0.373.
(d). Physiological parameters and resistance to oxidative stress
There was no relationship between resistance to oxidative stress and parasitaemia (F1,67 = 0.94, p = 0.337). Similarly, body mass was not affected by parasitaemia (F1,97 = 0.01, p = 0.942), sex (F1,97 = 0.49, p = 0.484) or treatment (F2,97 = 1.11, p = 0.332). By contrast, there was a negative relationship between the level of parasitaemia and haematocrit level for both sexes (males: F1,43 = 6.35, p = 0.015; females: F1,40 = 7.29, p = 0.010).
4. Discussion
Experimental manipulation of brood size had a significant impact on the level of malaria infection. Fourteen days after hatching, males rearing enlarged broods had higher parasitaemia when compared with control and reduced broods. These results are in accordance with previous experimental studies based on the prevalence of Plasmodium detected in blood smears, which found that males rearing enlarged broods, but not females, showed significantly higher rates of food provisioning to the chicks and had higher level of malaria prevalence than reduced and control groups [6]. In addition, the very high prevalence of Plasmodium observed in the present study (more than 90%), together with the detection and quantification of parasitaemia levels with qPCR, shows that PCR-based methods allow the detection of previously scarce quantitative data on parasitaemia and are more sensitive than studies based solely on microscopic examination of blood smears. This conclusion has been reached by most studies (but see [48]), which suggested that molecular methods are performing better when parasite intensities are low, which is common among chronic Plasmodium infections [33,34,49,50]. A meta-analysis on the links between reproductive effort and susceptibility to blood infection or level of immune defence has found a weak but positive and significant effect across studies. Interestingly, these studies found a stronger effect when parasitaemia is taken into account instead of prevalence [17].
Although the link between survival and haematozoan parasitaemia in the wild is somewhat uncertain [17], medication experiments have demonstrated that infection may have detrimental effects on important life-history traits [23–25,51–53]. The negative relationship between parasitaemia and haematocrit found in the present study further suggests that some haematozoan parasites negatively affected this important physiological parameter, as previously shown in experimental infestation and in empirical studies [54,55]. However, a review of the literature on the validity of the haematocrit as an indicator of condition in wild birds showed contrasting results concerning the relationships between haematocrit value and haematozoan parasites [56]. For example, haematocrit was not influenced by the presence of Haemoproteus parasites in great tits but increased in response to brood enlargement [57]. This last result was interpreted as the response to the requirement of elevated oxygen-carrying capacity owing to increased work load [57]. The negative relationship between parasitaemia and haematocrit in the present study suggests that Plasmodium parasites are more virulent than Haemoproteus and destroy a larger amount of erythrocytes (98% of Plasmodium sp. versus 2% of Haemoproteus sp. were present in the studied populations, identified by cyt b DNA sequencing (data not shown)).
Our results show that an increased brood size negatively affects individual ability to resist free-radical attack. These findings obtained in field conditions are in accordance with the results of two studies on captive zebra finches which showed that the activity of major antioxidant enzymes and resistance to oxidative stress decreased with increasing brood size [8,9]. From a proximal point of view, this may be explained by a strong increase in metabolism owing to physical activities, with the consequence of an increase in free-radical production as proposed by Alonso-Alvarez et al. [8] (but see [58]). The long-term consequences of a decreased ability to resist free-radical attack during offspring care are difficult to evaluate. As emphasized by Monaghan et al. [58], the time lapse during which oxidative stress occurs is an important factor to be considered when studying the evolution of life-history traits. However, even if the period of reproductive events is relatively short, the reduction of the ability to cope with oxidative stress may possibly be prolonged over a long period owing to the reallocation of antioxidant molecules involved in parasite defences. An increase in heterophil : lymphocyte ratio, a signal of stress, among birds making an intense parental effort further suggests a trade-off between reproductive effort and health state in great tits [57,59]. A study carried out by Horák et al. [57] in great tits tried to evaluate the effects of reproductive effort on health status by brood manipulation. The authors observed that the number of lymphocytes in the peripheral blood decreased with the increase of parental effort and suggested that reproductive effort could lead to suppression of the immune response. A similar observation occurred in a blue tit population where it was shown that birds forced to increase their parental effort by brood size manipulation suffered a reduction in immunoglobulin levels, thus potentially compromising immune function against parasites [60]. These findings are usually interpreted as a consequence of a reallocation of resources between physiological systems [61,62].
The higher susceptibility to parasites could also be interpreted as an alteration in metabolism in relation to changes and modification of abundance of food in different habitats [61]. The difference in parasitaemia and level of antioxidant defences between the two studied populations may thus be attributed to difference in habitat quality. Indeed, the population of Dorigny is located in an urban habitat with lower level of food abundance than the population located in the Marais de Monods, as shown by the lower body mass of nestlings reared in Dorigny. The difference in resistance to oxidative stress and in body mass of nestlings between the two years may be explained by weather conditions that were particularly harsh in spring 2006, with many cold and rainy days that are known to negatively affect caterpillar abundance, the main source of dietary carotenoids involved in antioxidant defences during the breeding season [63,64]. Variation in vector availability and in Plasmodium lineages between the two populations could also play a role in the observed differences.
A recent meta-analysis found that host's immune response generally increases the level of oxidative stress [12]. Indeed, in response to infection, immune cells produce and release cytotoxic compounds such as reactive oxygen and nitrogen species that help to counter pathogens [65]. The reactive oxygen species released by leucocytes play a major role in the immune response against Plasmodium parasites and have been associated with efficient parasite clearance [66–68]. Nevertheless, the exact mechanisms involved are controversial [69,70] and the release of reactive oxygen species in the circulation may on some occasions have more pathological than beneficial effects [29,71].
We were expecting that birds involved in a high reproductive investment would also be less able to counter the haematozoan outbreaks because they had to consume their resources of antioxidants to buffer the consequences of a high metabolic rate. Our results confirmed this prediction as a low resistance to oxidative stress and a high parasitaemia characterized males producing an important reproductive effort. Surprisingly, the resistances to oxidative stress and to parasites were not correlated, suggesting that the negative effects are additional. The effects of the production of free radicals owing to the increase in metabolism and to immune response combined with differential allocation of antioxidant molecules may explain the absence of a direct relationship between parasitaemia and level of oxidative defences. This result also suggests that reproductive effort triggers multiple physiological pathways and induces independently the consumption of resources otherwise allocated to the defences against oxidative stress on the one hand, and against the parasites on the other hand. For example, T-cell-mediated immune response leads to a depletion of total antioxidant defences and an increase in pro-oxidation concentration in wild nestlings of the Eurasian kestrel [72]. The absence of correlation between parasitaemia and level of resistance to oxidative stress may also reveal a diversity of strategy among individuals, some individuals investing in antioxidant-costly resistance against Plasmodium, whereas others may be more tolerant to parasitaemia [73]. Only a study combining measures of a large panel of antioxidant products, immune responses and both the production of free radicals and the resistance to oxidative stress would permit us to get more insights into the complex interaction between parasitaemia and oxidative stress. Whatever may be the exact physiological and immunological processes, increased susceptibilities to oxidative stress and to parasites can be a general phenomenon that participates in the overall deterioration of organisms following reproductive events.
Acknowledgements
All animals were treated in accordance with the Cantonal Veterinary Authorities of the Canton de Vaud, Switzerland, authorization 1730.
This research was supported by grant no. 31003A_120479 from the Swiss National Science Foundation. Special thanks to Daniel Croll and Anne-Lyse Ducrest who gave advice on qPCR analyses and to Pierre Bize and Fabrice Lalubin for statistics. We thank Florentino de Lope, Javier Balbontin and one anonymous reviewer whose comments greatly improved the quality of the manuscript.
References
- 1.Williams G. C. 1966. Natural selection, the costs of reproduction, and a refinement of Lack's principle. Am. Nat. 100, 687–690 10.1086/282461 (doi:10.1086/282461) [DOI] [Google Scholar]
- 2.Stearns S. C. 1992. The evolution of life histories. Oxford, UK: Oxford University Press [Google Scholar]
- 3.Barnes A. I., Partridge L. 2003. Costing reproduction. Anim. Behav. 66, 199–204 10.1006/anbe.2003.2122 (doi:10.1006/anbe.2003.2122) [DOI] [Google Scholar]
- 4.Harshman L. G., Zera A. J. 2007. The cost of reproduction: the devil in the details. Trends Ecol. Evol. 22, 80–86 10.1016/j.tree.2006.10.008 (doi:10.1016/j.tree.2006.10.008) [DOI] [PubMed] [Google Scholar]
- 5.Nilsson J. A. 2002. Metabolic consequences of hard work. Proc. R. Soc. Lond. B 269, 1735–1739 10.1098/rspb.2002.2071 (doi:10.1098/rspb.2002.2071) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Richner H., Christe P., Oppliger A. 1995. Paternal investment affects prevalence of malaria. Proc. Natl Acad. Sci. USA 92, 1192–1194 10.1073/pnas.92.4.1192 (doi:10.1073/pnas.92.4.1192) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Faivre B., Gregoire A., Preault M., Cezilly F., Sorci G. 2003. Immune activation rapidly mirrored in a secondary sexual trait. Science 300, 103. 10.1126/science.1081802 (doi:10.1126/science.1081802) [DOI] [PubMed] [Google Scholar]
- 8.Alonso-Alvarez C., Bertrand S., Devevey G., Prost J., Faivre B., Sorci G. 2004. Increased susceptibility to oxidative stress as a proximate cost of reproduction. Ecol. Lett. 7, 363–368 10.1111/j.1461-0248.2004.00594.x (doi:10.1111/j.1461-0248.2004.00594.x) [DOI] [Google Scholar]
- 9.Wiersma P., Selman C., Speakman J. R., Verhulst S. 2004. Birds sacrifice oxidative protection for reproduction. Proc. R. Soc. Lond. B 271, S360–S363 10.1098/rsbl.2004.0171 (doi:10.1098/rsbl.2004.0171) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bize P., Devevey G., Monaghan P., Doligez B., Christe P. 2008. Fecundity and survival in relation to resistance to oxidative stress in a free-living bird. Ecology 89, 2584–2593 10.1890/07-1135.1 (doi:10.1890/07-1135.1) [DOI] [PubMed] [Google Scholar]
- 11.Costantini D. 2008. Oxidative stress in ecology and evolution: lessons from avian studies. Ecol. Lett. 11, 1238–1251 10.1111/j.1461-0248.2008.01246.x (doi:10.1111/j.1461-0248.2008.01246.x) [DOI] [PubMed] [Google Scholar]
- 12.Costantini D., Møller A. P. 2009. Does immune response cause oxidative stress in birds? A meta-analysis. Comp. Biochem. Phys. A 153, 339–344 10.1016/j.cbpa.2009.03.010 (doi:10.1016/j.cbpa.2009.03.010) [DOI] [PubMed] [Google Scholar]
- 13.Dowling D. K., Simmons L. W. 2009. Reactive oxygen species as universal constraints in life-history evolution. Proc. R. Soc. B 276, 1737–1745 10.1098/rspb.2008.1791 (doi:10.1098/rspb.2008.1791) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Christe P., Arlettaz R., Vogel P. 2000. Variation in intensity of a parasitic mite (Spinturnix myoti) in relation to the reproductive cycle and immunocompetence of its bat host (Myotis myotis). Ecol. Lett. 3, 207–212 10.1046/j.1461-0248.2000.00142.x (doi:10.1046/j.1461-0248.2000.00142.x) [DOI] [Google Scholar]
- 15.Festa-Bianchet M. 1989. Individual differences, parasites, and the cost of reproduction for bighorn ewes (Ovis canadensis). J. Anim. Ecol. 58, 785–795 10.2307/5124 (doi:10.2307/5124) [DOI] [Google Scholar]
- 16.Norris K., Anwar M., Read A. F. 1994. Reproductive effort influences the prevalence of haematozoan parasites in great tits. J. Anim. Ecol. 63, 601–610 10.2307/5226 (doi:10.2307/5226) [DOI] [Google Scholar]
- 17.Knowles S. C. L., Nakagawa S., Sheldon B. C. 2009. Elevated reproductive effort increases blood parasitaemia and decreases immune function in birds: a meta-regression approach. Funct. Ecol. 23, 405–415 10.1111/j.1365-2435.2008.01507.x (doi:10.1111/j.1365-2435.2008.01507.x) [DOI] [Google Scholar]
- 18.Sheldon B. C., Verhulst S. 1996. Ecological immunology: costly parasite defences and trade-offs in evolutionary ecology. Trends Ecol. Evol. 11, 317–325 10.1016/0169-5347(96)10039-2 (doi:10.1016/0169-5347(96)10039-2) [DOI] [PubMed] [Google Scholar]
- 19.Viney M. E., Riley E. M., Buchanan K. L. 2005. Optimal immune responses: immunocompetence revisited. Trends Ecol. Evol. 20, 665–669 10.1016/j.tree.2005.10.003 (doi:10.1016/j.tree.2005.10.003) [DOI] [PubMed] [Google Scholar]
- 20.Nordling D., Andersson M., Zohari S., Gustafsson L. 1998. Reproductive effort reduces specific immune response and parasite resistance. Proc. R. Soc. Lond. B 265, 1291–1298 10.1098/rspb.1998.0432 (doi:10.1098/rspb.1998.0432) [DOI] [Google Scholar]
- 21.Oppliger A., Christe P., Richner H. 1996. Clutch size and malaria resistance. Nature 381, 565. 10.1038/381565a0 (doi:10.1038/381565a0) [DOI] [PubMed] [Google Scholar]
- 22.Oppliger A., Christe P., Richner H. 1997. Clutch size and malarial parasites in female great tits. Behav. Ecol. 8, 148–152 10.1093/beheco/8.2.148 (doi:10.1093/beheco/8.2.148) [DOI] [Google Scholar]
- 23.Marzal A., de Lope F., Navarro C., Møller A. P. 2005. Malarial parasites decrease reproductive success: an experimental study in a passerine bird. Oecologia 142, 541–545 10.1007/s00442-004-1757-2 (doi:10.1007/s00442-004-1757-2) [DOI] [PubMed] [Google Scholar]
- 24.Martinez-de la Puente J., Merino S., Tomas G., Moreno J., Morales J., Lobato E., Garcia-Fraile S., Belda E. J. 2010. The blood parasite Haemoproteus reduces survival in a wild bird: a medication experiment. Biol. Lett. 6, 663–665 10.1098/rsbl.2010.0046 (doi:10.1098/rsbl.2010.0046) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Knowles S. C. L., Palinauskas V., Sheldon B. C. 2010. Chronic malaria infections increase family inequalities and reduce parental fitness: experimental evidence from a wild bird population. J. Evol. Biol. 23, 557–569 10.1111/j.1420-9101.2009.01920.x (doi:10.1111/j.1420-9101.2009.01920.x) [DOI] [PubMed] [Google Scholar]
- 26.Finney O. C., Riley E. M., Walther M. 2010. Regulatory T-cells in malaria: friend or foe? Trends Immunol. 31, 63–70 10.1016/j.it.2009.12.002 (doi:10.1016/j.it.2009.12.002) [DOI] [PubMed] [Google Scholar]
- 27.Hansen D. S., Schofield L. 2010. Natural regulatory T-cells in malaria: host or parasite allies? PLoS Pathog. 6, e1000771. 10.1371/journal.ppat.1000771 (doi:10.1371/journal.ppat.1000771) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bertrand S., Criscuolo F., Faivre B., Sorci G. 2006. Immune activation increases susceptibility to oxidative tissue damage in zebra finches. Funct. Ecol. 20, 1022–1027 10.1111/j.1365-2435.2006.01191.x (doi:10.1111/j.1365-2435.2006.01191.x) [DOI] [Google Scholar]
- 29.Sorci G., Faivre B. 2009. Inflammation and oxidative stress in vertebrate host–parasite systems. Phil. Trans. R. Soc. B 364, 71–83 10.1098/rstb.2008.0151 (doi:10.1098/rstb.2008.0151) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bize P., Criscuolo F., Metcalfe N. B., Nasir L., Monaghan P. 2009. Telomere dynamics rather than age predict life expectancy in the wild. Proc. R. Soc. B 276, 1679–1683 10.1098/rspb.2008.1817 (doi:10.1098/rspb.2008.1817) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Isaksson C., Sheldon B., Uller T. 2011. The challenges of integrating oxidative stress into life-history biology. Bioscience 61, 194–202 10.1525/bio.2011.61.3.5 (doi:10.1525/bio.2011.61.3.5) [DOI] [Google Scholar]
- 32.Bentz S., Rigaud T., Barroca M., Martin-Laurent F., Bru D., Moreau J., Faivre B. 2006. Sensitive measure of prevalence and parasitaemia of haemosporidia from European blackbird (Turdus merula) populations: value of PCR-RFLP and quantitative PCR. Parasitology 133, 685–692 10.1017/S0031182006001090 (doi:10.1017/S0031182006001090) [DOI] [PubMed] [Google Scholar]
- 33.Fallon S. M., Ricklefs R. E. 2008. Parasitemia in PCR-detected Plasmodium and Haemoproteus infections in birds. J. Avian Biol. 39, 514–522 10.1111/j.2008.0908-8857.04308.x (doi:10.1111/j.2008.0908-8857.04308.x) [DOI] [Google Scholar]
- 34.Garamszegi L. Z. 2010. The sensitivity of microscopy and PCR-based detection methods affecting estimates of prevalence of blood parasites in birds. J. Parasitol. 96, 1197–1203 10.1645/GE-2531.1 (doi:10.1645/GE-2531.1) [DOI] [PubMed] [Google Scholar]
- 35.Christe P., Richner H., Oppliger A. 1996. Begging, food provisioning and nestling competition in great tit broods infested with ectoparasites. Behav. Ecol. 7, 127–131 10.1093/beheco/7.2.127 (doi:10.1093/beheco/7.2.127) [DOI] [Google Scholar]
- 36.Perrin N., Christe P., Richner H. 1996. On host life-history response to parasitism. Oikos 75, 317–320 10.2307/3546256 (doi:10.2307/3546256) [DOI] [Google Scholar]
- 37.Richner H., Oppliger A., Christe P. 1993. Effect of an ectoparasite on reproduction in great tits. J. Anim. Ecol. 62, 703–710 10.2307/5390 (doi:10.2307/5390) [DOI] [Google Scholar]
- 38.Stocker P., Lesgards J. F., Vidal N., Chalier F., Prost M. 2003. ESR study of a biological assay on whole blood: antioxidant efficiency of various vitamins. Biochim. Biophys. Acta Gen. Subj. 1621, 1–8 10.1016/S0304-4165(03)00008-4 (doi:10.1016/S0304-4165(03)00008-4) [DOI] [PubMed] [Google Scholar]
- 39.Girard A., Madani S., El Boustani E. S., Belleville J., Prost J. 2005. Changes in lipid metabolism and antioxidant defense status in spontaneously hypertensive rats and Wistar rats fed a diet enriched with fructose and saturated fatty acids. Nutrition 21, 240–248 10.1016/j.nut.2004.04.022 (doi:10.1016/j.nut.2004.04.022) [DOI] [PubMed] [Google Scholar]
- 40.Alonso-Alvarez C., Bertrand S., Devevey G., Gaillard M., Prost J., Faivre B., Sorci G. 2004. An experimental test of the dose-dependent effect of carotenoids and immune activation on sexual signals and antioxidant activity. Am. Nat. 164, 651–659 10.1086/424971 (doi:10.1086/424971) [DOI] [PubMed] [Google Scholar]
- 41.Alonso-Alvarez C., Bertrand S., Devevey G., Prost J., Faivre B., Chastel O., Sorci G. 2006. An experimental manipulation of life-history trajectories and resistance to oxidative stress. Evolution 60, 1913–1924 10.1111/j.0014-3820.2006.tb00534.x (doi:10.1111/j.0014-3820.2006.tb00534.x) [DOI] [PubMed] [Google Scholar]
- 42.Devevey G., Bruyndonckx N., von Houwald F., Studer-Thiersch A., Christe P. 2010. Age-specific variation of resistance to oxidative stress in the greater flamingo (Phoenicopterus ruber roseus). J. Ornithol. 151, 251–254 10.1007/s10336-009-0456-5 (doi:10.1007/s10336-009-0456-5) [DOI] [Google Scholar]
- 43.Rojas Wahl R. U., Zeng L., Madison S. A., De Pinto R. L., Shay B. J. M. 1998. Mechanistic studies on the decomposition of water soluble azo-radical-initiators. J. Chem. Soc. Perkin Trans. 2, 2009–2018 10.1039/A801624K (doi:10.1039/A801624K) [DOI] [Google Scholar]
- 44.Lesgards J. F., Durand P., Lassarre M., Stocker P., Lesgards G., Lanteaume A., Prost M., Lehucher-Michel M. P. 2002. Assessment of lifestyle effects on the overall antioxidant capacity of healthy subjects. Environ. Health Perspect. 110, 479–486 10.1289/ehp.02110479 (doi:10.1289/ehp.02110479) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Waldenstrom J., Bensch S., Hasselquist D., Ostman O. 2004. A new nested polymerase chain reaction method very efficient in detecting Plasmodium and Haemoproteus infections from avian blood. J. Parasitol. 90, 191–194 10.1645/GE-3221RN (doi:10.1645/GE-3221RN) [DOI] [PubMed] [Google Scholar]
- 46.Derveaux S., Vandesompele J., Hellemans J. 2010. How to do successful gene expression analysis using real-time PCR. Methods 50, 227–230 10.1016/j.ymeth.2009.11.001 (doi:10.1016/j.ymeth.2009.11.001) [DOI] [PubMed] [Google Scholar]
- 47.Crawley M. J. 2007. The R book. Chichester, UK: /Hoboken, NJ: Wiley [Google Scholar]
- 48.Valkiunas G., Iezhova T. A., Krizanauskiene A., Palinauskas V., Sehgal R. N. M., Bensch S. 2008. A comparative analysis of microscopy and PCR-based detection methods for blood parasites. J. Parasitol. 94, 1395–1401 10.1645/GE-1570.1 (doi:10.1645/GE-1570.1) [DOI] [PubMed] [Google Scholar]
- 49.Richard F. A., Sehgal R. N. M., Jones H. I., Smith T. B. 2002. A comparative analysis of PCR-based detection methods for avian malaria. J. Parasitol. 88, 819–822 10.2307/3285374 (doi:10.2307/3285374) [DOI] [PubMed] [Google Scholar]
- 50.Knowles S. C. L., Wood M. J., Alves R., Wilkin T. A., Bensch S., Sheldon B. C. 2011. Molecular epidemiology of malaria prevalence and parasitaemia in a wild bird population. Mol. Ecol. 20, 1062–1076 10.1111/j.1365-294X.2010.04909.x (doi:10.1111/j.1365-294X.2010.04909.x) [DOI] [PubMed] [Google Scholar]
- 51.Merino S., Moreno J., Sanz J. J., Arriero E. 2000. Are avian blood parasites pathogenic in the wild? A medication experiment in blue tits (Parus caeruleus). Proc. R. Soc. Lond. B 267, 2507–2510 10.1098/rspb.2000.1312 (doi:10.1098/rspb.2000.1312) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Marzal A., Bensch S., Reviriego M., Balbontin J., de Lope F. 2008. Effects of malaria double infection in birds: one plus one is not two. J. Evol. Biol. 21, 979–987 10.1111/j.1420-9101.2008.01545.x (doi:10.1111/j.1420-9101.2008.01545.x) [DOI] [PubMed] [Google Scholar]
- 53.Tomas G., Merino S., Moreno J., Morales J., Martinez-de la Puente J. 2007. Impact of blood parasites on immunoglobulin level and parental effort: a medication field experiment on a wild passerine. Funct. Ecol. 21, 125–133 10.1111/j.1365-2435.2006.01214.x (doi:10.1111/j.1365-2435.2006.01214.x) [DOI] [Google Scholar]
- 54.Cellier-Holzem E., Esparza-Salas R., Garnier S., Sorci G. 2010. Effect of repeated exposure to Plasmodium relictum (lineage SGS1) on infection dynamics in domestic canaries. Int. J. Parasitol. 40, 1447–1453 10.1016/j.ijpara.2010.04.014 (doi:10.1016/j.ijpara.2010.04.014) [DOI] [PubMed] [Google Scholar]
- 55.Christe P., Møller A. P., González G., de Lope F. 2002. Intraseasonal variation in immune defence, body mass and hematocrit in adult house martins Delichon urbica. J. Avian Biol. 33, 321–325 10.1034/j.1600-048X.2002.330317.x (doi:10.1034/j.1600-048X.2002.330317.x) [DOI] [Google Scholar]
- 56.Fair J., Whitaker S., Pearson B. 2007. Sources of variation in haematocrit in birds. Ibis 149, 535–552 10.1111/j.1474-919X.2007.00680.x (doi:10.1111/j.1474-919X.2007.00680.x) [DOI] [Google Scholar]
- 57.Hõrak P., Ots I., Murumagi A. 1998. Haematological health state indices of reproducing great tits: a response to brood size manipulation. Funct. Ecol. 12, 750–756 10.1046/j.1365-2435.1998.00244.x (doi:10.1046/j.1365-2435.1998.00244.x) [DOI] [Google Scholar]
- 58.Monaghan P., Metcalfe N. B., Torres R. 2009. Oxidative stress as a mediator of life history trade-offs: mechanisms, measurements and interpretation. Ecol. Lett. 12, 75–92 10.1111/j.1461-0248.2008.01258.x (doi:10.1111/j.1461-0248.2008.01258.x) [DOI] [PubMed] [Google Scholar]
- 59.Ots I., Hõrak P. 1996. Great tits Parus major trade health for reproduction. Proc. R. Soc. Lond. B 263, 1443–1447 10.1098/rspb.1996.0210 (doi:10.1098/rspb.1996.0210) [DOI] [PubMed] [Google Scholar]
- 60.Merino S., Moreno J., Tomas G., Martinez J., Morales J., Martinez-De la Puente J., Osorno J. L. 2006. Effects of parental effort on blood stress protein HSP60 and immunoglobulins in female blue tits: a brood size manipulation experiment. J. Anim. Ecol. 75, 1147–1153 10.1111/j.1365-2656.2006.01135.x (doi:10.1111/j.1365-2656.2006.01135.x) [DOI] [PubMed] [Google Scholar]
- 61.Pedersen B. K., Hoffman-Goetz L. 2000. Exercise and the immune system: regulation, integration and adaptation. Physiol. Rev. 80, 1055–1081 [DOI] [PubMed] [Google Scholar]
- 62.Norris K., Evans M. R. 2000. Ecological immunology: life history trade-offs and immune defense in birds. Behav. Ecol. 11, 19–26 10.1093/beheco/11.1.19 (doi:10.1093/beheco/11.1.19) [DOI] [Google Scholar]
- 63.Partali V., Liaaenjensen S., Slagsvold T., Lifjeld J. T. 1987. Carotenoids in food-chain studies. II. The food-chain of Parus spp. monitored by carotenoid analysis. Comp. Biochem. Physiol. B 87, 885–888 10.1016/0305-0491(87)90408-1 (doi:10.1016/0305-0491(87)90408-1) [DOI] [Google Scholar]
- 64.Tummeleht L., Magi M., Kilgas P., Mand R., Hõrak P. 2006. Antioxidant protection and plasma carotenoids of incubating great tits (Parus major L.) in relation to health state and breeding conditions. Comp. Biochem. Phys. C 144, 166–172 10.1016/j.cbpc.2006.08.004 (doi:10.1016/j.cbpc.2006.08.004) [DOI] [PubMed] [Google Scholar]
- 65.Stevenson M. M., Riley E. M. 2004. Innate immunity to malaria. Nat. Rev. Immunol. 4, 169–180 10.1038/nri1311 (doi:10.1038/nri1311) [DOI] [PubMed] [Google Scholar]
- 66.Schirmer R. H., Schöllhammer T., Eisenbrand G., Kraut-Siegel R. L. 1987. Oxidative stress as a defense mechanism against parasitic infections. Free Radical Res. 3, 3–12 10.3109/10715768709069763 (doi:10.3109/10715768709069763) [DOI] [PubMed] [Google Scholar]
- 67.Golenser J., Kamyl M., Tsafack A., Marva E., Cohen A., Kitrossky N., Chevion M. 1992. Correlation between destruction of malarial parasites by polymorphonuclear leukocytes and oxidative stress. Free Radic. Res. Commun. 17, 249–262 10.3109/10715769209079517 (doi:10.3109/10715769209079517) [DOI] [PubMed] [Google Scholar]
- 68.Greve B., Lehman L. G., Lell B., Luckner D., Schmidt-Ott R., Kremsner P. G. 1999. High oxygen radical production is associated with fast parasite clearance in children with Plasmodium falciparum malaria. J. Infect. Dis. 179, 1584–1586 10.1086/314780 (doi:10.1086/314780) [DOI] [PubMed] [Google Scholar]
- 69.Potter S. M., Mitchell A. J., Cowden W. B., Sanni L. A., Dinauer M., de Haan J. B., Hunt N. H. 2005. Phagocyte-derived reactive oxygen species do not influence the progression of murine blood-stage malaria infections. Infect. Immun. 73, 4941–4947 10.1128/IAI.73.8.4941-4947.2005 (doi:10.1128/IAI.73.8.4941-4947.2005) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Sobolewski P., Gramaglia I., Frangos J. A., Intaglietta M., Van der Heyde H. 2005. Plasmodium berghei resists killing by reactive oxygen species. Infect. Immun. 73, 6704–6710 10.1128/IAI.73.10.6704-6710.2005 (doi:10.1128/IAI.73.10.6704-6710.2005) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Postma N. S., Mommers E. C., Eling W. M. C., Zuidema J. 1996. Oxidative stress in malaria; implications for prevention and therapy. Pharm. World Sci. 18, 121–129 10.1007/BF00717727 (doi:10.1007/BF00717727) [DOI] [PubMed] [Google Scholar]
- 72.Costantini D., Dell'Omo G. 2006. Effects of T-cell-mediated immune response on avian oxidative stress. Comp. Biochem. Phys. A 145, 137–142 10.1016/j.cbpa.2006.06.002 (doi:10.1016/j.cbpa.2006.06.002) [DOI] [PubMed] [Google Scholar]
- 73.Råberg L., Graham A. L., Read A. F. 2009. Decomposing health: tolerance and resistance to parasites in animals. Phil. Trans. R. Soc. B 364, 37–49 10.1098/rstb.2008.0184 (doi:10.1098/rstb.2008.0184) [DOI] [PMC free article] [PubMed] [Google Scholar]