Abstract
During the process of plant domestication, below-ground communities are rarely considered. Some studies have attempted to understand the changes in root symbionts owing to domestication, but little is known about how it influences mycorrhizal response in domesticated crops. We hypothesized that selection for above-ground traits may also result in decreased mycorrhizal abundance in roots. Breadfruit (Artocarpus sp.) has a long domestication history, with a strong geographical movement of cultivars from west to east across the Melanesian and Polynesian islands. Our results clearly show a decrease in arbuscular mycorrhizas (AMs) along a domestication gradient from wild to recently derived cultivars. We showed that the vesicular and arbuscular colonization rate decreased significantly in more recently derived breadfruit cultivars. In addition, molecular analyses of breadfruit roots indicated that AM fungal species richness also responded along the domestication gradient. These results suggest that human-driven selection for plant cultivars can have unintended effects on below-ground mutualists, with potential impacts on the stress tolerance of crops and long-term food security.
Keywords: mycorrhiza, domestication, breadfruit, Artocarpus
1. Introduction
Domestication is a complex evolutionary process in which human use of plant and animal species leads to morphological and physiological changes that distinguish domesticated taxa from their wild ancestors [1]. A plant is considered to be domesticated when its native characteristics are altered such that it cannot grow and reproduce without human intervention. As a result, many domesticated species are differentiated from their wild ancestors based on an assortment of morphological, phenological and reproductive traits [2–4]. In general, the domestication process for plants involves directional selection for lineages with increased yields, prolonged length of fruiting season or accretion of edible parts. However, most studies have focused on grain crops, which typically have low mycorrhizal responsiveness (i.e. the degree to which the plant growth is enhanced by mycorrhizal fungi). We have a much less comprehensive knowledge of domestication in other types of crops, in particular the fruit crops [3,5], even though these species make up an important component of human nutrition throughout the world.
During the process of domestication, effects on below-ground processes are rarely considered. These include interactions with root symbionts that allow plants to access limiting nutrients and protect themselves from pathogens [6,7]. Here, we focus on the diversity and community structure of arbuscular mycorrhizal fungi (AM fungi), an abundant group of below-ground root symbionts that is known to play an important role in agricultural ecosystems [8].
The AM symbiosis is the most widespread mycorrhizal association, and affects approximately 70 to 90 per cent of land plants in both natural and agricultural ecosystems [9]. AM fungi play a key role in soil fertility, structure and ecology [10–12]. Recent studies have shown that AM fungal species are functionally distinct, in that some taxa provide disease resistance and others enhance nutrient supply to plants [13]. Furthermore, the diversity of AM fungi can potentially influence plant fitness, community structure, biodiversity, ecosystem productivity and variability [11,13–15]. The beneficial effects of AM fungi on plant growth have led to the development of AM fungi as bioinoculants for forestry, agriculture and horticulture [16].
While it is clear that almost all important food crops are highly mycorrhizal [17], it is thought that modern breeding practices have selected for crops that are less mycorrhizal-dependent, but this remains unclear [18]. There is evidence for loss of mycorrhizal responsiveness in modern lines of plants such as wheat [19–21] and maize [22–24]. While this relationship has not been observed for all crops, similar trends have been noted for another key root symbiosis: the nitrogen-fixing association between rhizobial bacteria and leguminous plants [25]. In many cases, the AM fungal symbiosis is maintained in crop plants after decades of breeding new varieties with no consideration of the presence or role of the symbiosis [26–28].
Breadfruit (Artocarpus altils Parkinson (Fosberg), Moraceae) is an important staple food crop in Oceania and throughout much of the tropics [29]. During the process of its domestication, hundreds of cultivars have been selected for and named [30]. Zerega et al. [31] examined the species limits within the breadfruit complex and recognized three species in the genus Artocarpus—Artocarpus altilis (domesticated breadfruit), Artocarpus camansi (breadnut) and Artocarpus mariannensis—and verified the existence of hybrids A. altilis × A. mariannensis. As part of the domestication process over the past 2000–3000 years, breadfruit (A. altilis) has changed considerably from its ancestor, breadnut (A. camansi). For example, domesticated cultivars of A. altilis have larger, fleshier fruit and fewer seeds [30]. In addition, some cultivars have extended fruiting season and fruit loads [32]. Also, breadfruit is dependent on humans for dispersal since many cultivars are seedless.
Because of the questions surrounding breadfruit origins and the role of humans in its dispersal, Zerega et al. [33] traced human-mediated breadfruit dispersal through Oceania. Their results agree with the theory that humans colonized Polynesia via Melanesia. With regard to breadfruit, most Melanesian and Polynesian cultivars appear to have arisen over generations of vegetative propagation and selection from A. camansi [33]. Wild populations of A. camansi have been recorded from primary forests only in and around New Guinea (NG) [34].
Breadfruit presents a unique opportunity to study the effects of domestication because its domestication falls conveniently along a spatial gradient (longitude). This unusual situation arose because breadfruit was dispersed throughout Oceania exclusively by human migrants who carried with them vegetative cuttings from choice cultivars. Thus, each island along the west-to-east route represents a distinct ‘bottleneck’, which increasingly differentiated cultivars from their ancestral state. These derived cultivars remained relatively unchanged along the gradient because breadfruit also lost the ability to sexually reproduce along the migration route. Breadfruit growing closer to NG experienced a greater degree of gene flow, with more ancestral cultivars.
Using the hypothesized eastward migration from its origins in NG [33], we set out to investigate how the domestication process affected the AM symbiosis in breadfruit. We asked the following questions. (i) Has domestication reduced the intensity of the AM symbiosis? (ii) Has domestication affected the identity of the AM fungal community?
2. Results
(a). Effect of the domestication distance on number of seeds
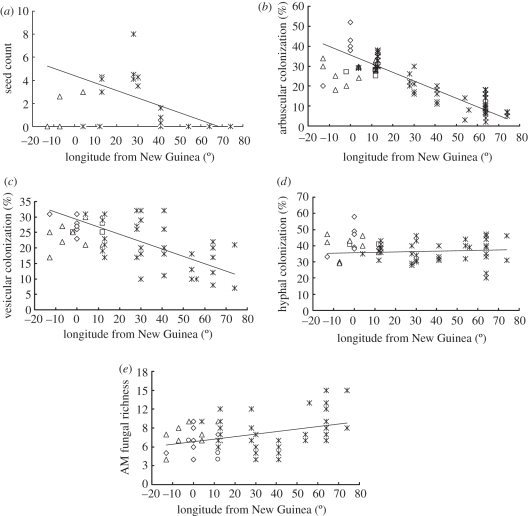
We observed a significant decrease of seed counts per individual fruit along the eastward domestication from NG (figure 1a). From a total of 56 samples, only 16 samples contained seeds. Starting with average seed counts of 2.37 in cultivars around NG (longitude ±13°), cultivars become increasingly seedless past 30° E of NG, and none of the fruit past 40° E contained seeds.
Figure 1.
(a) Relationship between longitude and seed count in breadfruit; line represents y = −0.0643x + 4.4128, R2 = 0.37, p < 0.001. (b) Relationship between longitude and arbuscular colonization; line represents y = −0.432x + 35.524, R2 = 0.773, p < 0.001. (c) Relationship between longitude and vesicular colonization; line represents y = −0.239x + 29.182, R2 = 0.399, p < 0.001. (d) Relationship between longitude and hyphal colonization (HC); line represents y = 0.026x + 35.553, R2 = 0.007, p = 0.627. (e) Relationship between longitude and AM fungal richness. A linear increase of AM fungal richness is shown for eastward domestication from New Guinea. Line represents y = 0.040x + 6.810, R2 = 0.097, p = 0.057. AM fungal richness is assessed as the number of AM fungi sequences detected in each sample. Linear regression was performed including all 56 samples to visualize and complement the statistical results presented in table 1. (a–d) Diamonds, A. camansi; squares, A. mariannensis; triangles, A × M; asterisks, A. altilis. (e) Diamonds, A. camansi; circles, A. mariannensis; triangles, A × M; asterisks, A. altilis.
(b). Does domestication reduce the intensity of arbuscular mycorrhiza symbiosis?
(i). Longitude versus root colonization
Two of the three measures of fungal root colonization levels were negatively correlated with longitude. Eastward from NG, both AM fungal-specific abundance measures—arbuscular colonization (AC) and vesicular colonization (VC)—were significantly reduced (figure 1b,c), while changes in hyphal colonization (HC) were negligible (table 1 and figure 1d). Both partial Mantel tests (PMTs) and analysis of covariance (ANCOVA) confirmed significant effects of longitude on the AC and VC, after controlling for potentially confounding distance (PMT)/site (ANOVA) and plant age effects. Thus, these longitudinal effects cannot be explained by tree age nor the spatial arrangement of individual trees within the common garden, even though some soil properties varied among different cultivating sites (electronic supplementary material, table S2).
Table 1.
Effect of longitude, plant age and cultivating site on AM fungal richness and root colonization (only A. altilis samples). Age refers to the host plant age; longitude refers to the eastward longitude increasing from New Guinea; site refers to the pairwise distance among individual trees. Bold values are significant at p < 0.05. AC, root arbuscular colonization; VC, root vesicular colonization; HC, soil hyphal colonization.
| partial Mantel test (error d.f. = 30) |
ANCOVA (error d.f. = 30) |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| longitude |
age |
site |
longitude |
age |
site |
|||||||
| R | p | R | p | R | p | F | p | F | p | F | p | |
| AC | 0.708 | <0.001 | 0.059 | 0.419 | −0.167 | 0.008 | 62.67 | <0.001 | 0.58 | 0.453 | 0.63 | 0.682 |
| VC | 0.311 | <0.001 | 0.042 | 0.540 | −0.098 | 0.068 | 14.06 | <0.001 | 1.50 | 0.231 | 1.76 | 0.151 |
| HC | 0.0002 | 0.996 | −0.128 | 0.115 | 0.055 | 0.398 | 0.89 | 0.353 | 0.00 | 0.954 | 0.75 | 0.593 |
| sequence richness | 0.031 | 0.55 | 0.092 | 0.331 | 0.061 | 0.412 | 10.75 | 0.003 | 0.08 | 0.783 | 4.96 | 0.002 |
| phylotype richness | −0.06 | 0.179 | 0.139 | 0.043 | 0.168 | 0.008 | 7.86 | 0.009 | 2.51 | 0.123 | 7.11 | <0.001 |
(c). Does domestication affect the identity of the arbuscular mycorrhiza fungal community?
(i). Longitude versus arbuscular mycorrhiza fungal species richness
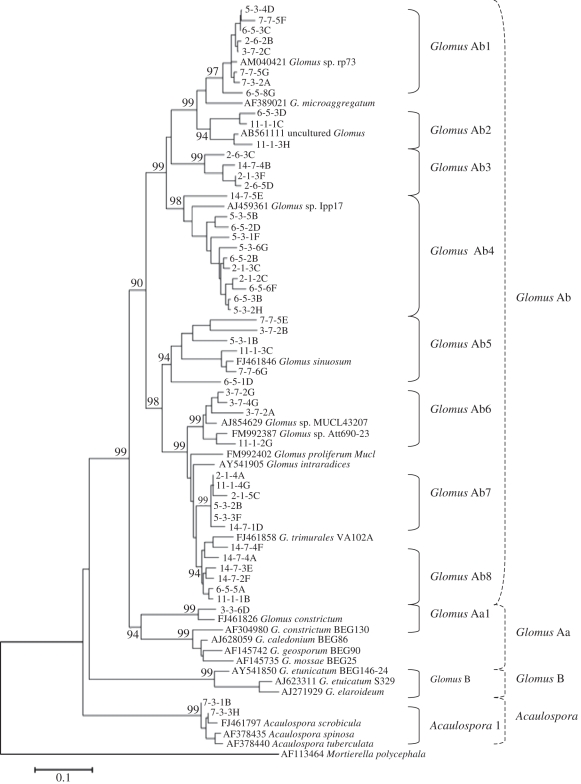
Blast searches in the GenBank database showed that all sequences obtained in this study had a high similarity to AM fungi and belonged to the Glomeromycota phylum. These sequences and those corresponding to the closest matches from GenBank were used to determine phylogenetic relationships. A total of ten AM fungal phylotypes were detected in breadfruit roots, with one to seven phylotypes per plant. The majority of these sequences (figure 2) belonged to Glomus genus, and mainly to the Glomus A group, except for one sequence (7-3-1B), an Acaulospora sp. with a 99 per cent bootstrap support. The phylogenetic relationships among the sequences belonging to the Glomus genus allowed the delineation of nine monophyletic groups, supported by significant bootstraps (greater than or equal to 94%). Among the total 59 root samples, the most common AM fungal phylotype Glomus Ab1 was found in 45 samples, while the very low-frequency AM fungal phylotype Glomus Ab2 was only found in four samples. Most of the root samples contain three to five AM fungal phylotypes.
Figure 2.
Phylogram derived from partial 28S rDNA sequences (LR1/FLR2 and FLR3-FLR4). Phylotype names are indicated to the right of the tree. Sequences obtained from this study are indicated by three numbers followed by a letter (e.g. 6-5-5A). Sequences obtained from GenBank are recognizable by containing a full species name (e.g. FJ Acaulospora spinosa). Taxonomic clades were numbered, and their affiliation to the Glomus groups as defined by Schüβler et al. [35] is indicated within dashed brackets. User-defined clades, based on support values, are indicated within solid brackets.
Both AM fungal sequence and phylotype richness strongly correlated with each other (R = 0.7085, p < 0.001) and positively related with longitude (figure 1e). While ANCOVA also indicated a positive correlation between these measures of AM fungal richness and longitude, PMT did not indicate a significant relationship after accounting for the plant age and cultivation site.
We also tested for differences among the three breadfruit species and hybrids close to NG (longitude < 13°), the native and ancestral habitat of breadfruit. We found no significant effects of age, site, species or longitude on any of the traits (electronic supplementary material, table S3), with the only exception a significant age effect of AM sequence and phylotype richness (PMT).
3. Discussion
We studied the effects of domestication on mycorrhizal symbiosis using the west-to-east movement of breadfruit cultivars as our model. Our results clearly show directionality to step-wise changes that occurred during breadfruit domestication, from oldest to most recent cultivars. The roots of domesticated breadfruit cultivars support fewer, but more diverse, communities of AM fungi than their wild ancestors, illustrating a biogeographic pattern that coincides with the presumed domestication route of breadfruit and the colonization of the Pacific islands by humans.
Our results support the hypothesis that selection of cultivars can lead to the breakdown of the AM symbiosis. Regardless of soil nutrient status and age of tree, both root AC and VC showed a clear effect of longitude on root colonization. This trend was robust despite the fact that breadfruit has been not been subjected to deliberate breeding programmes as have other crops in modern agriculture. Some studies have looked at the effect of domestication on a shorter time scale and have found similar results [23,24,36].
(a). Colonization by arbuscular mycorrhiza fungi
Based on colonization intensity, our study shows that modern breadfruit cultivars are less able to support AM fungi than wild ancestors. Domestication typically favours above-ground traits (i.e. large fruits) over below-ground traits. Compared with their wild relatives, edible fruits of domesticated taxa tend to be larger and sweeter, and are higher in oil [37]. In some cases, seeds are bigger and more numerous [4], or reduced/absent in others (i.e. banana or watermelon). In the case of breadfruit, A. altilis has fewer seeds, and a higher proportion of edible fruit with cultivar development and human migration [38]. In this study, we found that these changes concurred with a significant reduction in seed count per fruit along the domestication gradient; at a longitude beyond 40° east of NG breadfruit became increasingly seedless. This is somewhat expected since breadfruit cultivars with a high flesh/seed ratio were more likely to be propagated by people. Seedless fruits are also likely to coincide with the emergence of triploidy in some A. altilis cultivars, but the origins of this event are not clear [38,39].
The observed reduced colonization intensity may be an unintended result of selection for high-yielding breadfruit. Because fruit are strong sinks for photosynthate [40], it is possible that selection for high-fruit-yielding trees might impose limits on the amount of photosynthate available below ground for root colonization. The consequences of this trade-off in resource allocation between root and shoot may have many unintended consequences. AM fungi can confer pathogen resistance to hosts [13], and this effect may be related to the extent of root colonization [41]. Reduced allocation to mycorrhizas means that these trees may be more vulnerable to pathogens, an effect that could be magnified with industrial-scale propagation of single genotypes.
Reduced root allocation will also make it more difficult for trees to access nutrients and water in stressful conditions. AM fungi are well known to increase root depletion zones [42] and aid in the uptake of many essential nutrients, including water [43]. Given that breadfruit production is poised to become an international crop under wide-scale propagation in many new environments, it is not known how well breadfruit will do in places that have nutrient limitations or drought stress.
Some studies have shown that mycorrhizal responsiveness (the degree to which mycorrhizas improve plant growth), rather than colonization intensity, can change in response to selection. However, mycorrhizal responsiveness is strongly linked to environmental conditions, and can fluctuate greatly within a short time frame for one individual [18,44–46]. During domestication, we expect mycorrhizal responsiveness to decrease simply because cultivated plants are grown in relatively stress-free environments compared with their wild ancestors [47]. Our study was different from many studies examining the effect of domestication on root mutualisms because our trees were not grown under conditions of high nutrient availability (see electronic supplementary material, table S2). The fact that we were able to detect a change in mycorrhizal status even when nutrients were not in excess suggests that domestication effects go beyond simply alleviating nutrient stress and indicates a deeper disruption of the mutualism that is independent from soil conditions.
(b). Arbuscular mycorrhiza fungal community identity
Whereas domestication reduced the intensity of root mycorrhization, it had the opposite effect on AM sequence and phylotype richness. While this seems counterintuitive, there are some examples of increased species richness coinciding with reduced colonization intensity [48,49]. For example, if dominant AM fungi require high resource levels, then a plant's reduction in carbon allocation to roots may increase the persistence of subdominant fungi through competitive release, thereby increasing fungal species richness, despite overall lower abundance of fungi in the roots. In addition, differences in colonization strategies (spores versus runner hyphae) among AM fungi may support higher/lower fungal diversity when fungi are competing for new roots [48]. Finally, domestication could inadvertently have selected for ‘generalist’ hosts capable of forming symbiosis with a large number of fungi, since these plants were typically moved between sites.
The most common fungi in this study were affiliated with taxa belonging to Glomus group A, a finding that has been reported in agricultural fields [50,51], grassland [52] and other temperate ecosystems [53]. When we looked at AM fungal communities as a whole there were only slight differences among trees based on domestication gradient, age of tree and location in the garden. This is probably because the trees had access to the same suite of AM fungi present at the National Tropical Botanical Garden (NTBG) field site. It would be interesting to see if the trees maintained similar communities if given different cohorts of fungi to choose from.
4. Conclusion
Domestication of breadfruit provides a unique opportunity to study the effects of selection on the AM symbiosis with living representatives along the domestication trajectory. We were able to detect a biogeographic pattern along the domestication gradient in breadfruit by controlling for environmental heterogeneity in a common garden. Selection for favourable above-ground traits seemingly concurred with increased host defection from cornerstone below-ground mutualisms. This leaves the possibility that modern breadfruit cultivars may have become more susceptible to environmental stress such as droughts or pathogens, which may impact long-term food security of this and other crops. Future research should now focus on the functional effects of AM fungi on breadfruit cultivars. We still know little about changes to AM fungi in the soil resulting from domestication. This study is a starting point from which to further investigate the mechanisms and ecological consequences that are involved in the domestication process of highly mycotrophic plant crops.
5. Methods
(a). Breadfruit collection
Samples were collected in January 2010 at Kahanu Garden, part of the NTBG near Hana, Maui, Hawaii (20°47′57.07″ N, 156°02′18.42″ W). The extensive breadfruit collection (http://ntbg.org/breadfruit) is managed by NTBG's Breadfruit Institute. It contains more than 270 accessions of breadfruit (A. altilis and A. altilis × A. mariannensis hybrids), breadnut (A. camansi) and dugdug (A. mariannensis) originating from 34 Pacific islands, the Philippines, Indonesia and the Seychelles [30]. Trees were planted from 1978 to 2003 in a grid pattern orchard contained in less than 12 acres. As such it represents an unrivalled opportunity to test how the domestication process affects below-ground communities.
(b). Tree and soil characteristics
Information on the tree age, name of cultivar, origin of cultivar and number of seeds is listed in the electronic supplementary material, table S1. Information on chemical characteristics of the soils (pH, organic matter, content of phosphorus and other nutrients) obtained from the six cultivation sites (A, B, C, D, E and H) is listed in electronic supplementary material, table S2 and figure S4. Seed number was established in a previous study, using the same trees [38].
(c). Sample collection and determination of fungal colonization
We sampled 59 individual trees (electronic supplementary material, table S1) to obtain sufficient replication across species and geographical origin of cultivars. The majority of trees were A. altilis, for which we sampled cultivars with an origin spanning 74° east–west. At each tree, we sampled root soils at 15 cm from three random locations around the bole. For each sample, we located a young root that could be decisively traced to the parent tree. Young roots and any adhering rhizosphere were collected along with approximately 100 ml of bulk soil. For each tree, the three samples were then pooled. Samples were refrigerated immediately, and shipped to the University of British Columbia for further processing.
To determine AM fungal colonization of roots, we subsampled three pooled replicates from each tree. These roots were cut into small fragments (1 cm) and stained with Tryptan blue following a modified protocol [54]. Stained roots were mounted on slides and inspected for signs of AM colonization, including HC, AC and VC. For soil HC, all fungal hyphae were extracted from pooled samples [55] and hyphal length measured using a gridline intersect method [56].
(d). Molecular analysis of root arbuscular mycorrhiza fungal communities
DNA was extracted from the root fragments using the Extract-N-Amp Plant PCR Kit (Sigma-Aldrich, USA) following the manufacturer's instructions. DNA extractions and analyses were performed in duplicate. DNA extracts were diluted 10× in PCR-grade water before nested PCR.
The community structure of root-colonizing AM fungi was determined by terminal-restriction fragment length polymorphism (T-RFLP) analyses of the fragments of the LSU rRNA gene [57,58]. This procedure involves nested PCR with the primer pair LR1-FLR2 and a nested reaction with the AM fungal-specific primer pair FLR3-FLR4 [59], which were labelled with fluorescent dyes 6-FAM (FLR3) and NED (FLR4). The PCR cycling was performed as by Mummey & Rillig [57], except that the first PCR was run for 30 cycles, and the first PCR products were diluted 500× before a second PCR. After the second PCR, 50 µl of product was purified by Qiagen quick PCR purification kit (Qiagen, USA). Ten microlitres of the purified PCR product were digested by incubation with 1 U MboI in the manufacturer's recommended buffer for 4 h at 37°C, followed by 10 min at 65°C for MboI inactivity. Digests were purified by sodium acetate and ethanol precipitation, and diluted in 50 µl of H2O. Terminal restriction fragment (T-RF) sizes in each sample were determined using an ABI 3100 automated capillary DNA sequencer (Applied Biosystems, USA) with 500LIZ as size standard. T-RF size determination and quantification was performed using Genemapper software (Applied Biosystems). When the strongest peaks in the electropherograms were not in the range 2000–8000 relative fluorescence units (rfu), the sample concentration was adjusted accordingly and rerun.
T-RF size calling and binning was performed using the same method as Verbruggen et al. [51]. The resulting T-RF profiles were uploaded to the T-REX web application [60] for final dataset-side T-RF binning with a clustering threshold of 1.2 bp. All singletons (across all samples) and T-RFs present in less than three samples were excluded to reduce ‘background noise’ [61].
(e). Clone libraries
We generated an experiment-wide clone library using amplicons pooled across the six cultivation sites. These were purified using the QiAquick PCR purification kit (Qiagen) and cloned using the pGEM-T vector (Promega, USA) and Escherichia coli JM109 High Efficiency Competent cells (Promega). Ninety-six clones per library were chosen randomly, and the inserts were re-amplified. PCR products of each clone were digested using MboI as described above. Products of the reaction were examined by 1.0 per cent agarose gels. Representative clones of restriction types were sequenced in both directions. Samples were run on an ABI-310 capillary sequencer. Electropherograms of 93 successfully sequenced clones were checked in Sequencer (v. 4.10.1; Gene Codes Corporation, USA). All non-redundant sequences were deposited in GenBank under the accession numbers JF798521–JF798571.
Sequences were edited using BioEdit Sequence Alignment Editor and reference sequences of the SSU region were obtained from GenBank. Multiple alignments of sequences were processed using the Clustalx algorithm and a neighbour-joining distance tree was constructed in MEGA v. 4.0 [62] with 1000 bootstrap replicates. We finally defined ten AM fungal phylotypes in the clone sequence based on the phylogenetic tree. T-RFLP analysis of clones was used to generate fragment length profiles of known sequences to identify T-RFLP profiles of experimental samples.
(f). Data analysis
(i). T-RF data
Because we used dual-labelled primers, each sequence theoretically gave rise to two peaks. AM fungal sequence richness was therefore assessed as the number of T-RF lengths per group divided by two. Based on the phylogenetic tree and after comparing the T-RF size of each clone sequence to the original T-RFLP data, AM fungal phylotype richness was assessed as the number of different phylotypes detected in a sample.
(ii). Data handling and statistical analyses
Prior to all analyses, the response variables (richness, AC and VC) were Box–Cox-transformed using (x + 1)z (z = 0.2, 0.8 and 0.8, respectively).
The main goal of the analyses was to establish whether we could detect significant longitudinal effects along the west–east domestication gradient on AM fungal colonization rate and fungal community composition inside roots. We used two approaches: PMT and ANCOVA. In both tests, the significance of all the factors was tested after variability by other factors was accounted for. For the PMTs, we used the ecodist package in R v. 2.10.0 (http://www.R-project.org) and n = 10 000 permutations. Dissimilarity matrices were calculated with the function distance, using Euclidean distance metric for continuous variables, and a binary 0/1 coding for models that included different Artocarpus species. To account for any potential spatial autocorrelation within the common garden the pairwise distances of all the sampled trees were calculated based on their x, y coordinates on a local site map. For ANCOVAs, site and Artocarpus species were considered as fixed categorical factors. Age and longitude were included as covariates. All possible interaction terms were omitted because preliminary models indicated that most interaction terms were not significant and did not affect the main effect findings of a significant longitude effect.
To test for the effect of domestication without the effect of different Artocarpus species along a west–east gradient (oldest to most recently derived cultivars), we first analysed only A. altilis samples (n = 39). In a second analysis, we wanted to see whether the four Artocarpus species differed among samples that originated within ±13° longitude from NG. Therefore, these analyses only included trees that came from a region within or close to NG, the native habitat of breadfruit (n = 26). In figures, linear regression was used to visualize the relationship between domestication distance and seed count, root colonization and AM fungal richness, respectively (using all 56 samples).
Acknowledgements
The authors would like to thank the Breadfruit Institute for access to their collection and support throughout the project. Thanks also to the staff at Kahanu Garden for their time and advice. We would also like to thank Alex Lane for his generous help in Hawaii. This research was supported by an NSERC Discovery Grant to M.M.H., and A.M.K. was supported by a grant from the Swiss National Science Foundation (PAOOA-119519).
References
- 1.Hancock J. F. 2005. Contributions of domesticated plant studies to our understanding of plant evolution. Ann. Bot. (Lond.) 96, 953–963 10.1093/aob/mci259 (doi:10.1093/aob/mci259) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Benz B. F. 2001. Archaeological evidence of teosinte domestication from Guilá Naquitz, Oaxaca. Proc. Natl Acad. Sci. USA 98, 2104–2106 10.1073/pnas.98.4.2104 (doi:10.1073/pnas.98.4.2104) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Purugganan M. D., Fuller D. Q. 2009. The nature of selection during plant domestication. Nature 457, 843–848 10.1038/nature07895 (doi:10.1038/nature07895) [DOI] [PubMed] [Google Scholar]
- 4.Parker I. M., López I., Petersen J. J., Anaya N., Cubilla-Rios L., Potter D. 2010. Domestication syndrome in Caimito (Chrysophyllum cainito L.): fruit and seed characteristics. Econ. Bot. 64, 161–175 10.1007/s12231-010-9121-4 (doi:10.1007/s12231-010-9121-4) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fuller D. Q. 2007. Contrasting patterns in crop domestication and domestication rates: recent archaeobotanical insights from the old world. Ann. Bot. 100, 903–924 10.1093/aob/mcm048 (doi:10.1093/aob/mcm048) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wardle D. A., Bardgett R. D., Klironomos J. N., Setala H., van der Putten W. H., Wall D. H. 2004. Ecological linkages between aboveground and belowground biota. Science 304, 1629–1633 10.1126/science.1094875 (doi:10.1126/science.1094875) [DOI] [PubMed] [Google Scholar]
- 7.Sapp J. 2004. The dynamics of symbiosis: an historical overview. Can. J. Bot. 82, 1046–1056 10.1139/b04-055 (doi:10.1139/b04-055) [DOI] [Google Scholar]
- 8.Gosling P., Hodge A., Goodlass G., Bending G. D. 2006. Arbuscular mycorrhizal fungi and organic farming. Agr. Ecosyst. Environ. 113, 17–35 10.1016/j.agee.2005.09.009 (doi:10.1016/j.agee.2005.09.009) [DOI] [Google Scholar]
- 9.Smith S. E., Read D. J. 2008. Mycorrhizal symbiosis. London, UK: Academic Press [Google Scholar]
- 10.Rillig M. C., Mummey D. L. 2006. Mycorrhizas and soil structure. New Phytol. 171, 41–53 10.1111/j.1469-8137.2006.01750.x (doi:10.1111/j.1469-8137.2006.01750.x) [DOI] [PubMed] [Google Scholar]
- 11.van der Heijden M. G., Streitwolf-Engel R., Riedl R., Siegrist S., Neudecker A., Ineichen K., Boller T., Wiemken A., Sanders I. R. 2006. The mycorrhizal contribution to plant productivity, plant nutrition and soil structure in experimental grassland. New Phytol. 172, 739–752 10.1111/j.1469-8137.2006.01862.x (doi:10.1111/j.1469-8137.2006.01862.x) [DOI] [PubMed] [Google Scholar]
- 12.Bouwmeester H. J., Roux C., Lopez-Raez J. A., Beard G. 2007. Rhizosphere communication of plants, parasitic plants and AM fungi. Trends Plant Sci. 12, 224–230 10.1016/j.tplants.2007.03.009 (doi:10.1016/j.tplants.2007.03.009) [DOI] [PubMed] [Google Scholar]
- 13.Maherali H., Klironomos J. N. 2007. Influence of phylogeny on fungal community assembly and ecosystem functioning. Science 316, 1746–1748 10.1126/science.1143082 (doi:10.1126/science.1143082) [DOI] [PubMed] [Google Scholar]
- 14.Hart M. M., Klironomos J. N. 2002. Diversity of arbuscular mycorrhizal fungi and ecosystem functioning. In Mycorrhizal ecology: ecological studies (eds van der Heijden M. G. A., Sander I.). Berlin, Germany: Springer [Google Scholar]
- 15.Wilson G. W. T., Rice C. W., Rillig M. C., Springer A., Hartnett D. C. 2009. Soil aggregation and carbon sequestration are tightly correlated with the abundance of arbuscular mycorrhizal fungi: results from long-term field experiments. Ecol. Lett. 12, 452–461 10.1111/j.1461-0248.2009.01303.x (doi:10.1111/j.1461-0248.2009.01303.x) [DOI] [PubMed] [Google Scholar]
- 16.Schwartz M. W., Hoeksema J. D., Gehring C. A., Johnson N. C., Klironomos J. N., Abbott L. K., Pringle A. 2006. The promise and the potential consequences of the global transport of mycorrhizal fungal inoculum. Ecol. Lett. 9, 501–515 10.1111/j.1461-0248.2006.00910.x (doi:10.1111/j.1461-0248.2006.00910.x) [DOI] [PubMed] [Google Scholar]
- 17.Hamel C. 1996. Prospects and problems pertaining to the management of arbuscular mycorrhizae in agriculture. Agric. Ecosyst. Environ. 60, 197–210 10.1016/S0167-8809(96)01071-7 (doi:10.1016/S0167-8809(96)01071-7) [DOI] [Google Scholar]
- 18.Sawers R. J. H., Gutjahr C., Paszkowski U. 2008. Cereal mycorrhiza: an ancient symbiosis in modern agriculture. Trends Plant Sci. 13, 93–97 10.1016/j.tplants.2007.11.006 (doi:10.1016/j.tplants.2007.11.006) [DOI] [PubMed] [Google Scholar]
- 19.Hetrick B. A. D., Wilson G. W. T., Cox T. S. 1993. Mycorrhizal dependence of modern wheat cultivars and ancestors: a synthesis. Can. J. Bot. 71, 512–518 10.1139/b93-056 (doi:10.1139/b93-056) [DOI] [Google Scholar]
- 20.Zhu Y. G., Smith S. E., Barritt A. R., Smith F. A. 2001. Phosphorus (P) efficiencies and mycorrhizal responsiveness of old and modern wheat cultivars. Plant Soil 237, 249–255 10.1023/A:1013343811110 (doi:10.1023/A:1013343811110) [DOI] [Google Scholar]
- 21.Grace E. J., Cotsaftis O., Tester M., Smith F. A., Smith S. E. 2009. Arbuscular mycorrhizal inhibition of growth in barley cannot be attributed to extent of colonization, fungal phosphorus uptake or effects on expression of plant phosphate transporter genes. New Phytol. 181, 938–949 10.1111/j.1469-8137.2008.02720.x (doi:10.1111/j.1469-8137.2008.02720.x) [DOI] [PubMed] [Google Scholar]
- 22.Khalil S., Loynachan T. E., Tabatabai M. A. 1994. Mycorrhizal dependency and nutrient-uptake by improved and unimproved corn and soybean cultivars. Agron. J. 86, 949–958 10.2134/agronj1994.00021962008600060005x (doi:10.2134/agronj1994.00021962008600060005x) [DOI] [Google Scholar]
- 23.Wright D. P., Scholes J. D., Read D. J., Rolfe S. A. 2005. European and African maize cultivars differ in their physiological and molecular responses to mycorrhizal infection. New Phytol. 167, 881–896 10.1111/j.1469-8137.2005.01472.x (doi:10.1111/j.1469-8137.2005.01472.x) [DOI] [PubMed] [Google Scholar]
- 24.An G. H., Kobayashi S., Enoki H., Sonobe K., Muraki M., Karasawa T., Ezawa T. 2010. How does arbuscular mycorrhizal colonization vary with host plant genotype? An example based on maize (Zea mays) germplasms. Plant Soil 327, 441–453 10.1007/s11104-009-0073-3 (doi:10.1007/s11104-009-0073-3) [DOI] [Google Scholar]
- 25.Kiers E. T., Hutton M. G., Denison R. F. 2007. Human selection and the relaxation of legume defences against ineffective rhizobia. Proc. R. Soc. B 274, 3119–3126 10.1098/rspb.2007.1187 (doi:10.1098/rspb.2007.1187) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Koide R., Li M., Lewis J., Irby C. 1988. Role of mycorrhizal infection in the growth and reproduction of wild vs cultivated plants. 1. Wild vs cultivated oats. Oecologia 77, 537–543 10.1007/BF00377271 (doi:10.1007/BF00377271) [DOI] [PubMed] [Google Scholar]
- 27.Bryla D. R., Koide R. T. 1990. Role of mycorrhizal infection in the growth and reproduction of wild vs cultivated plants. 2. 8 wild accessions and 2 cultivars of Lycopersicon esculentum Mill. Oecologia 84, 82–92 10.1007/BF00665599 (doi:10.1007/BF00665599) [DOI] [PubMed] [Google Scholar]
- 28.Estaún V., Calvet C., Camprubi A. 2010. Effect of differences among crop species and cultivars on the arbuscular mycorrhizal symbiosis. In Arbuscular mycorrhizas: physiology and function (eds Koltai H., Kapulnik Y.), pp. 279–298 Berlin, Germany: Springer Sciences + Business Media BV [Google Scholar]
- 29.Jagtap U. B., Bapat V. A. 2010. Artocarpus: a review of its traditional uses, phytochemistry and pharmacology. J. Ethnopharmacol. 129, 142–166 10.1016/j.jep.2010.03.031 (doi:10.1016/j.jep.2010.03.031) [DOI] [PubMed] [Google Scholar]
- 30.Ragone D. 1997. Breadfruit. Artocarpus altilis (Parkinson) Fosberg. In Promoting the conservation and use of underutilized and neglected crops. Rome, Italy: International Plant Genetic Resources Institute [Google Scholar]
- 31.Zerega N. J. C., Ragone D., Moltley T. J. 2005. Systematics and species limits of Breadfruit (Artocarpus, Moraceae). Syst. Bot. 30, 603–615 10.1600/0363644054782134 (doi:10.1600/0363644054782134) [DOI] [Google Scholar]
- 32.Jones A. M. P., Murch S., Ragone D. 2010. Diversity of breadfruit (Artocarpus altilis, Moraceae) seasonality: a resource for year-round nutrition. Econ. Bot. 64, 340–351 10.1007/s12231-010-9134-z (doi:10.1007/s12231-010-9134-z) [DOI] [Google Scholar]
- 33.Zerega N. J. C., Ragone D., Motley T. J. 2004. Complex origins of breadfruit (Artocarpus altilis, Moraceae): implications for human migrations in Oceania. Am. J. Bot. 91, 760–766 10.3732/ajb.91.5.760 (doi:10.3732/ajb.91.5.760) [DOI] [PubMed] [Google Scholar]
- 34.Jarrett F. M. 1959. Studies in Artocarpus and allied genera. III. A revision of Artocarpus subgenus Artocarpus. J. Arnold Arboretum 40, 114–155 (327–368) [Google Scholar]
- 35.Schüβler A., Schwarzott D., Walker C. 2001. A new fungal phylum, the Glomeromycota: phylogeny and evolution. Mycol. Res. 105, 1413–1421 10.1017/S0953756201005196 (doi:10.1017/S0953756201005196) [DOI] [Google Scholar]
- 36.Toth R., Toth D., Starke D., Smith D. R. 1990. Vesicular-arbuscular mycorrhizal colonization in Zea mays affected by breeding for resistance to fungal pathogens. Can. J. Bot. 68, 1039–1044 10.1139/b90-131 (doi:10.1139/b90-131) [DOI] [Google Scholar]
- 37.Maranz S., Wiesman Z. 2003. Evidence for indigenous selection and distribution of the shea tree, Vitellaria paradoxa, and its potential significance to prevailing parkland savanna tree patterns in Sub-Saharan Africa north of the equator. J. Biogeogr. 30, 1505–1516 10.1046/j.1365-2699.2003.00892.x (doi:10.1046/j.1365-2699.2003.00892.x) [DOI] [Google Scholar]
- 38.Ragone D. 2001. Chromosome numbers and pollen stainability of three species of Pacific Island breadfruit (Artocarpus, Moraceae). Am. J. Bot. 88, 693–696 10.2307/2657070 (doi:10.2307/2657070) [DOI] [PubMed] [Google Scholar]
- 39.Murch S. J., Ragone D., Shi W. L., Alan A. R., Saxena P. K. 2008. In vitro conservation and sustained production of Breadfruit (Artocarpus altilis, Moraceae): modern technologies for a traditional tropical crop. Naturwissenschaften 95, 99–107 10.1007/s00114-007-0295-2 (doi:10.1007/s00114-007-0295-2) [DOI] [PubMed] [Google Scholar]
- 40.Guo Y., Fourcaud T., Jaeger M., Zhang X. P., Li B. G. 2011. Plant growth and architectural modelling and its applications. Ann. Bot. 107, 723–727 10.1093/aob/mcr073 (doi:10.1093/aob/mcr073) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Newsham K. K., Fitter A. H., Watkinson A. R. 1995. Arbuscular mycorrhiza protect an annual grass from root pathogenic fungi in the field. J. Ecol. 83, 991–1000 10.2307/2261180 (doi:10.2307/2261180) [DOI] [Google Scholar]
- 42.Marschner H., Dell B. 1994. Nutrient uptake in mycorrhizal symbiosis. Plant Soil 159, 89–102 [Google Scholar]
- 43.Auge R. M. 2001. Water relations, drought and vesicular–arbuscular mycorrhizal symbiosis. Mycorrhiza 11, 3–42 10.1007/s005720100097 (doi:10.1007/s005720100097) [DOI] [Google Scholar]
- 44.Janos D. P. 2007. Plant responsiveness to mycorrhizas differs from dependence upon mycorrhizas. Mycorrhiza 17, 75–91 10.1007/s00572-006-0094-1 (doi:10.1007/s00572-006-0094-1) [DOI] [PubMed] [Google Scholar]
- 45.Hoeksema J. D., et al. 2010. A meta-analysis of context-dependency in plant response to inoculation with mycorrhizal fungi. Ecol. Lett. 13, 394–407 10.1111/j.1461-0248.2009.01430.x (doi:10.1111/j.1461-0248.2009.01430.x) [DOI] [PubMed] [Google Scholar]
- 46.Varga S., Kytoviita M. M. 2010. Mycorrhizal benefit differs among the sexes in a gynodioecious species. Ecology 91, 2583–2593 10.1890/09-1383.1 (doi:10.1890/09-1383.1) [DOI] [PubMed] [Google Scholar]
- 47.Rao P. S. K., Tilak K., Arunachalam V. 1990. Genetic-variation for VA mycorrhiza-dependent phosphate mobilization in groundnut (Arachis hypogaea L). Plant Soil 122, 137–142 10.1007/BF02851921 (doi:10.1007/BF02851921) [DOI] [Google Scholar]
- 48.Johnson D., Vandenkoornhuyse P. J., Leake J. R., Gilbert L., Booth R. E., Grime J. P., Young J. P. W., Read D. J. 2004. Plant communities affect arbuscular mycorrhizal fungal diversity and community composition in grassland microcosms. New Phytol. 161, 503–515 10.1046/j.1469-8137.2003.00938.x (doi:10.1046/j.1469-8137.2003.00938.x) [DOI] [PubMed] [Google Scholar]
- 49.Johnson D., Anderson I. C., Williams A., Whitlock R., Grime J. P. 2010. Plant genotypic diversity does not beget root-fungal species diversity. Plant Soil 336, 107–111 10.1007/s11104-010-0452-9 (doi:10.1007/s11104-010-0452-9) [DOI] [Google Scholar]
- 50.Hijri I., Sykorova Z., Oehl F., Ineichen K., Mader P., Wiemken A., Redecker D. 2006. Communities of arbuscular mycorrhizal fungi in arable soils are not necessarily low in diversity. Mol. Ecol. 15, 2277–2289 10.1111/j.1365-294X.2006.02921.x (doi:10.1111/j.1365-294X.2006.02921.x) [DOI] [PubMed] [Google Scholar]
- 51.Verbruggen E., Röling W. F. M., Gamper H. A., Kowalchuk G. A., Verhoef H. A., van der Heijden M. G. A. 2010. Positive effects of organic farming on belowground mutualists: large-scale comparison of mycorrhizal fungal communities in agricultural soils. New Phytol. 186, 968–979 10.1111/j.1469-8137.2010.03230.x (doi:10.1111/j.1469-8137.2010.03230.x) [DOI] [PubMed] [Google Scholar]
- 52.van de Voorde T. F. J., van der Puttern W. H., Gamper H. A., Gera Hol W. H., Bezemer T. M. 2010. Comparing arbuscular mycorrhizal communities of individual plants in a grassland biodiversity experiment. New Phytol. 186, 746–754 10.1111/j.1469-8137.2010.03216.x (doi:10.1111/j.1469-8137.2010.03216.x) [DOI] [PubMed] [Google Scholar]
- 53.Öpik M., Moora M., Liira J., Zobel M. 2006. Composition of root-colonizing arbuscular mycorrhizal fungal communities in different ecosystems around the globe. J. Ecol. 94, 778–790 10.1111/j.1365-2745.2006.01136.x (doi:10.1111/j.1365-2745.2006.01136.x) [DOI] [Google Scholar]
- 54.McGonigle T. P., Miller M. H., Evans D. G., Fairchild G. L., Swan J. A. 1990. A new method which gives an objective measure of colonization of roots by vesicular–arbuscular mycorrhizal fungi. New Phytol. 115, 495–501 10.1111/j.1469-8137.1990.tb00476.x (doi:10.1111/j.1469-8137.1990.tb00476.x) [DOI] [PubMed] [Google Scholar]
- 55.Miller R. M., Reinhardt D. R., Jastrow J. D. 1995. External hyphal production of vesicular-arbuscular mycorrhizal fungi in pasture and tallgrass prairie communities. Oecologia 103, 17–23 10.1007/BF00328420 (doi:10.1007/BF00328420) [DOI] [PubMed] [Google Scholar]
- 56.Neuman E. I. 1966. A method of estimating the total length of root in a sample. J. Appl. Ecol. 3, 139–145 10.2307/2401670 (doi:10.2307/2401670) [DOI] [Google Scholar]
- 57.Mummey D. L., Rillig M. C. 2007. Evaluation of LSU rRNA-gene PCR primers for analysis of arbuscular mycorrhizal fungal communities via terminal restriction fragment length polymorphism analysis. J. Microbiol. Method 70, 200–204 10.1016/j.mimet.2007.04.002 (doi:10.1016/j.mimet.2007.04.002) [DOI] [PubMed] [Google Scholar]
- 58.Krüger M., Stockinger H., Krüger C., Schüßler A. 2009. DNA-based species level detection of Glomeromycota: one PCR primer set for all arbuscular mycorrhizal fungi. New Phytol. 183, 212–223 10.1111/j.1469-8137.2009.02835.x (doi:10.1111/j.1469-8137.2009.02835.x) [DOI] [PubMed] [Google Scholar]
- 59.Gollotte A., van Tuinen D., Atkinson D. 2004. Diversity of arbuscular mycorrhizal fungi colonizing roots of the grass species Agrostis capillaris and Lolium perenne in a field experiment. Mycorrhiza 14, 111–117 10.1007/s00572-003-0244-7 (doi:10.1007/s00572-003-0244-7) [DOI] [PubMed] [Google Scholar]
- 60.Culman S. W., Bukowski R., Gauch H. G., Cadillo-Quiroz H., Buckley D. H. 2009. T-REX: software for the processing and analysis of T-RFLP data. BMC Bioinform. 10, 10. 10.1186/1471-2105-10-171 (doi:10.1186/1471-2105-10-171) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Culman S. W., Gauch H. G., Blackwood C. B., Thies J. E. 2008. Analysis of T-RFLP data using analysis of variance and ordination methods: a comparative study. J. Microbiol. Method 75, 55–63 10.1016/j.mimet.2008.04.011 (doi:10.1016/j.mimet.2008.04.011) [DOI] [PubMed] [Google Scholar]
- 62.Tamura K., Dudley J., Nei M., Kumar S. 2007. MEGA4: molecular evolutionary genetics analysis (MEGA) software version 4.0. Mol. Biol. Evol. 24, 1596–1599 10.1093/molbev/msm092 (doi:10.1093/molbev/msm092) [DOI] [PubMed] [Google Scholar]