Abstract
Structural colours, the most intense, reflective and pure colours in nature, are generated when light is scattered by complex nanostructures. Metallic structural colours are widespread among modern insects and can be preserved in their fossil counterparts, but it is unclear whether the colours have been altered during fossilization, and whether the absence of colours is always real. To resolve these issues, we investigated fossil beetles from five Cenozoic biotas. Metallic colours in these specimens are generated by an epicuticular multi-layer reflector; the fidelity of its preservation correlates with that of other key cuticular ultrastructures. Where these other ultrastructures are well preserved in non-metallic fossil specimens, we can infer that the original cuticle lacked a multi-layer reflector; its absence in the fossil is not a preservational artefact. Reconstructions of the original colours of the fossils based on the structure of the multi-layer reflector show that the preserved colours are offset systematically to longer wavelengths; this probably reflects alteration of the refractive index of the epicuticle during fossilization. These findings will allow the former presence, and original hue, of metallic structural colours to be identified in diverse fossil insects, thus providing critical evidence of the evolution of structural colour in this group.
Keywords: structural colour, taphonomy, fossil insects, epicuticle, fossil preservation
1. Introduction
Biophotonic nanostructures generate structural colours by scattering light [1]. They comprise alternating materials of different refractive indices organized into one- (e.g. multi-layer reflectors), two- (e.g. diffraction gratings) or three-dimensional (e.g. gyroids and cubic and diamond lattices) arrays [1]. Such structures are widespread among modern animals [2], where they function primarily in inter- and intraspecific signalling [3–5]. Research on biological structural colours has focused on insects, especially butterflies [3] and beetles [5]. Preservation of colour-producing structures in fossil insects offers the possibility of testing hypotheses on the evolution of structural colours [4–7] and communication strategies in this group, but only if the original structures and colours are known. Evidence of structural colour (including metallic colours [8]) has been reported from insects (typically beetles) from various fossil biotas [9–20]. Survival of the original colours in such fossils is often assumed (e.g. [14,17]), but rarely tested using computer modelling. The only studies to do so have yielded conflicting results: claims that original structural colour survives in beetles from the Pleistocene (less than 600 ka) [12] and the Mid-Eocene (ca 47 Ma) [13] contrast with evidence from Mid-Eocene fossil lepidopterans in which original colours are not preserved [20]. The preservation potential of structural colours over geological timescales, and their usefulness for evolutionary reconstructions, is therefore uncertain. Here, we address these issues by investigating the ultrastructural- and spectral fidelity of fossil beetles from five lacustrine-hosted Lagerstätten ranging from 15 to 47 million years in age. In our novel approach, we combine data from electron microscopic-, microspectrophotometric- and two-dimensional Fourier analyses to determine the origin and the fidelity of preserved metallic colours in the beetles. Further, by placing preservation of biophotonic nanostructures within the context of the fidelity of preservation of cuticule as a whole, we present, to our knowledge, the first predictive model for the presence of biophotonic nanostructures in fossil insects.
2. Material and methods
Fossil beetle specimens were studied from the following Lagerstätten: Clarkia, Middle Miocene (15 Ma), Idaho, USA [21]; Randecker Maar, Early Miocene (17 Ma), Germany; Enspel, Late Oligocene (25 Ma), Germany [22]; Eckfeld, Middle Eocene (40 Ma), Germany [23] and Messel, Middle Eocene (47 Ma), Germany [24] (figure 1). These Lagerstätten are hosted within organic-rich, finely laminated lacustrine mudstones [21,25]. The specimens are held by the following institutions: Generaldirektion Kulturelles Erbe, Mainz, Germany (GKE); Naturhistorisches Museum Mainz (Germany) (NMM); Senckenberg Forschungsinstitut und Naturmuseum, Forschungsstation Grube Messel, Germany (MeI); Staatliches Museum für Naturkunde Stuttgart, Germany (SMNS) and the Yale Peabody Museum of Natural History, USA (YPM). Specimens are stored in glycerine (100%, or 70–99% in water) or ethanol (70%). There is no evidence that immersion in these solutions affects the hue of the observed colour. The material studied includes unidentified beetle specimens with metallic colours and specimens of beetle taxa known to exhibit metallic colours (Buprestidae, Chrysomelidae and Tenebrionidae; the specimens studied are not identified to species level, but examples from different Lagerstätten probably represent conspecifics or, at the very least, closely related species). The lustre and cohesiveness of the cuticle were assessed visually and used as a proxy for the quality of preservation: the cuticle was coded as (i) ‘well preserved’ if highly reflective, solid and cohesive, and (ii) ‘poorly preserved’ if not reflective and friable. Metallic colours are bright where the cuticle is well preserved, and dull or absent where the cuticle is poorly preserved; these colours are apparent in sunlight, white light and under the optical microscope, and their hue does not alter upon drying of the specimen (at least on timescales of hours to days). Well-preserved curculionid beetles from Eckfeld that do not exhibit metallic coloration were studied for comparison; their cuticles are typically black, highly reflective, solid and cohesive. Specimens for digital photography were washed in water and allowed to dry (the storage medium was replaced subsequently).
Figure 1.
Beetle specimens from Cenozoic Lagerstätten analysed using scanning- and transmission electron microscopy, and reflectance microspectrophotometry in this study. (a–h) Well-preserved specimens; the cuticle is highly reflective and metallic colours, bright. (a) Buprestid from Enspel (GKE 2009 PE 5889). (b) Chrysomelid elytron from Clarkia (YPM 2010 P37b 005). (c) Unidentified beetle from Clarkia (YPM 2010 P37b 010). (d) Chrysomelid from Messel (MeI 15 552). (e) Chrysomelid from Messel (MeI 15 553). (f–h) Chrysomelids from Eckfeld ((f) NMM PE 2000 364, (g) NMM PE 2000 385 and (h) NMM PE 2000 636). (i) Poorly preserved chrysomelid elytron from Eckfeld (NMM PE 2000 470); metallic colour is dull. (j) Poorly preserved chrysomelid from Eckfeld; cuticle is matt and friable and metallic colour is not preserved (NMM PE 2000 449). Scale bars: (a,c) 5 mm; (b,d–j) 1 mm.
Small (2–3 mm2) samples of cuticle were removed from selected specimens using sterile tools and prepared for scanning- and transmission electron microscopy (SEM and TEM) using the method in McNamara et al. [20]. Particular care was taken to orient samples for TEM orthogonal to the cutting surface to ensure that sections through the cuticle were as close to vertical as possible. Samples for SEM were examined using a FEI XL-30 ESEM-FEG microscope equipped with an EDAX energy dispersive X-ray spectrometer. Observations were made at an accelerating voltage of 15 kV, with acquisition times of 60 s for electron dispersive spectra of carbon-coated samples. Samples for TEM were examined using a Zeiss EM900 TEM at 80 kV with an objective aperture of 90 µm diameter.
Entire specimens or cuticle samples for reflectance microspectrophotometry were washed in water and allowed to dry. Spectra collected from a 70 µm diameter spot were recorded in air using an epi-illumination Nikon Optiphot 66 microscope and an Ocean Optics HR2000+ spectrophotometer. Recorded spectra were normalized against the spectrum of the light source from a white standard. Predicted reflection spectra were obtained by analysing variation in the refractive index of structures within the cuticle using two-dimensional Fourier analysis of digital TEM micrographs [1]. This analysis was performed using a freely available Fourier tool (http://www.yale.edu/eeb/prum/fourier.htm) that is implemented in the matrix algebra program Matlab and uses the distribution of lighter and darker areas in the TEM image (and therefore the distribution of materials of different refractive index) to estimate the average refractive index of the structure. The fossil cuticles exhibit subtle vertical undulations over distances of 10–100 µm owing to minor deformation around small sedimentary particles. This results in small variations in the thicknesses of cuticular structures in TEM sections. Care was taken to select TEM micrographs that are representative of each sample; in particular, micrographs of regions of cuticle where structures appear unusually thick were not used for two-dimensional Fourier analysis.
3. Results
(a). Preservation of cuticular ultrastructure
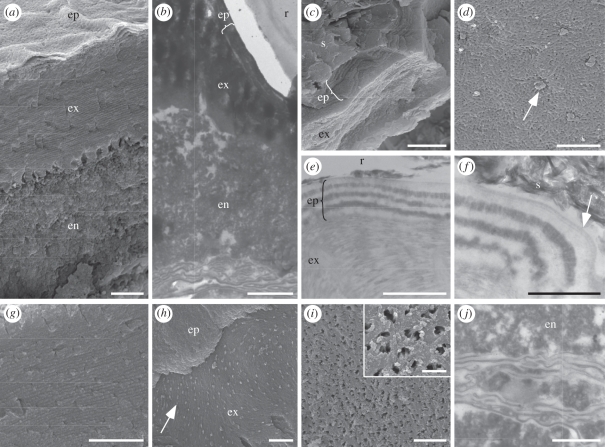
All the fossil beetles studied that show metallic colours (regardless of taxon and locality) retain ultrastructures diagnostic of extant beetle cuticle (figure 2, [26]), including horizontal divisions between the epi-, exo- and endocuticle (figure 2a,b). The epicuticle (0.3–0.7 µm thick) in the fossil beetles comprises two layers (figure 2c–f). The finely laminate outermost layer (total thickness 15–30 nm; lamina thickness 5–10 nm) is of moderate electron density (figure 2f, arrow) and corresponds in thickness and structure to the outer epicuticle (sensu [27]) [27,28] in extant insects. It is underlained by 6–14 thicker laminae of alternating electron contrast; lamina thickness varies between specimens, but is typically between 100 and 150 nm (figure 2c–f). Similar laminar arrays occur in the inner epicuticle of many extant beetles [5,28–30]. Each lamina comprises a reticulate network of filaments (each 150–750 nm long and 75 nm wide; figure 2d) that resembles the fibrillar lipid network in the inner epicuticle of extant beetles [31]. The exocuticle in the fossil beetles comprises thinner, uniformly electron-dense laminae (each 90–100 nm thick; figure 2e,g) and exhibits arcuate patterns in oblique section (figure 2h). Such patterns also occur in the exocuticle of extant insects, where they are generated by helicoidal chitin fibrils [26]. The exocuticle of the fossil beetles is perforated by pore canals (each 200–400 nm across) that each contain two to three spiral filaments (each 60 nm across; figure 2h,i), as in extant beetles [26]. The endocuticle of the fossil beetles shows a granular texture (figure 2a,b,j) and is underlain by membranous material (figure 2b,j) that represents the degraded remains of the extracellular membranes of the basal endocuticle and basement membrane of the cuticle [26].
Figure 2.
(a,c,d,g–i) Scanning and (b,e,f,j) transmission electron micrographs of cuticle ultrastructures in fossil beetles with well-preserved metallic colours. (a,b) Vertical sections through the cuticle showing epicuticle (ep), exocuticle (ex) and endocuticle (en). r, resin. (c) Fractured vertical section through the cuticle showing laminated epicuticle (ep) and exocuticle (ex). s, sediment. (d) Detail of surface of lamina in epicuticle showing fibrillar network perforated by pore canals (arrow). (e) Vertical section through the cuticle showing alternating electron-dense and electron-lucent laminae in epicuticle (ep), and uniformly electron-dense laminae in exocuticle (ex). r, resin. (f) Vertical section through the epicuticle showing multi-laminate outer epicuticle (arrow). s, sediment. (g) Vertical section through the exocuticle showing laminations. (h) Oblique fractured section through the epicuticle (ep) and exocuticle (ex) showing arcuate patterns and pore canals (arrow) in the exocuticle. (i) Internal-facing surface of the base of the exocuticle showing pore canals and, inset, pore canal filaments. (j) Membranes of the basal endocuticle (en) and/or basement membrane. Note, granular texture of the endocuticle. Scale bars: (a,b,e) 1 µm; (c,d,g,h) 2 µm; (f,j) 500 nm; (i) 5 µm, inset, 1 µm.
The fidelity of preservation of cuticular ultrastructures is significantly lower in specimens where metallic coloration is dull or not preserved. Where metallic coloration is dull, there is little or no evidence of the outer epicuticle, exocuticular lamination or pore canals (figure 3a), lamination of the inner epicuticle is typically reduced to a subtle banding (figure 3b), and details of fibrillar and helicoidal textures in the epi- and exocuticle, respectively, are not preserved. The endocuticle in these specimens typically shows a granular texture, but may exhibit a subtle banding. Where metallic coloration is not preserved (in taxa that are known to be coloured), only the division between the exo- and endocuticle is apparent (figure 3c,d). Such specimens exhibit an orthogonal arrangement of fibrous bundles in the endocuticle (figure 3c,d). This is identical to the cross-ply organization of bundles of chitin microfibrils in the endocuticle of extant beetles [26] and is characteristic of degraded insect cuticle [32]. The curculionid beetles from Eckfeld lack metallic colour, but exhibit most or all of the cuticular ultrastructures present in the specimens of other taxa with well-preserved colour, except for lamination within the epicuticle (figure 3e,f).
Figure 3.
(a,c,e,f) Scanning and (b,d) transmission electron micrographs of beetle specimens that do not exhibit well-preserved metallic colours. (a,b) Cuticle from a specimen with poorly preserved metallic colour. (a) Vertical fractured section through the cuticle showing textural distinction between exocuticle (ex) and endocuticle (en), and pore canals (arrow). (b) Subtle banding in the epicuticle (ep). Note lamination in the exocuticle is not preserved. s, sediment. (c,d) Cuticle from a specimen where metallic colours are not preserved, showing orthogonally arranged bundles of chitin fibrils in the endocuticle (en). No ultrastructures are preserved in the exocuticle (ex) or epicuticle. r, resin. (e,f) Cuticle from a curculionid from Eckfeld. (e) Preserved lamination in the exocuticle; there is no evidence for a laminated epicuticle. (f) Internal-facing surface of the exocuticle showing pore canals. Scale bars: (a,e) 1 µm; (b) 500 nm; (c) 5 µm; (d,f) 2 µm and inset in (d) 500 nm.
(b). Reflectance spectrophotometry of colour-producing nanostructures
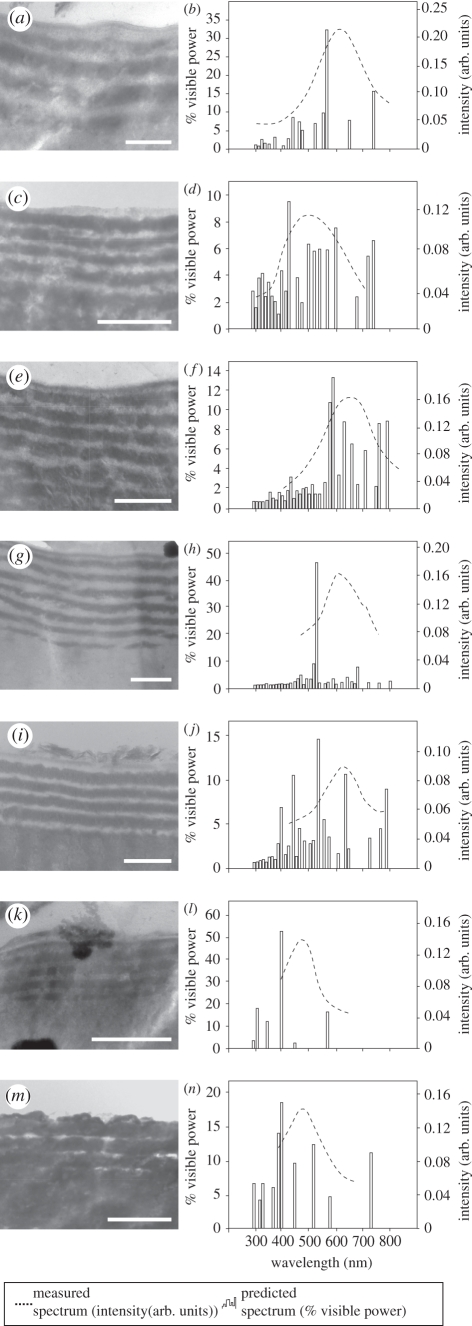
There is no evidence in any of the fossil beetles of either diffraction grating on the surface of the cuticle, or three-dimensional photonic crystals within it; the preserved colour is therefore generated by one or more laminar structures within the cuticle. In extant beetles, laminar colour-producing nanostructures, i.e. multi-layer reflectors, can occur in the epicuticle, exocuticle or endocuticle [5]. The periodicity of the layering in the fossil cuticles is not sufficiently high in either the outer epicuticle (approx. 5–10 nm) or the exocuticle (approx. 90–100 nm) to scatter light in the visible part of the spectrum [33] (electronic supplementary material, figure S1). The laminar array within the inner epicuticle, however, has a periodicity of 150–300 nm and is therefore capable of generating a structural colour [33], i.e. it represents a fossil multi-layer reflector. This feature can be used to determine whether the preserved colours of the fossil beetles are original, but only if its structure has not been modified during fossilization [4]. There is no evidence that the fossil cuticles experienced diagenetic shrinkage (owing to dehydration), expansion (owing to absorption of diagenetic fluids) or loading (during burial): features such as polygonal cracking, separation of cuticle layers, swelling and ptygmatic folding are absent. Further, it is highly unlikely that the cuticle experienced isotropic volume changes during diagenesis: the dimensions of preserved cuticular ultrastructures correspond to those in extant insects. Collectively, these data indicate that the structure of the fossil cuticles has survived fossilization with little or no modification. The fossil multi-layer reflector, therefore, provides a reliable basis for determining whether the colours of the fossil beetles are original. The electron-dense (high-index) and electron-lucent (low-index) layers of the multi-layer reflector were assigned refractive index values of 1.73 and 1.4, respectively, based on the available data for multi-layer reflectors within the epicuticle of extant, and other fossil, insects [12,30,34]. Two-dimensional Fourier analysis of TEM images of the fossil multi-layer reflectors from various beetle specimens reveals that the predicted peak wavelengths of reflectance are consistently shorter (12.97 ± 3.84%) than the measured reflectance peaks (figure 4 and table 1).
Figure 4.
Two-dimensional Fourier analysis and reflectance microspectrophotometry of fossil multi-layer reflectors in beetle specimens with well-preserved metallic colours. (a,c,e,g,i,k,m) transmission electron micrographs of epicuticular multi-layer reflectors preserved in specimens in figure 1(a–g). (b,d,f,h,j,l,n) Measured and Fourier predicted reflectance spectra for nanostructures in parts (a,c,e,g,i,k,m). Scale bars: (a,c,e,g,i,k,m) 500 nm.
Table 1.
Measured and expected values for the peak wavelength generated by the fossil multi-layer reflectors in figure 4.
| specimen | reflectance peak (nm) |
|
|---|---|---|
| measured | predicted | |
| GKE 2009 PE 5889 | 606 | 560 |
| YPM 2010 P37b 005 | 498 | 430 |
| YPM 2010 P37b 010 | 640 | 590 |
| MeI 15 552 | 631 | 530 |
| MeI 15 553 | 610 | 530 |
| NMM 2000 364 | 482 | 400 |
| NMM 2000 385 | 474 | 400 |
4. Discussion
(a). Determining the presence or absence of multi-layer reflectors in fossil insects
The state of preservation of various ultrastructural features of the cuticle correlates with that of the epicuticular multi-layer reflector. These features include the epicuticular cement layer, fibrillar textures in the epicuticular laminae, nanometre-scale lamination of the exocuticle, helicoidal textures in the exocuticle, exocuticular pore canals and their filaments, and extracellular membranes of the lowermost endocuticle and basement membrane of the cuticle. As the preserved colour deteriorates (based on visual assessment of cuticles performed using an optical microscope), so too does the preservation of the epicuticular multi-layer reflector and other ultrastructural features of the cuticle. The only feature that is more clearly evident in specimens with poor or no colour preservation is the orthogonal arrangement of bundles of chitin microfibrils in the endocuticle, but this arrangement becomes only evident when the protein matrix surrounding the chitin is degraded [32].
The various ultrastructural features listed above are ‘markers’ for high-fidelity preservation of the cuticle and provide a basis for determining the occurrence of multi-layer reflectors in fossil beetle taxa. The absence of a multi-layer reflector in a fossil is likely to be real, i.e. the beetle was not structurally coloured in vivo, if the marker ultrastructural features are well preserved. The curculionids from Eckfeld provide a test of this model. The high-fidelity preservation of marker ultrastructural features in their cuticle echoes the visual evidence of high reflectivity and cohesiveness. The absence of an epicuticular multi-layer reflector is therefore real and confirms that these Eocene beetles lacked metallic colours. This is not unexpected as epicuticular multi-layer reflectors have not been reported from extant curculionids.
(b). The original structural colours of fossil insects
The difference between the observed and predicted reflectance peaks is not an artefact of dehydration or other processes during sample preparation. Both reflectance measurements and TEM analyses used dry fossil material. There is no evidence that the dehydration process altered the ultrastructure of the multi-layer reflector: the hue of the cuticle does not change upon drying (if the low-index layers had contained abundant free water, dehydration of the multi-layer reflector would have resulted in a decrease in the spacing of the layers and in the wavelength of the observed colour). Further, there is no evidence that the ultrastructural features of the fossil cuticles were modified during fossilization (see §3). Nonetheless, the difference between the measured and predicted reflectance peaks reveals that the colours of the beetles have been altered during diagenesis. The wavelength of visible light produced by a biophotonic nanostructure is a function of the periodicity of the structure and its refractive index [5]; as there was no modification of the cuticle ultrastructure, the refractive index of the material must have changed. The refractive index of the cuticle in extant insects is determined by its precise biochemistry [28]. Alteration of its refractive index during fossilization must reflect changes in its biomolecular composition; most fossil arthropod cuticles are chemically altered during diagenesis [35,36]. This hypothesis explains the results of previous investigations of structurally coloured fossil beetles. The preservation of original structural colour in Pleistocene beetles reflects limited diagenetic alteration: geochemical analyses of the cuticle demonstrated that many of its original biomacromolecules survived [12]. By contrast, investigation of a structurally coloured fossil beetle from the Mid-Eocene demonstrated that the wavelength of the predicted reflectance peak was slightly less than that measured (a similar result to ours). This difference was attributed to limitations of the experimental technique used [13], but is also probably the result of diagenetic alteration of the composition of the cuticle.
5. Conclusions and wider implications
The fossil beetle specimens analysed in this study differ in age, precise depositional context, geological history and their phylogenetic position. Nonetheless, structural colours commonly survive owing to preservation of multi-layer reflectors in the epicuticle. Our data show that the original structural colours do not survive fossilization, but can be reconstructed based on the structure of the preserved multi-layer reflector. The offset between the measured and predicted reflectance peaks for the fossil multi-layer reflectors is strikingly similar for all specimens (see above); in each case, the colour has been shifted to longer wavelengths (‘redshifted’) during fossilization. These results could be applied to measured reflectance data from fossils of related insect taxa, and from a similar depositional context, to those used herein in order to predict the original colour. Future investigations will constrain the extent to which the colour shift varies between taxa and with the age and diagenetic history of the biota. Importantly, the preserved biophotonic nanostructures also allow the former presence or absence of structural colour to be determined even when fossils are non-metallic.
Multi-layer reflectors are particularly common in extant representatives of the beetle groups investigated in this study, and also in Scarabaeidae, Carabidae and Cerambycidae [5]. Fossil examples of these last three groups, however, rarely exhibit metallic colours. TEM analyses of cuticle from these taxa will determine whether the absence of colour is real, or an artefact of preservation. A dull, matt colour in other fossil insect taxa need not be the result of a poorly preserved reflector: in some extant beetles, modification of the cuticle above a multi-layer reflector can produce a weakly reflecting matt effect [8]. Among extant insects, epicuticular multi-layer reflectors also occur in orders other than beetles [37,38]. Our findings therefore could be used to determine the former presence and (where the original structure of multi-layer reflectors is preserved [4]) to estimate original hues of metallic structural colours in non-coleopteran insect fossils.
Numerous Mesozoic and Cenozoic insect-rich Lagerstätten are known [39]. These biotas are an underused resource for understanding the evolution of structural colour in insects. Colour-producing nanostructures are hypothesized to have arisen via multiple convergence events in at least some insect groups, e.g. beetles [5] and some odonate [40] and lepidopteran [41] taxa. Evolution towards progressively more complex (but not necessarily more optically efficient) structures has been postulated for lepidoptera [41] (and also other invertebrates [42]). Characterization of colour-producing nanostructures in fossil insects will test these evolutionary hypotheses and will identify the extent to which they can be extrapolated to other insect groups. Further, fossils will provide insights into the evolution of the function(s) of structural coloration, particularly when combined with data from related extant groups [8]. The evolution of colour-producing nanostructures is linked to sexual signalling in several insect groups [4,29,30,43–45] and in other invertebrate taxa [4,46]. Structural colour in insects is known or postulated to have various other visual (e.g. crypsis [47], aposematism) and non-visual (thermoregulation, [48], friction reduction and water repellency [49], aerodynamics [41] and antireflection [4]) functions. Reconstruction of the original colours and colour patterns of structurally coloured insect fossils, particularly those for which an ecological and environmental context can be provided, will help to determine the function(s) of the colour. Comprehensive investigation of structural colour in various insect taxa from different fossil biotas will provide crucial insights into the evolution of structural colour and its functions.
Acknowledgements
We thank the Eckfeld, Enspel and Senckenberg excavation teams, Bill Rember for field assistance at the Clarkia sites, Günter Bechly, Herbert Lutz, Markus Poschmann, Michael Rasser, Sonja Wedmann and Michael Wuttke for access to specimens, Uta Kiel and Petra Schäfers for preparation and photographing of fossils, Thomas Engel for assistance with photography, Barry Piekos and Zhenting Zhang for assistance with EM techniques, and Larry Gall for useful discussion. The research is funded by an IRCSET-Marie Curie International Mobility Fellowship awarded to M.McN. and by NSF grant PHY-0957680 to H.C.
References
- 1.Prum R. O., Torres R. H. 2003. A Fourier tool for the analysis of coherent light scattering by bio-optical nanostructures. Integr. Comp. Biol. 43, 591–602 10.1093/icb/43.4.591 (doi:10.1093/icb/43.4.591) [DOI] [PubMed] [Google Scholar]
- 2.Vukusic P., Sambles J. R. 2003. Photonic structures in biology. Nature 424, 852–855 10.1038/nature01941 (doi:10.1038/nature01941) [DOI] [PubMed] [Google Scholar]
- 3.Oshima N. 2005. Light reflection in motile iridophores of fish. In Structural colors in biological systems: principles and applications (eds Kinoshita S., Yoshioka S.), pp. 211–229 Osaka, Japan: Osaka University Press [Google Scholar]
- 4.Parker A. R. 2000. 515 million years of structural colour. J. Opt. A Pure Appl. Opt. 2, R15–R28 10.1088/1464-4258/2/6/201 (doi:10.1088/1464-4258/2/6/201) [DOI] [Google Scholar]
- 5.Seago A. E., Brady P., Vigneron J.-P., Schultz T. D. 2009. Gold bugs and beyond: a review of iridescence and structural colour mechanisms in beetles (Coleoptera). J. R. Soc. Interface 6, S165–S184 10.1098/rsif.2008.0354.focus (doi:10.1098/rsif.2008.0354.focus) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wilts B. D., Leertouwer H. L., Stavenga D. G. 2009. Imaging scatterometry and microspectrophotometry of lycaenid butterfly wing scales with perforated multilayers. J. R. Soc. Interface 6, S185–S192 10.1098/rsif.2008.0299.focus (doi:10.1098/rsif.2008.0299.focus) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tilley R. J. D., Eliot J. N. 2002. Scale microstructure and its phylogenetic implications in lycaenid butterflies (Lepidoptera, Lycaenidae). Trans. Lepid. Soc. Jpn 53, 153–180 [Google Scholar]
- 8.Parker A. R., McKenzie D. R., Large M. C. J. 1998. Multilayer reflectors in animals using green and gold beetles as contrasting examples. J. Exp. Biol. 201, 1307–1313 [DOI] [PubMed] [Google Scholar]
- 9.Parker A. R., Hegedus Z., Watts R. A. 1998. Solar-absorber antireflector on the eye of an Eocene fly (45 Ma). Proc. R. Soc. Lond. B 265, 811–815 10.1098/rspb.1998.0364 (doi:10.1098/rspb.1998.0364) [DOI] [Google Scholar]
- 10.Parker A. R. 1998. Colour in Burgess Shale animals and effect of light on evolution in the Cambrian. Proc. R. Soc. Lond. B 265, 967–972 10.1098/rspb.1998.0385 (doi:10.1098/rspb.1998.0385) [DOI] [Google Scholar]
- 11.Hinojosa-Díaz I. A., Engel M. S. 2007. A new fossil orchid bee in Colombian copal (Hymenoptera: Apidae). Am. Mus. Novit. 3589, 1–7 10.1206/0003-0082(2007)3589[1:ANFOBI]2.0.CO;2 (doi:10.1206/0003-0082(2007)3589[1:ANFOBI]2.0.CO;2) [DOI] [Google Scholar]
- 12.Tanaka G., Taniguchi H., Maeda H., Nomura S. 2010. Original structural colour preserved in an ancient leaf beetle. Geology 38, 127–130 10.1130/G25353.1 (doi:10.1130/G25353.1) [DOI] [Google Scholar]
- 13.Parker A. R., McKenzie D. R. 2003. The cause of 50 million-year-old colour. Proc. R. Soc. Lond. B 270, S151–S153 10.1098/rsbl.2003.0055 (doi:10.1098/rsbl.2003.0055) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lutz H. 1990. Systematische und paleökologische Untersuchungen an Insekten aus dem Mittel-Eozän der Grube Messel bei Darmstadt. Cour. Forschungsinst. Senckenb. 124, 1–165 [Google Scholar]
- 15.Engel M. S., Rightmyer M. G. 2000. A new augochlorine bee species in Tertiary amber from the Dominican Republic (Hymenoptera: Halictidae). Apidologie 31, 431–436 10.1051/apido:2000133 (doi:10.1051/apido:2000133) [DOI] [Google Scholar]
- 16.Coope G. R. 1959. A Late Pleistocene insect fauna from Chelford, Cheshire. Proc. R. Soc. Lond. B 151, 70–86 10.1098/rspb.1959.0051 (doi:10.1098/rspb.1959.0051) [DOI] [Google Scholar]
- 17.Wedmann S., Poschmann M., Hörnschemeyer T. 2010. Fossil insects from the Late Oligocene Enspel Lagerstätte and their palaeobiogeographic and palaeoclimatic significance. Palaeobiol. Palaeoenviron. 90, 49–58 10.1007/s12549-009-0013-5 (doi:10.1007/s12549-009-0013-5) [DOI] [Google Scholar]
- 18.Yang H., Yang S. 1994. The Shanwang fossil biota in eastern China: a Miocene Konservat-Lagerstätte in lacustrine deposits. Lethaia 27, 345–354 10.1111/j.1502-3931.1994.tb01585.x (doi:10.1111/j.1502-3931.1994.tb01585.x) [DOI] [Google Scholar]
- 19.Hopkins D. M., Matthews J. V., Wolfe J. A., Silberman M. L. 1970. A Pliocene flora and insect fauna from the Bering Strait region. Palaeogeogr. Palaeoclim. Palaeoecol. 9, 211–231 10.1016/0031-0182(71)90032-0 (doi:10.1016/0031-0182(71)90032-0) [DOI] [Google Scholar]
- 20.McNamara M. E., Briggs D. E. G., Orr P. J., Wedmann S., Noh H., Cao H. In press Fossilized biophotonic nanostructures reveal the original colors of 47 million-year-old moths. PLoS Biol. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Smiley C. J., Gray J., Huggins M. 1975. Preservation of Miocene fossils in unoxidised lake deposits, Clarkia, Idaho. J. Paleontol. 49, 833–844 [Google Scholar]
- 22.Wuttke M., Uhl D., Schindler T. 2010. The fossil-Lagerstätte Enspel: exceptional preservation in an Upper Oligocene maar. Palaeobiol. Palaeoenviron. 90, 1–2 10.1007/s12549-010-0026-0 (doi:10.1007/s12549-010-0026-0) [DOI] [Google Scholar]
- 23.Lutz H., et al. 2010. Eckfeld maar: window into an Eocene terrestrial habitat in Central Europe. Acta Geol. Sin. 84, 984–1009 10.1111/j.1755-6724.2010.00237.x (doi:10.1111/j.1755-6724.2010.00237.x) [DOI] [Google Scholar]
- 24.Schaal S., Ziegler W. 1992. Messel: an insight into the history of life and the Earth. Oxford, UK: Clarendon Press [Google Scholar]
- 25.Zimmerle W. 1993. Some aspects of Cenozoic maar sediments in Europe: the source-rock potential and their exceptionally good fossil preservation. In Paleolimnology of European maar lakes (eds Negendank J. F. W., Zolitschka B.), pp. 467–476 Berlin, Germany: Springer [Google Scholar]
- 26.Neville A. C. 1975. Biology of the arthropod cuticle. New York, NY: Springer [Google Scholar]
- 27.Locke M. 1966. The structure and formation of the cuticulin layer in the epicuticle of an insect, Calpodes ethlius (Lepidoptera, Hesperiidae). J. Morph. 118, 461–494 10.1002/jmor.1051180403 (doi:10.1002/jmor.1051180403) [DOI] [PubMed] [Google Scholar]
- 28.Schultz T. D., Rankin M. A. 1985. The ultrastructure of the epicuticular interference reflectors of tiger beetles (Cicindela). J. Exp. Biol. 117, 87–110 [Google Scholar]
- 29.Hariyama T., Takaku Y., Hironaka M., Horiguchi H., Komiya Y., Kurachi M. 2002. The origin of the iridescent colors in Coleopteran elytron. Forma 17, 123–132 [Google Scholar]
- 30.Kurachi M., Takaku Y., Komiya Y., Hariyama T. 2002. The origin of extensive colour polymorphism in Plateumaris sericea (Chrysomelidae: Coleoptera). Naturwissenschaften 89, 295–298 10.1007/s00114-002-0332-0 (doi:10.1007/s00114-002-0332-0) [DOI] [PubMed] [Google Scholar]
- 31.Hadley N. F. 1982. Cuticle ultrastructure with respect to the lipid waterproofing barrier. J. Exp. Zool. 222, 239–248 10.1002/jez.1402220306 (doi:10.1002/jez.1402220306) [DOI] [Google Scholar]
- 32.Stankiewicz B. A., Briggs D. E. G., Evershed R. P., Duncan I. J. 1997. Chemical preservation of insect cuticle from the Pleistocene asphalt deposits of California, USA. Geochim. Cosmochim. Acta 61, 2247–2252 10.1016/S0016-7037(97)00078-1 (doi:10.1016/S0016-7037(97)00078-1) [DOI] [Google Scholar]
- 33.Land M. F. 1972. The physics and biology of animal reflectors. Prog. Biophys. Mol. Biol. 24, 75–106 10.1016/0079-6107(72)90004-1 (doi:10.1016/0079-6107(72)90004-1) [DOI] [PubMed] [Google Scholar]
- 34.Bernard G. D., Miller W. H. 1968. Interference filters in the corneas of Diptera. Invest. Ophthalmol. 7, 416–434 [PubMed] [Google Scholar]
- 35.Briggs D. E. G. 1999. Molecular taphonomy of animal and plant cuticles: selective preservation and diagenesis. Phil. Trans. R. Soc. Lond. B 354, 7–17 10.1098/rstb.1999.0356 (doi:10.1098/rstb.1999.0356) [DOI] [Google Scholar]
- 36.Gupta N. S., Briggs D. E. G., Collinson M. E., Evershed R. P., Michels R., Pancost R. D. 2007. Molecular preservation of plant and insect cuticles from the Oligocene Enspel Formation, Germany: evidence against derivation of aliphatic polymer from sediment. Org. Geochem. 38, 404–418 10.1016/j.orggeochem.2006.06.012 (doi:10.1016/j.orggeochem.2006.06.012) [DOI] [Google Scholar]
- 37.Fitzstephens D. M., Getty T. 2000. Colour, fat and social status in male damselflies, Calopteryx maculata. Anim. Behav. 60, 851–855 10.1006/anbe.2000.1548 (doi:10.1006/anbe.2000.1548) [DOI] [PubMed] [Google Scholar]
- 38.Kroiss J., Strohm E., Vandenbem C., Vigneron P. 2009. An epicuticular multilayer reflector generates the iridescent coloration in chrysidid wasps (Hymenoptera: Chrysididae). Naturwissenschaften 96, 983–986 10.1007/s00114-009-0553-6 (doi:10.1007/s00114-009-0553-6) [DOI] [PubMed] [Google Scholar]
- 39.Grimaldi D. A., Engel M. S. 2005. Evolution of the insects. Cambridge, UK: Cambridge University Press [Google Scholar]
- 40.Prum R. A., Cole J. A., Torres R. H. 2004. Blue integumentary structural colours in dragonflies (Odonata) are not produced by incoherent Tyndall scattering. J. Exp. Biol. 207, 3999–4009 10.1242/jeb.01240 (doi:10.1242/jeb.01240) [DOI] [PubMed] [Google Scholar]
- 41.Wickham S., Large M. C. J., Poladian L., Jermiin L. S. 2006. Exaggeration and suppression of iridescence: the evolution of two-dimensional butterfly structural colours. J. R. Soc. Interface 3, 99–109 10.1098/rsif.2005.0071 (doi:10.1098/rsif.2005.0071) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Parker A. R. 1995. Discovery of functional iridescence and its coevolution with eyes in the phylogeny of Ostracoda (Crustacea). Proc. R. Soc. Lond. B 262, 349–355 10.1098/rspb.1995.0216 (doi:10.1098/rspb.1995.0216) [DOI] [Google Scholar]
- 43.Vulinec K. 1997. Iridescent dung beetles: a different angle. Florida Entomol. 80, 132–142 10.2307/3495550 (doi:10.2307/3495550) [DOI] [Google Scholar]
- 44.Shevtsova E., Hansson C., Janzen D. H., Kjærandesen J. 2011. Stable structural color patterns displayed on transparent insect wings. Proc. Natl Acad. Sci. USA 108, 668–673 10.1073/pnas.1017393108 (doi:10.1073/pnas.1017393108) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kemp D. J., Rutowski R. L., Mendoza M. 2005. Colour pattern evolution in butterflies: a phylogenetic analysis of structural ultraviolet and melanic markings in North American sulphurs. Evol. Ecol. Res. 7, 133–141 [Google Scholar]
- 46.Ingram A. L., Parker A. R. 2008. A review of the diversity and evolution of photonic structures in butterflies, incorporating the work of John Huxley (The Natural History Museum, London from 1961 to 1990). Phil. Trans. R. Soc. B 363, 2465–2480 10.1098/rstb.2007.2258 (doi:10.1098/rstb.2007.2258) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Crowson R. A. 1981. The biology of the Coleoptera. London, UK: Academic Press [Google Scholar]
- 48.Schultz T. D., Hadley N. F. 1987. Structural colors of tiger beetles and their role in heat transfer through the integument. Physiol. Zool. 60, 737–745 [Google Scholar]
- 49.Hinton H. E. 1970. Some little known surface structures. In Insect ultrastructure (ed. Neville A. C.), pp. 41–58 London, UK: Royal Entomological Society [Google Scholar]