Abstract
Introduction
Primary ovarian carcinoid is a very rare disease. Carcinoid heart disease occurs in about one-third of patients with carcinoid syndrome. Cardiac involvement may be a cause of death in this syndrome.
Presentation of case
We presents the unfavourable outcome of a 78-year-old woman admitted to the hospital complaining severe dyspnea and peripheral oedemas. Because of the presence of a large pericardial effusion with compression of cardiac chambers, an evacuative pericardiocentesis was performed. Computed tomography showed a solid pelvic mass with ascites. She underwent a complete surgical staging. Histological findings revealed an insular carcinoid stage IA. Three weeks later she was readmitted to the hospital and echocardiography demonstrated a right tricuspid valvular involvement with stenosis and severe regurgitation with a 2 cm masses in the posterior mitral leaflet. Hemoculture was positive for Staphylococcus aureus. Patient was not suitable for surgical intervention and decease due to sepsis and because secondary complications of the mitral endocarditis.
Discussion
High level of 5-HIAA has a rule in the development and progression of the carcinoid heart syndrome and could lead the right tricuspid valvular involvement. In the case we presented the bacterial endocarditis worsened the cardiac functions and the clinical conditions before she deceased.
Conclusion
Ovarian primary carcinoid tumors are very rare tumors that require appropriate preoperative diagnosis. Even if survival is usually excellent, when carcinoid syndrome with heart involvement is present, a high level of attention is mandatory to prevent and limit damage caused by the vasoactive amine secreted by the tumor.
Keywords: Primary ovarian carcinoid, Carcinoid heart disease
1. Introduction
Primary ovarian carcinoid is very uncommon neoplasm. Carcinoid heart disease occurs in about one-third of patients with carcinoid syndrome, and cardiac involvement may be a cause of death in this syndrome. Surgical removing of tumor is associated with rapid remission of the symptoms with good prognosis. In this report we describe the unfavourable outcome of primary insular ovarian carcinoid with associated carcinoid heart disease.
2. Presentation of case
A 78-year-old woman was admitted to the Department of Internal Medicine, Sacco Hospital, Milan, complaining severe dyspnea and peripheral oedemas gradually developed over the course of two months. Her medical history was significant for hypertension, and heavy obesity, while surgical history included appendectomy and right mastectomy for stage I breast cancer with a 20-year negative follow-up.
Soft heart sound and diffuse mild grade holosystolic murmur (3/6) could be heard. During the accurate physical examination, heart rate was 78 beats/min and arterial blood pressure was 100/60 mmHg.
Laboratory investigations demonstrated an increase of the inflammatory markers, and serum testing for tumor markers revealed elevates carbohydrated antigen 125 (CA 125) levels 312 U/ml, with normal levels of CA 19.9, CA 15.3 and carcinoembryonic antigen (CEA).
Echocardiography showed a large pericardial effusion with compression of cardiac chambers and mild to moderate aortic stenosis in addition to mild pleural effusion.
Evacuative pericardiocentesis was performed, resulting in 1500 ml of yellow serous exudate, negative for tumor cells.
Echocardiography performed after drainage showed persistent mild pericardial effusion, mild bi-atrial enlargement, left ventricular hypertrophy with normal systolic function, right ventricular enlargement with apical hypokinesia and severe tricuspid regurgitation.
Diuretic therapy was started in addition to beta-blocker treatment for the obstructive cardiomiopathy, with improvement of symptoms.
Chest and abdominal computed tomography (CT) showed perihepatic and perisplenic ascitic layers, 1.5 cm thick in mean dimension, and a solid pelvic mass with a mean size of 14 cm × 10 cm × 11 cm. Pelvic ultrasonography confirmed the left solid ovarian mass measuring 70 mm × 61 mm × 48 mm. Colonoscopy was negative. She was then referred to the Department of Obstetrics and Gynecology, San Gerardo Hospital, Monza. Preoperative positron emission tomography PET/CT scan showed 18-fluorodeoxyglucose (F-18 FDG) uptake in correspondence to the pelvic mass. At explorative laparotomy we found a smooth-surfaced, left adnexal mass, approximately of 13 cm in diameter, with solid and cystic components. A total abdominal hysterectomy, bilateral salpingo-oophorectomy, with surgical staging were performed without any complications. Intraoperative frozen section was requested and response of the specimen from pathologist was teratocarcinoma (carcinoid ovarian tumor versus cutaneous adnexa tumor) in benign ovarian teratoma. At this point, ovarian carcinoid tumor with cardiac involvement was considered. Further exploration of the entire abdomen with particular attention to the gastrointestinal tract was negative for suspicious tumors.
Left ovarian mass had bumpy surface and measured 12 cm × 12 cm × 8 cm; on cut section it presented a cystic part of benign teratoma, and a firm whitish solid part and a third solid component of about 8 cm in diameter with grayish surface and little cystic hemorrhagic areas. Histological findings revealed an insular carcinoid associated with benign cystic teratoma, with an isolated focus of struma ovarii. The International Federation of Gynecology and Obstetrics (FIGO) surgical staging was IA. Immunohistochemical staining was positive for chromogranin, synaptophysin, neuron-specific enolase (NSE), and cytokeratin pool and negative for cytocheratine-7 (Fig. 1, Fig. 2). Postoperatively, the 24-h urinary excretion of 5-hydroxyindolacetic acid (5-HIAA) was elevated at 85 mg, promptly normalized before discharge. Postoperative course was uneventful and she was discharged 4 days after surgery. She was readmitted to the Department of Internal Medicine twenty days after surgery because of severe weariness, weakness and lack of appetite. Laboratory investigation demonstrated hypokalemia, associated with increase of inflammatory markers. Echocardiography confirmed the right tricuspid valvular involvement with stenosis and severe regurgitation and revealed a 2 cm masses in the posterior mitral leaflet. Hemoculture was positive for Staphylococcus aureus and antibiotics therapy with ceftriaxone, gentamycin and vancomycin was started. CT scan of abdomen and chest was negative. Because worsening of general conditions patient was not suitable for surgical intervention to correct the double valvular defect. Her decease was due to sepsis and because of secondary complications of the mitral S. aureus endocarditis.
Fig. 1.
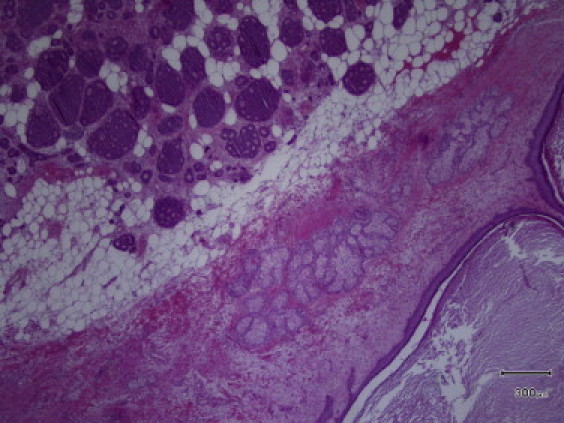
Histological examination of the tumor (H&E, original magnification 40×).
Fig. 2.
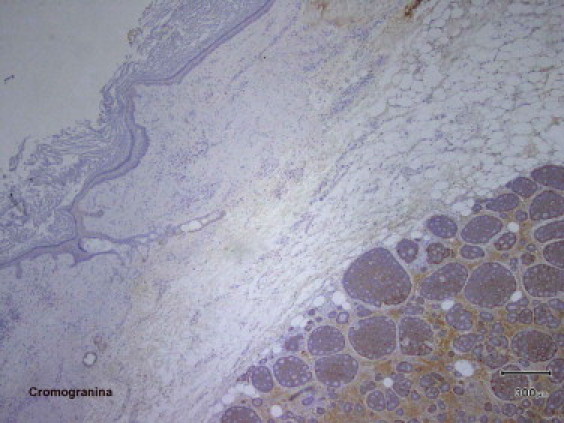
Immunohistochemical staining (chromogranin, original magnification 40×).
3. Discussion
Carcinoid tumors, mostly of bowel origin, are very rare neoplasms occurring with an incidence of 8/100,000,1 and only 1–2% arise from the ovaries, account for less than 0.3–0.5% of all ovarian malignancy.2, 3 Insular carcinoid is the most common type of primary ovarian carcinoid neoplasms. Carcinoid syndrome and carcinoid heart disease are believed to complicate fewer than 10% of these rare ovarian tumors.4
Women with primary ovarian carcinoid develop carcinoid syndrome in 43% and 25% of primary insular and primary insular associated with mature cystic teratoma, respectively.5, 6 Typical manifestations of carcinoid syndrome usually presented with episodic flushing, diarrhea, bronchospasm, abdominal pain, and right-side valvular lesions.7
Ovarian carcinoid is unique because the serotonin-like substances are released directly into the systemic circulation through the ovarian venous system that may lead cardiac lesions without hepatic metastasis.
Severity of cardiac lesions seems to be proportion to the level of circulating 5-HIAA. Some author suggested that the cardiac plaques can be determined by the elevated level of serotonin secreted by the tumor on the endocardium.8 However, the exact etiology of the heart lesions is still unclear.
No regression of heart lesions was observed in the largest published series.8 Cardiac involvement in those patients includes right-side and left valvular lesions, myocardial metastases, and pericardial effusions. Diagnosis is always difficult because symptoms occur only in the late stage of disease. Worsening of cardiac lesions, including right ventricular failure secondary to tricuspid stenosis and regurgitation, may be fatal.9, 10 Hendel et al. described a good correlation between tumor size and presence of carcinoid syndrome.11 Even if survival is usually excellent for patients with disease confined to one ovary,6 with echocardiographic evidence of cardiac involvement, the three years survival is reported to be 31%.8
This rare case of primary ovarian carcinoid with heart involvement had an unusual presentation with a severe pericardial effusion of 1500 ml associated to classic clinical manifestations. The woman was admitted to the hospital because of severe dyspnea and peripheral oedemas over the course of two months with evidence of severe right-side valvular involvement.
Since high level of vasoactive substances has a rule in the development and progression of the carcinoid heart syndrome, most cardiac findings can be explained by the systematic effects of 5-HIAA and could lead the right tricuspid valvular involvement with stenosis and severe regurgitation in the case described.
Surgical correction of tricuspid valve could be undertaken even in mildly symptomatic patients, to prevent heart failure progression and to achieve a better outcome.8, 11, 12
Radical surgical removal of a stage IA ovarian carcinoid tumor was not sufficient to treat carcinoid heart disease or to enhance regression of damaged valve. In our patients heart lesions progressed and patient was not suitable for valvular replacement. The superimposed mitral endocarditis impaired both clinical conditions and ventricular systolic function before she deceased.
In conclusion, the case presented suggests that, we should consider a rare condition such as carcinoid heart disease and a high index of suspicion must be maintained in the differential diagnosis to avoid a diagnostic delay and the detrimental cardiac effects of vasoactive substances secreted by the tumor.
Author contributions
All authors contributed.
Funding
None.
Ethical approval
Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the Editor-in-Chief of this journal on request.
Conflicts of interest statement
None.
References
- 1.Miller J.K. Carcinoid syndrome and the APUD concept. Semin Anesth. 1987;3:228–237. [Google Scholar]
- 2.Telerman A. In: Blaunstein's pathology of the female genital tract. Kurman R.J., editor. Springer-Verlag; New York: 2002. Germ cell tumor of the ovary; pp. 1006–1008. [Google Scholar]
- 3.Stewart M.J., Willis R.A., Saram G.S.W. Argentaffin carcinoma arising in ovarian teratomas – a report of two cases. J Pathol Bacteriol. 1939;49:207–212. [Google Scholar]
- 4.Strickman N.E., Rossi P.A., Massumkhnai G.A., Hall R.J. Carcinoid heart disease: a clinical, pathologic, and therapeutic update. Curr Probl Cardiol. 1982;6:1–42. doi: 10.1016/0146-2806(82)90013-5. [DOI] [PubMed] [Google Scholar]
- 5.Robboy S.J., Norris H.J., Scully R.D. Insular carcinoid primary in the ovary: a clinopathologic analysis of 48 cases. Cancer. 1975;36:404–418. doi: 10.1002/1097-0142(197508)36:2<404::aid-cncr2820360216>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- 6.Davis K.P., Hartmann L.K., Keeney G.L., Shapiro H. Primary ovarian carcinoid tumors. Gynecol Oncol. 1996;61:259–265. doi: 10.1006/gyno.1996.0136. [DOI] [PubMed] [Google Scholar]
- 7.Botero M., Fuchs R., Paulus D.A. Carcinoid heart disease: a case report and literature review. J Clin Anesth. 2002;14:57–63. doi: 10.1016/s0952-8180(01)00353-1. [DOI] [PubMed] [Google Scholar]
- 8.Pellikka P.A., Tajik A.J., Khandheria B.K., Seward J.B., Callahan J.A., Pitot H.C., et al. Carcinoid heart disease. Clinical and echocardiographic spectrum in 74 patients. Circulation. 1993;87:1188–1196. doi: 10.1161/01.cir.87.4.1188. [DOI] [PubMed] [Google Scholar]
- 9.Robiolio P.A., Rigolin V.H., Wilson J.S., Harrison J.K., Sanders L.L., Bashore T.M., et al. Carcinoid heart disease. Correlation of high serotonin levels with valvular abnormalities detected by cardiac catheterization and echocardiography. Circulation. 1995;92:790–795. doi: 10.1161/01.cir.92.4.790. [DOI] [PubMed] [Google Scholar]
- 10.Denney W.D., Kemp W.E., Nthony L.B., Oates J.A., Burd B.F. Echocardiographid and biochemical evaluation of the development and progression of carcinoid heart disease. J Am Coll Cardiol. 1998;32:1017–1022. doi: 10.1016/s0735-1097(98)00354-4. [DOI] [PubMed] [Google Scholar]
- 11.Hendel N., Leckie B., Richards J. Carcinoid heart disease: eight-year survival following tricuspid valve replacement and pulmonary valvotomy. Ann Thorac Surg. 1980;30:391–395. doi: 10.1016/s0003-4975(10)61280-5. [DOI] [PubMed] [Google Scholar]
- 12.Knott-Craig C.J., Schaff H.V., Mullany C.J., Kvols L.K., Moertel C.G., Edwards W.D., et al. Carcinoid disease of the heart: surgical management of 10 patients. J Thorac Cardiovasc Surg. 1992;104:475–481. [PubMed] [Google Scholar]


