Abstract
INTRODUCTION
Solid pseudopapillary neoplasms are rare pancreatic neoplasms with low malignant potential and favorable prognosis that are typically seen in young women.
PRESENTATION OF CASE
We report a case of two large solid pseudopapillary neoplasms in a 23-year old woman who was treated successfully with a total pancreatectomy.
CONCLUSION
To the best of our knowledge, this is the first report of two discrete solid pseudopapillary neoplasms in the same patient.
Keywords: Solid pseudopapillary neoplasm, Hamoudi tumor, Pancreas, Pancreatectomy
1. Introduction
Solid pseudopapillary neoplasms (SPNs) of the pancreas (also known as Hamoudi or Frantz tumors) are rare epithelial tumors of the exocrine pancreas with low malignant potential that primarily affect young women in the third and fourth decades of life.1, 2 They account for only 1% to 2% of all exocrine pancreatic tumors.3 SPNs typically present with non-specific symptoms of abdominal pain and/or a palpable abdominal mass.2 Characteristic features on computed tomography (CT) are a well-circumscribed, heterogeneous cystic mass with areas of necrosis and internal hemorrhage.4 Surgical resection can provide cure for localized disease and, hence, should be the treatment of choice.5 We present a case of a 23-year old female with two large distinct SPNs who underwent a successful total pancreatectomy.
2. Presentation of case
A previously healthy 23-year old African American female, G2P2, presented with a sudden onset of severe epigastric pain with radiation to her back. She had no associated changes in appetite or jaundice. She denied previous history of abdominal trauma or pancreatitis. Family history was negative for pancreatic diseases. The exam was unremarkable, except for epigastric tenderness.
She underwent an abdominal ultrasound and CT at an outside hospital, which demonstrated two large masses in or abutting the pancreas, one in the head and the other in the body/tail. Upon the patient's transfer to our institution, a pancreas protocol CT scan was performed and showed two large, heterogeneous, multi-septated cystic pancreatic masses (Fig. 1). The masses were predominantly cystic, with multiple internal septations and enhancing solid components. There was no evidence of pancreatic ductal dilatation. The portal vein confluence, splenic vein, and superior mesenteric vein were compressed by the two adjacent pancreatic/peripancreatic masses without obstruction or invasion. The celiac, superior mesenteric, hepatic, and splenic arteries were patent.
Fig. 1.
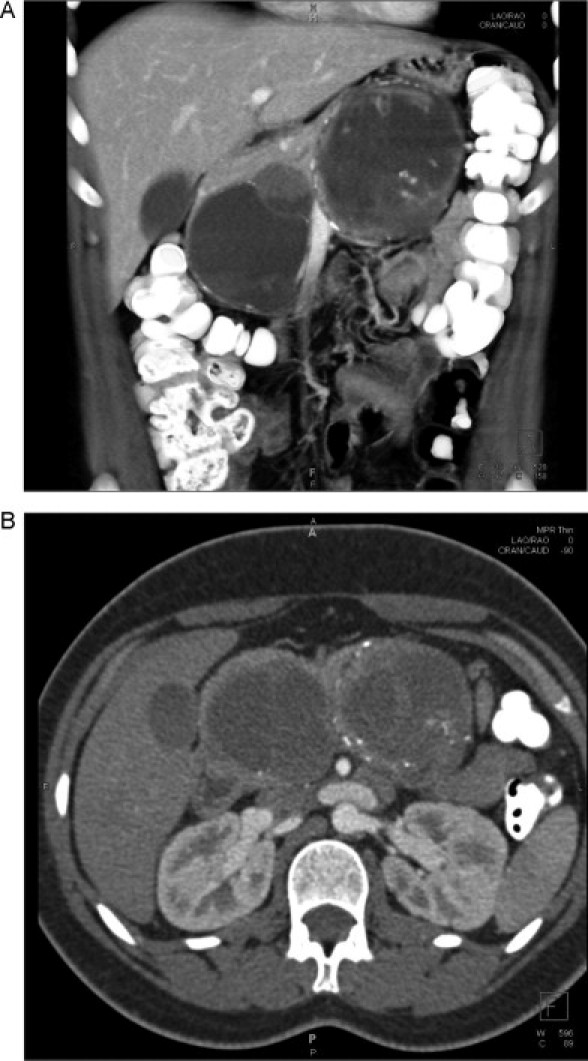
(A) Coronal and (B) axial CT images showing two large complex multiseptated cystic masses.
The patient subsequently underwent upper endoscopic ultrasonography (EUS) for further characterization of the pancreatic masses (Fig. 2). Two heterogeneous and mixed (solid and cystic) masses were seen: one mass was located in the head and measured 8 cm × 6 cm, and the second separate mass was located in the body/tail and measured 6.6 cm × 5.8 cm. The mass in the head of the pancreas abutted the portal vein without invading it. The pancreatic duct was not dilated in the head, body or tail. EUS examination was consistent with pancreatic SPN.
Fig. 2.
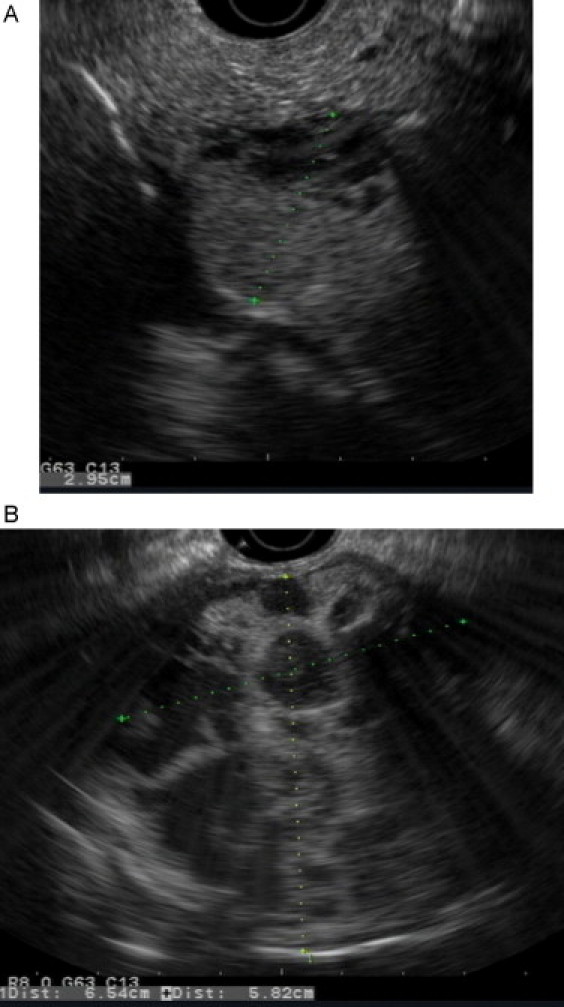
Upper endoscopic ultrasound: (A) mixed mass in the head of the pancreas, (B) mixed mass in the body of the pancreas.
The patient underwent total pancreatectomy with splenectomy. There was no evidence of direct tumor invasion of adjacent structures and vasculature, or tumor metastases. The overlying pancreatic parenchyma appeared to be relatively normal with a thin, translucent pancreatic capsule. There was a bridge of relatively normal, somewhat attenuated pancreas between the two large masses, which were separate and not anatomically linked to one another (Fig. 3). Diagnosis of SPN was confirmed by intraoperative frozen section.
Fig. 3.
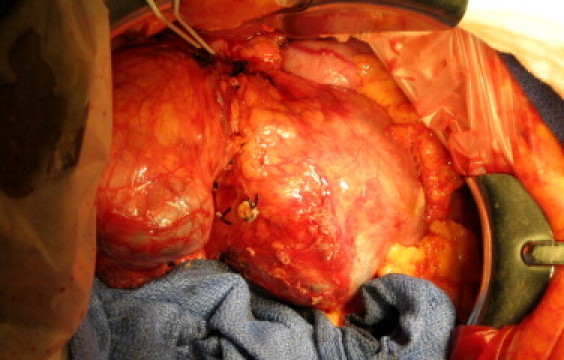
Intraoperative photo of SPNs in head and body/tail of the pancreas with thick fibrotic capsule.
On gross pathology, the tumors were 9 cm and 7 cm in maximum diameter, respectively, and were confined to the pancreas (Fig. 4). The masses were grossly distinct and had multicystic and solid components. All 19 harvested lymph nodes were negative for tumor. Immunohistochemical analysis was positive for beta-catenin and CD10 and was negative for chromogranin, trypsin and chymotrypsin. The final histopathologic diagnosis was consistent with two large and separate benign pancreatic SPNs. Postoperative course was unremarkable and patient was discharged home on post-operative day 13 in good condition and on subcutaneous insulin therapy.
Fig. 4.
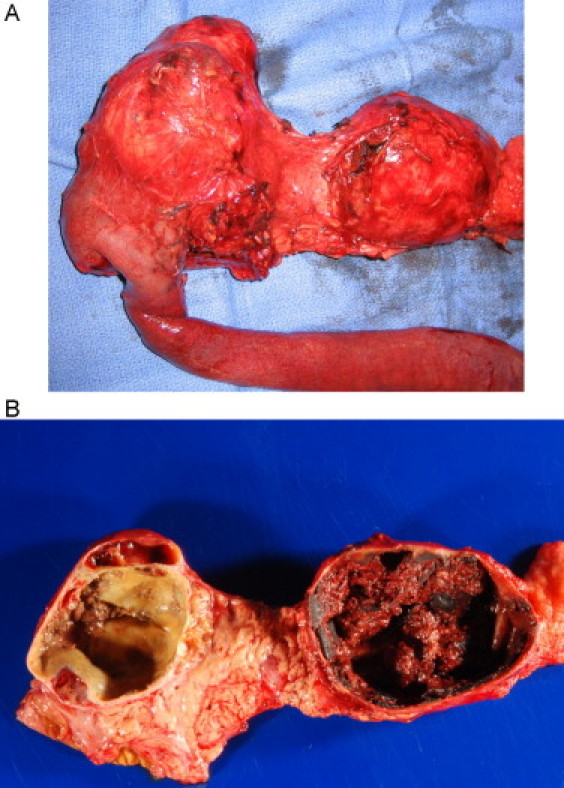
Gross appearance of the resected specimen: (A) the masses were grossly distinct, (B) the masses had multicystic and solid components.
3. Discussion
SPNs of the pancreas are typically solitary encapsulated masses with low malignant potential and favorable prognosis. More than 95% of patients are cured with complete surgical resection2. Factors that may be associated with tumor recurrence include tumor size greater than 5 cm, venous invasion, nuclear grade, and prominent necrobiotic nests6, 7; however, the predictive value of these features have not been consistent across all series.3, 8, 9
The genetic pathway of SPN formation is well known. SPNs are characterized by the presence of activating β-catenin gene mutations that interfere with phosphorylation of the protein product.10, 11 This results in the translocation of β-catenin into the nucleus, where it functions as a transcriptional regulator by binding to targets including the growth regulatory genes cyclin D1 and c-myc.5 In addition, abnormal β-catenin function may also explain the poorly cohesive nature of SPN as it has been shown that β-catenin interacts with the cell adhesion molecule E-cadherin, preventing the formation of normal cell-to-cell interactions.12
No prior reports have described two distinct pancreatic SPNs in the same patient. The etiology behind the formation of two lesions of this size is unclear. One theory is that a primary tumor initially formed at one location and metastasized to a separate part of the gland to seed another location. This will be determined by further genetic analyses of both tumors. Another theory is that the two tumors spontaneously and independently formed, perhaps due to same underlying predisposition in our patient.
Despite the extreme rarity of the patient's presentation, the prognosis is favorable. Complete excision of the tumor, and metastatic disease if present, can result in excellent long term survival3, 5, 13. Perhaps of greater risk than that of recurrence or long term disease burden is that of iatrogenic diabetes mellitus as a consequence of total pancreatectomy. Recent studies,14, 15, 16 however, have shown that glycemic control after total pancreatectomy is managable and is associated with fewer glycemic complications than had been reported in the past.17, 18, 19 With adequate education and support, our patient's pronosis is good for recovery and adaptation to a new way of life.
4. Conclusion
To the best of our knowledge, this is the first report of two discrete SPNs in the same patient. The patient underwent a successful total pancreatectomy. Surgical resection can provide cure for localized disease and, hence, should be the treatment of choice.
Ethical approval
Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the Editor-in-Chief of this journal on request.
Conflict of interest statement
None.
Funding
None.
Author contributions
All authors have contributed to patient care, writing and editing of the manuscript. IVG wrote the draft and performed the literature search. MAK and SAG provided radiological analysis of the ultrasounds and edited the manuscript. CB, MW, and CW edited the manuscript. FEE reviewed and finalized the final version of the manuscript.
References
- 1.Ng K.H., Tan P.H., Thng C.H., Ooi L.L. Solid pseudopapillary tumour of the pancreas. ANZ J Surg. 2003;73(June (6)):410–415. doi: 10.1046/j.1445-2197.2003.t01-1-02634.x. [DOI] [PubMed] [Google Scholar]
- 2.Papavramidis T., Papavramidis S. Solid pseudopapillary tumors of the pancreas: review of 718 patients reported in English literature. J Am Coll Surg. 2005;200(June (6)):965–972. doi: 10.1016/j.jamcollsurg.2005.02.011. [DOI] [PubMed] [Google Scholar]
- 3.Martin R.C., Klimstra D.S., Brennan M.F., Conlon K.C. Solid-pseudopapillary tumor of the pancreas: a surgical enigma? Ann Surg Oncol. 2002;9(January–February (1)):35–40. doi: 10.1245/aso.2002.9.1.35. [DOI] [PubMed] [Google Scholar]
- 4.Mortenson M.M., Katz M.H., Tamm E.P., Bhutani M.S., Wang H., Evans D.B., et al. Current diagnosis and management of unusual pancreatic tumors. Am J Surg. 2008;196(July (1)):100–113. doi: 10.1016/j.amjsurg.2008.02.005. [DOI] [PubMed] [Google Scholar]
- 5.Reddy S., Cameron J.L., Scudiere J., Hruban R.H., Fishman E.K., Ahuja N., et al. Surgical management of solid-pseudopapillary neoplasms of the pancreas (Franz or Hamoudi tumors): a large single-institutional series. J Am Coll Surg. 2009;208(May (5)):950–957. doi: 10.1016/j.jamcollsurg.2009.01.044. [discussion 957–9] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kang C.M., Kim K.S., Choi J.S., Kim H., Lee W.J., Kim B.R. Solid pseudopapillary tumor of the pancreas suggesting malignant potential. Pancreas. 2006;32(April (3)):276–280. doi: 10.1097/01.mpa.0000202956.41106.8a. [DOI] [PubMed] [Google Scholar]
- 7.Nishihara K., Nagoshi M., Tsuneyoshi M., Yamaguchi K., Hayashi I. Papillary cystic tumors of the pancreas. Assessment of their malignant potential. Cancer. 1993;71(January (1)):82–92. doi: 10.1002/1097-0142(19930101)71:1<82::aid-cncr2820710114>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- 8.Choi S.H., Kim S.M., Oh J.T., Park J.Y., Seo J.M., Lee S.K. Solid pseudopapillary tumor of the pancreas: a multicenter study of 23 pediatric cases. J Pediatr Surg. 2006;41(December (12)):1992–1995. doi: 10.1016/j.jpedsurg.2006.08.024. [DOI] [PubMed] [Google Scholar]
- 9.Machado M.C., Machado M.A., Bacchella T., Jukemura J., Almeida J.L., Cunha J.E. Solid pseudopapillary neoplasm of the pancreas: distinct patterns of onset, diagnosis, and prognosis for male versus female patients. Surgery. 2008;143(January (1)):29–34. doi: 10.1016/j.surg.2007.07.030. [DOI] [PubMed] [Google Scholar]
- 10.Abraham S.C., Klimstra D.S., Wilentz R.E., Yeo C.J., Conlon K., Brennan M., et al. Solid-pseudopapillary tumors of the pancreas are genetically distinct from pancreatic ductal adenocarcinomas and almost always harbor beta-catenin mutations. Am J Pathol. 2002;160(April (4)):1361–1369. doi: 10.1016/s0002-9440(10)62563-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tanaka Y., Kato K., Notohara K., Hojo H., Ijiri R., Miyake T., et al. Frequent beta-catenin mutation and cytoplasmic/nuclear accumulation in pancreatic solid-pseudopapillary neoplasm. Cancer Res. 2001;61(December (23)):8401–8404. [PubMed] [Google Scholar]
- 12.Chetty R., Serra S. Membrane loss and aberrant nuclear localization of E-cadherin are consistent features of solid pseudopapillary tumour of the pancreas. An immunohistochemical study using two antibodies recognizing different domains of the E-cadherin molecule. Histopathology. 2008;52(February (3)):325–330. doi: 10.1111/j.1365-2559.2007.02949.x. [DOI] [PubMed] [Google Scholar]
- 13.Hassan I., Celik I., Nies C., Zielke A., Gerdes B., Moll R., et al. Successful treatment of solid-pseudopapillary tumor of the pancreas with multiple liver metastases. Pancreatology. 2005;5:2–3. doi: 10.1159/000085285. [289–294] [DOI] [PubMed] [Google Scholar]
- 14.Jethwa P., Sodergren M., Lala A., Webber J., Buckels J.A., Bramhall S.R., et al. Diabetic control after total pancreatectomy. Dig Liver Dis. 2006;38(June (6)):415–419. doi: 10.1016/j.dld.2006.01.022. [DOI] [PubMed] [Google Scholar]
- 15.Billings B.J., Christein J.D., Harmsen W.S., Harrington J.R., Chari S.T., Que F.G., et al. Quality-of-life after total pancreatectomy: is it really that bad on long-term follow-up? J Gastrointest Surg. 2005;9(November (8)):1059–1066. doi: 10.1016/j.gassur.2005.05.014. [discussion 1066-7] [DOI] [PubMed] [Google Scholar]
- 16.Pezzilli R. Diabetic control after total pancreatectomy. Dig Liver Dis. 2006;38(June (6)):420–422. doi: 10.1016/j.dld.2006.02.007. [DOI] [PubMed] [Google Scholar]
- 17.Kiviluoto T., Schroder T., Karonen S.L., Kuusi T., Lempinen M., Taskinen M.R. Glycemic control and serum lipoproteins after total pancreatectomy. Ann Clin Res. 1985;17(3):110–115. [PubMed] [Google Scholar]
- 18.Dresler C.M., Fortner J.G., McDermott K., Bajorunas D.R. Metabolic consequences of (regional) total pancreatectomy. Ann Surg. 1991;214(August (2)):131–140. doi: 10.1097/00000658-199108000-00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Duron F., Duron J.J. Pancreatectomy and diabetes. Ann Chir. 1999;53(5):406–411. [PubMed] [Google Scholar]


