Abstract
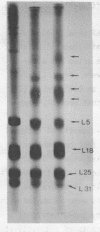
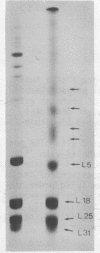
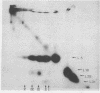
5S RNA-protein complexes were prepared in vitro using partially purified E. coli 5S RNA and total E. coli 70S ribosomal proteins. The complexes were isolated from sucrose gradients and shown to contain proteins L5, L18, L25 and a fourth protein not heretofore characterized and designed L31. The complexes were treated with the crosslinking reagents dimethyl suberimidate and dimethyl-3,3'-dithiobispropionimidate. Both reagents gave identical patterns of crosslinked proteins when analyzed by one-dimensional polyacrylamide/dodecylsulfate gel electrophoresis. Dimers of L5-L31', L5-L18 and L18-L18 and a trimer containing L5, L18 and L31' were identified by diagonal polyacrylamide/dodecylsulfate gel electrophoresis of the proteins crosslinked with dimethyl-3,3'-dithiobispropionimidate. No crosslinking was detected between L25 and the other three proteins.
Full text
PDF



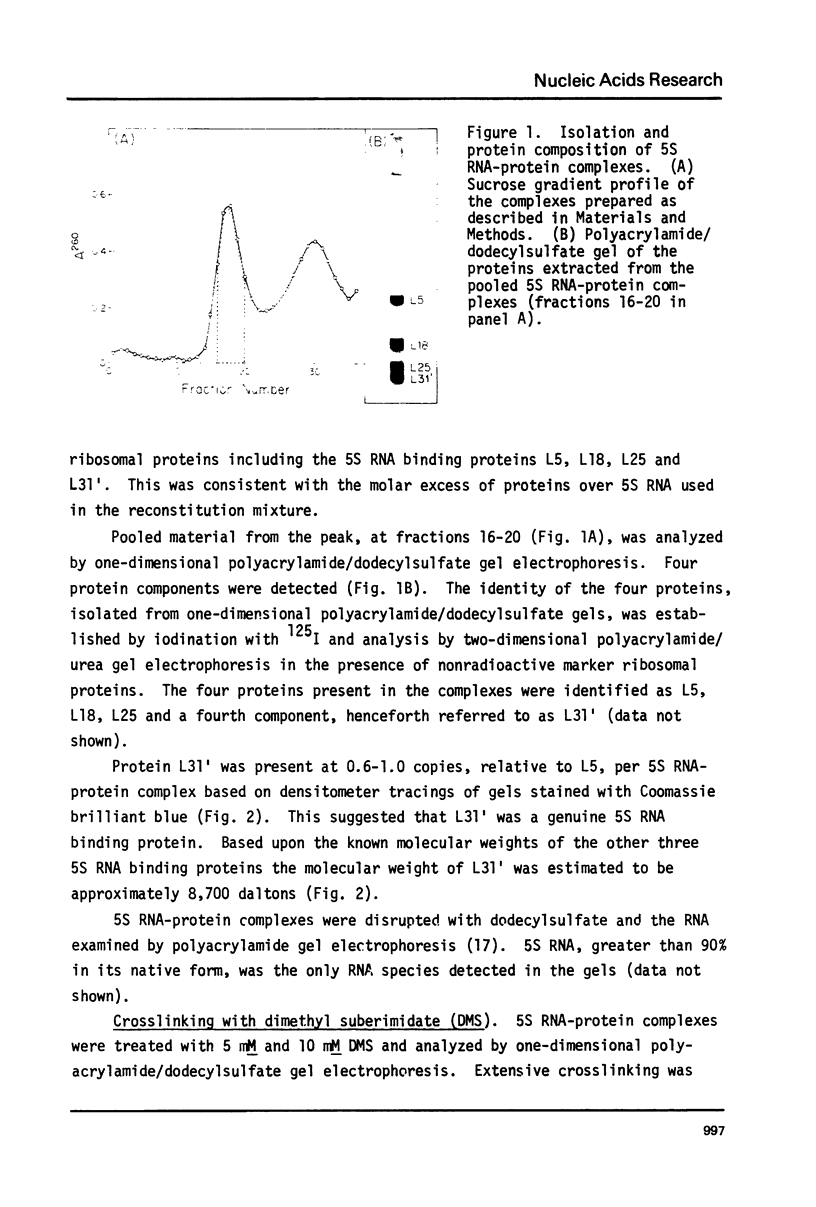
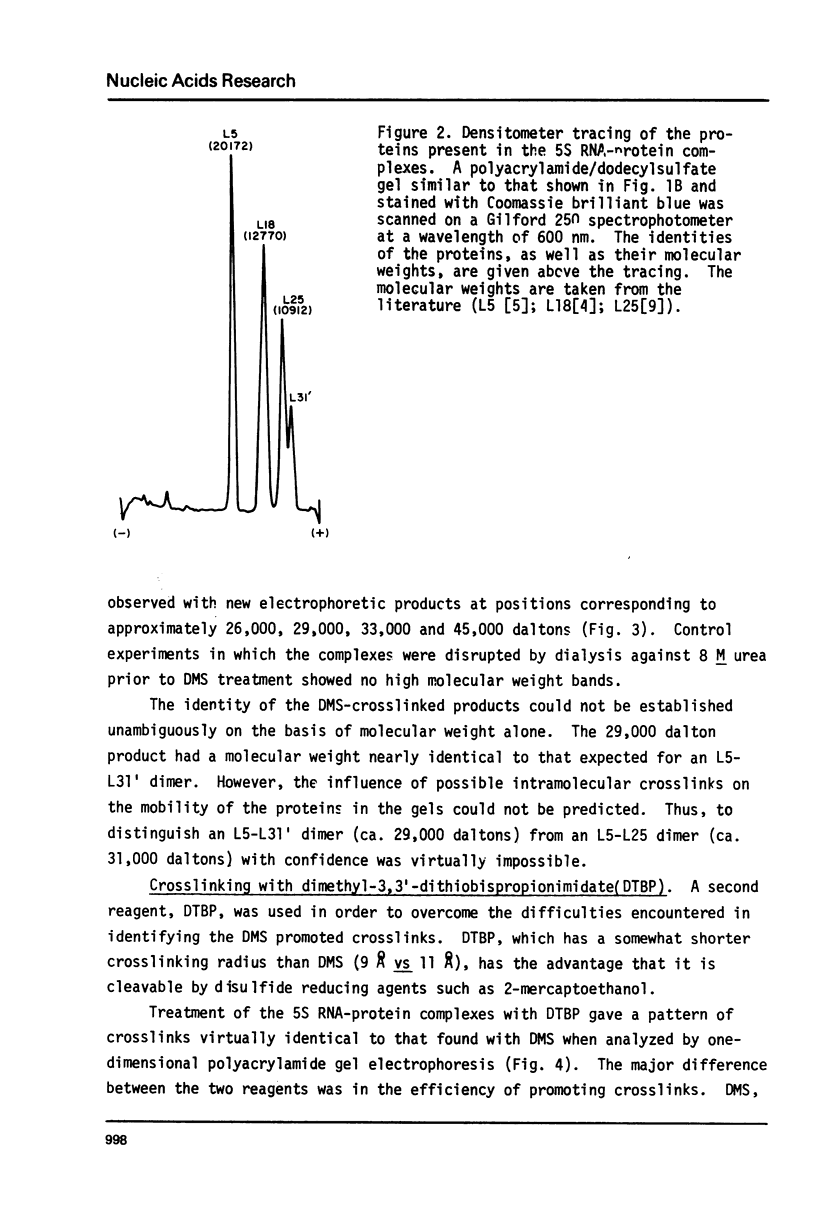






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bear D. G., Schleich T., Noller H. F., Garrett R. A. Alteration of 5S RNA conformation by ribosomal proteins L18 and L25. Nucleic Acids Res. 1977 Jul;4(7):2511–2526. doi: 10.1093/nar/4.7.2511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Brosius J. Primary structure of Escherichia coli ribosomal protein L31. Biochemistry. 1978 Feb 7;17(3):501–508. doi: 10.1021/bi00596a020. [DOI] [PubMed] [Google Scholar]
- Brosius J., Schiltz E., Chen R. The primary structure of the 5S RNA binding protein L18 from Escherichia coli ribosomes. FEBS Lett. 1975 Aug 15;56(2):359–361. doi: 10.1016/0014-5793(75)81127-6. [DOI] [PubMed] [Google Scholar]
- Chen-Schmeisser U., Garrett R. A. A new method for the isolation of a 5 S RNA complex with proteins L5, L18 and L25 from Escherichia coli ribosomes. FEBS Lett. 1977 Mar 1;74(2):287–291. doi: 10.1016/0014-5793(77)80866-1. [DOI] [PubMed] [Google Scholar]
- Chen R., Ehrke G. The primary structure of the 5 S RNA binding protein L5 of Escherichia coli ribosomes. FEBS Lett. 1976 Oct 15;69(1):240–245. doi: 10.1016/0014-5793(76)80695-3. [DOI] [PubMed] [Google Scholar]
- Comb D. G., Zehavi-Willner T. Isolation, purification and properties of 5 s ribosomal RNA: a new species of cellular RNA. J Mol Biol. 1967 Feb 14;23(3):441–458. doi: 10.1016/s0022-2836(67)80117-7. [DOI] [PubMed] [Google Scholar]
- Dohme F., Nierhaus K. H. Role of 5S RNA in assembly and function of the 50S subunit from Escherichia coli. Proc Natl Acad Sci U S A. 1976 Jul;73(7):2221–2225. doi: 10.1073/pnas.73.7.2221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dovgas N. V., Markova L. F., Mednikova T. A., Vinokurov L. M., Alakhov Y. B., Ovhinnikov Y. A. The primary structure of the 5 S RNA binding protein L25 from Escherichia coli ribosomes. FEBS Lett. 1975 May 15;53(3):351–354. doi: 10.1016/0014-5793(75)80053-6. [DOI] [PubMed] [Google Scholar]
- Feunteun J., Monier R. Accessibility of 5S RNA to ribonucleases in Escherichia coli ribosomes. Biochimie. 1971;53(5):657–660. doi: 10.1016/s0300-9084(71)80021-4. [DOI] [PubMed] [Google Scholar]
- Fox J. W., Wong K. P. Changes in the conformation and stability of 5 S RNA upon the binding of ribosomal proteins. J Biol Chem. 1978 Jan 10;253(1):18–20. [PubMed] [Google Scholar]
- Garrett R. A., Noller H. F. Structures of complexes of 5S RNA with ribosomal proteins L5, L18 and L25 from Escherichia coli: identification of kethoxal-reactive sites on the 5S RNA. J Mol Biol. 1979 Aug 25;132(4):637–648. doi: 10.1016/0022-2836(79)90379-6. [DOI] [PubMed] [Google Scholar]
- Gray P. N., Bellemare G., Monier R., Garrett R. A., Stöffler G. Identification of the nucleotide sequences involved in the interaction between Escherichia coli 5 RNA and specific 50 S subunit proteins. J Mol Biol. 1973 Jun 15;77(1):133–152. doi: 10.1016/0022-2836(73)90367-7. [DOI] [PubMed] [Google Scholar]
- Hardy S. J. The stoichiometry of the ribosomal proteins of Escherichia coli. Mol Gen Genet. 1975 Oct 3;140(3):253–274. doi: 10.1007/BF00334270. [DOI] [PubMed] [Google Scholar]
- Hershey J. W., Yanov J., Johnston K., Fakunding J. L. Purification and characterization of protein synthesis initiation factors IF1, IF2, and IF3 from Escherichia coli. Arch Biochem Biophys. 1977 Aug;182(2):626–638. doi: 10.1016/0003-9861(77)90543-4. [DOI] [PubMed] [Google Scholar]
- Howard G. A., Traugh J. A., Croser E. A., Traut R. R. Ribosomal proteins from rabbit reticulocytes: number and molecular weights of proteins from ribosomal subunits. J Mol Biol. 1975 Apr 15;93(3):391–404. doi: 10.1016/0022-2836(75)90285-5. [DOI] [PubMed] [Google Scholar]
- Kenny J. W., Lambert J. M., Traut R. R. Cross-linking of ribosomes using 2-iminothiolane (methyl 4-mercaptobutyrimidate) and identification of cross-linked proteins by diagonal polyacrylamide/sodium dodecyl sulfate gel electrophoresis. Methods Enzymol. 1979;59:534–550. doi: 10.1016/0076-6879(79)59112-5. [DOI] [PubMed] [Google Scholar]
- Kenny J. W., Traut R. R. Identification of fifteen neighboring protein pairs in the Escherichia coli 50 S ribosomal subunit crosslinked with 2-iminothiolane. J Mol Biol. 1979 Jan 25;127(3):243–263. doi: 10.1016/0022-2836(79)90328-0. [DOI] [PubMed] [Google Scholar]
- LEBOY P. S., COX E. C., FLAKS J. G. THE CHROMOSOMAL SITE SPECIFYING A RIBOSOMAL PROTEIN IN ESCHERICHIA COLI. Proc Natl Acad Sci U S A. 1964 Dec;52:1367–1374. doi: 10.1073/pnas.52.6.1367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lambert J. M., Jue R., Traut R. R. Disulfide cross-linking of Escherichia coli ribosomal proteins with 2-iminothiolane (methyl 4-mercaptobutyrimidate): evidence that the cross-linked protein pairs are formed in the intact ribosomal subunit. Biochemistry. 1978 Dec 12;17(25):5406–5416. doi: 10.1021/bi00618a014. [DOI] [PubMed] [Google Scholar]
- Maassen J. A., Möller W. Identification by photo-affinity labeling of the proteins in Escherichia coli ribosomes involved in elongation factor G-dependent GDP binding. Proc Natl Acad Sci U S A. 1974 Apr;71(4):1277–1280. doi: 10.1073/pnas.71.4.1277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore P. B., Traut R. R., Noller H., Pearson P., Delius H. Ribosomal proteins of Escherichia coli. II. Proteins from the 30 s subunit. J Mol Biol. 1968 Feb 14;31(3):441–461. doi: 10.1016/0022-2836(68)90420-8. [DOI] [PubMed] [Google Scholar]
- Newberry V., Brosius J., Garrett R. Fragment of protein L18 from the Escherichia coli ribosome that contains the 5S RNA binding site. Nucleic Acids Res. 1978 Jun;5(6):1753–1766. doi: 10.1093/nar/5.6.1753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osterberg R., Garrett R. A. Small-angle X-ray titration study on the complex formation between 5-S RNA and the L18 protein of the Escherichia coli 50-S ribosome particle. Eur J Biochem. 1977 Sep 15;79(1):56–72. doi: 10.1111/j.1432-1033.1977.tb11784.x. [DOI] [PubMed] [Google Scholar]
- Pierce J., Suelter C. H. An evaluation of the Coomassie brillant blue G-250 dye-binding method for quantitative protein determination. Anal Biochem. 1977 Aug;81(2):478–480. doi: 10.1016/0003-2697(77)90723-0. [DOI] [PubMed] [Google Scholar]
- Richards E. G., Lecanidou R., Geroch M. E. The kinetics of renaturation of 5-S RNA from Escherichia coli in the presence of Mg 2+ ions. Eur J Biochem. 1973 Apr;34(2):262–267. doi: 10.1111/j.1432-1033.1973.tb02755.x. [DOI] [PubMed] [Google Scholar]
- Richter D., Erdmann V. A., Sprinzl M. Specific recognition of GTpsiC loop (loop IV) of tRNA by 50S ribosomal subunits from E. coli. Nat New Biol. 1973 Dec 5;246(153):132–135. doi: 10.1038/newbio246132a0. [DOI] [PubMed] [Google Scholar]
- Sommer A., Traut R. R. Diagonal polyacrylamide-dodecyl sulfate gel electrophoresis for the identification of ribosomal proteins crosslinked with methyl-4-mercaptobutyrimidate. Proc Natl Acad Sci U S A. 1974 Oct;71(10):3946–3950. doi: 10.1073/pnas.71.10.3946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spierer P., Zimmermann R. A. Stoichiometry, cooperativity, and stability of interactions between 5S RNA and proteins L5, L18, and L25 from the 50S ribosomal subunit of Escherichia coli. Biochemistry. 1978 Jun 27;17(13):2474–2479. doi: 10.1021/bi00606a002. [DOI] [PubMed] [Google Scholar]
- Weber H. J. Stoichiometric measurements of 30S and 50S ribosomal proteins from Escherichia coli. Mol Gen Genet. 1972;119(3):233–248. doi: 10.1007/BF00333861. [DOI] [PubMed] [Google Scholar]
- Yu R. S., Wittmann H. G. The sequence of steps in the attachment of 5-S RNA to cores of Escherichia coli ribosomes. Biochim Biophys Acta. 1973 Oct 26;324(3):375–385. doi: 10.1016/0005-2787(73)90282-7. [DOI] [PubMed] [Google Scholar]
- van Kley H., Hale S. M. Assay for protein by dye binding. Anal Biochem. 1977 Aug;81(2):485–487. doi: 10.1016/0003-2697(77)90725-4. [DOI] [PubMed] [Google Scholar]