Abstract
Previous research demonstrates that repeated intravenous (IV) nicotine injection resulted in increased locomotor sensitization in female relative to male rats. In order to determine if increased nicotine levels are detected in females compared to males the present experiment examined the plasma nicotine levels of male, female, castrated (CAST), and ovariectomized (OVX) rats (n = 7-11 rats/group) following repeated IV nicotine injection (50 μg/kg/injection). All rats received 14 IV nicotine injections, one/day. Approximately one minute after the 14th nicotine injection, rats were rapidly decapitated and trunk blood was collected immediately. Gas chromatography revealed a sex difference in nicotine content: higher plasma nicotine levels were measured from female rats (>10X increase) relative to males, and the sex difference was attenuated by gonadectomy. These data suggest that the sex difference in plasma nicotine levels is due to alteration in distribution or nicotine metabolism as a function of circulating gonadal hormones. These findings indicate that gonadal hormones may influence nicotine pharmacokinetics and therefore nicotine-induced sex differences in behavior.
Keywords: nicotine, ovariectomy, castration, gas chromatography, intravenous, rats
1. Introduction
Persistent use and dependence on tobacco is mediated primarily, but not exclusively, by nicotine (Chaudhri et al., 2005b; Wonnacott et al., 2005). Sex is an important factor that mediates the neurobiological response to nicotine, and ultimately the course of dependence behavior in humans that abuse cigarettes. Basic science research has demonstrated nicotine-induced sex differences in rats by measuring various behavioral and non-behavioral indices (Rosecrans, 1971, 1972; Battig, 1981; Grunberg et al., 1986; 1987; Levin et al., 1987; Kanyt et al., 1999; Booze, et al., 1999; Donny et al., 2000; Harrod et al., 2004; Chaudhri et al., 2005a), and epidemiological research strongly suggests sex differences in tobacco use and abuse (Waldron, 1991; Kandel et al., 1998; Westermeyer & Boedicker, 2000). Although there may be multiple factors which influence initiation and use of tobacco smoking, the neurobiological processes which mediate sex specific differences in response to nicotine are not well known.
Research investigating the behavioral and neuropharmacological effects of repeated intravenous (IV) nicotine injection demonstrates sex differences in the expression of behavioral sensitization (Booze et al., 1999; Harrod et al., 2004; Harrod et al., 2006). Behavioral sensitization refers to an augmentation of a behavioral response following repeated administration of psychostimulant drugs (Downs and Eddy, 1932; Post, 1980; Post and Contel, 1983; Kalivas and Weber, 1988; Robinson and Berridge, 2003). Studies examining nicotine-induced sex differences by passively administering nicotine via IV injection (50 μg/kg/injection) indicate that female rats exhibit increased behavioral sensitization of locomotor activity compared to males (Booze et al., 1999; Harrod et al., 2004). Sex differences in the expression of D3 receptors and dopamine transporters (DAT) in the nucleus accumbens (N Acc) were also observed following repeated IV nicotine injection. Thus, females exhibited increased expression of D3 receptors and decreased DAT in the N Acc relative to males; and there were no changes in the expression of D1 or D2 receptors in either sex. Multiple regression analyses suggest that D3 receptors and DAT in various striatal and N Acc subregions differentially predicted nicotine-induced behaviors for males and females (Harrod et al., 2004). These findings demonstrate that repeated IV nicotine injections produce sex differences in the expression of behavioral sensitization, and suggest that nicotine-induced changes in D3 receptors and DAT are, in part, responsible for increased behavioral sensitization in female rats.
Previous research examined the contribution of sex and gonadal hormones on the expression of behavioral sensitization following repeated IV nicotine in rats (Booze et al., 1999). Repeated IV nicotine injections (50 μg/kg/injection) produced behavioral sensitization in both sexes and observational data revealed that females exhibited increased sensitivity to repeated IV nicotine as measured by a more rapid increase in the grooming response. Gonadectomy had modest effects on the expression of behavioral sensitization, suggesting some role for gonadal hormones (Booze et al., 1999). These behavioral observations were associated with peak arterial levels of nicotine (∼25 ng/ml) that are similar to venous levels maintained by cigarette smokers (Benowitz et al., 1982). Moreover, pharmacokinetic analysis determined that IV nicotine produced peak arterial nicotine levels within one minute of a 30-s bolus injection and that the disappearance of nicotine from plasma was biexponential, with a distribution and elimination half-life of 5 and 50 min, respectively. Plasma nicotine levels were determined from intact male and female, and ovariectomized (OVX) and castrated (CAST) rats; however, pharmacokinetic data were derived from a pool of 2-3 rats/group, and did not allow the determination of nicotine levels for male, female, OVX, or CAST groups separately. Thus, the contribution of gonadal hormones to plasma nicotine levels was not determined.
In the present study, male, female, OVX, and CAST rats were administered 14 injections of IV nicotine (50 μg/kg/injection) and trunk blood was collected approximately 1 minute after administration of nicotine on the final day of the experiment. As blood collection occurred at the time one would find peak arterial plasma nicotine levels (i.e., ∼ 1 min; Booze et al., 1999), data from current study are associated with peak distribution of arterial nicotine in rats. Plasma nicotine levels were measured using gas chromatography (GC; Jacob et al., 1981; Jacob et al., 1991). The aim of the present experiment was to determine if plasma nicotine levels differ as a function of sex and gonadectomy under conditions in which rats were passively injected with repeated IV nicotine. Previous in vitro and in vivo data indicate that male rats metabolize nicotine faster than female rats after an acute IV injection (Kyerematen et al., 1988). Given the evidence that female rats exhibit increased locomotor sensitization relative to males following repeated IV nicotine injection, we investigated if the animal's sex or gonadal status are important factors which mediate maintenance of plasma nicotine levels in rats. Based on previous research (Kyerematen et al., 1988; Harrod et al., 2004), it was hypothesized that female rats would exhibit higher nicotine plasma levels compared to males following repeated IV nicotine administration.
2. Method
2.1. Animals
Thirty four, adult male and female, Sprague–Dawley rats (70 days old) were obtained from Harlan Laboratories, Inc. (Indianapolis, IN). Prior to delivery, all rats were surgically implanted with an Intracath IV catheter (22 ga, Becton/Dickinson General Medical Corp., Grand Prarie, TX), which was used as a SC, dorsally implanted port for chronic IV injections. Ovariectomy, castration, or sham surgeries were also performed by Harlan Laboratories, Inc. The SC implantable access port was developed and described by Mactutus et al., (1994). Upon arrival at the animal care facilities, rats were placed in quarantine for 7 days, and then transferred to the colony. Animals were pair housed throughout the experiment and the catheters were flushed daily with 0.2 ml of heparinized (2.5%) saline. Rats were pair housed by sex and group (i.e., OVX or CAST). Rodent food (Pro-Lab Rat, Mouse Hamster Chow #3000) and water were provided ad lib. The colony was maintained at 21 ± 2° C, 50% ± 10% relative humidity and a 12L:12D cycle with lights on at 0700 h (EST). The protocol for this research methodology was approved by the Institutional Animal Care and Use Committee (IACUC).
2.2. Experimental Design and Procedures
Four groups of rats were used in the present experiment: Female (n = 7), male (n = 11), OVX (n = 9), and CAST (n = 7). After one-week of habituation to the colony room, rats were injected with 14 daily nicotine bolus injections (50 μg/kg/injection) over 15-s duration, followed by a 15-s heparinized saline flush. Approximately one minute after the last nicotine injection, rats were rapidly decapitated with a guillotine designed for rodents. Trunk blood was collected in glass collection vials immediately after decapitation. Blood was immediately frozen in a -80°C freezer. Samples were shipped to Clinical Pharmacology Laboratories USCF-SFGH for GC analysis.
2.3. Drug Treatment
The nicotine treatment was always administered as a bolus injection delivered in a volume of 1 ml/kg body weight (15 s), and was followed by flushing (15 s) with 0.2 ml heparinized (2.5%) saline (i.e., the approximate volume of the catheter). The dose of nicotine bitartrate (50 μg/kg/ injection) is calculated on the weight of the base and dissolved in saline for an injection volume of 1 ml/kg. The IV dosing regimen used in the present experiment has been shown to produce plasma nicotine levels comparable to levels demonstrated in tobacco smokers (Booze et al., 1999; Benowitz, 1982).
2.4. Gas Chromatography
In conjunction with Dr. Neil Benowitz and his colleagues, GC analysis of plasma samples was performed using their published methodology (Jacob et al., 1981; Jacob et al., 1991). The concentration of nicotine was determined by GC with nitrogen-phosphorus detection (Jacob et al., 1981). This method has been modified for simultaneous extraction of nicotine and cotinine, and determination using capillary GC (Jacob et al., 1991). The internal standards, 5-methylnicotine and 1-methyl-5-(2-pyridyl)-pyrrolidin-2-one (“ortho-cotinine”), were obtained from Peyton Jacob, III, Ph.D., Division of Clinical Pharmacology of the Department of Medicine, University of California, San Francisco.
2.5. Data Analysis
The main analytic approach was to ascertain, via planned orthogonal contrasts, 1) the significance of the sex difference in intact animals, and 2) the significance of the sex difference in gonadectomized animals. The data were also analyzed using a 2 × 2 univariate analysis of variance (ANOVA), with sex and gonadectomy as between subject factors (Winer, 1970; SPSS, 2003), to asess the potential higher order interaction effect. The significance level was set at α=0.05.
3. Results
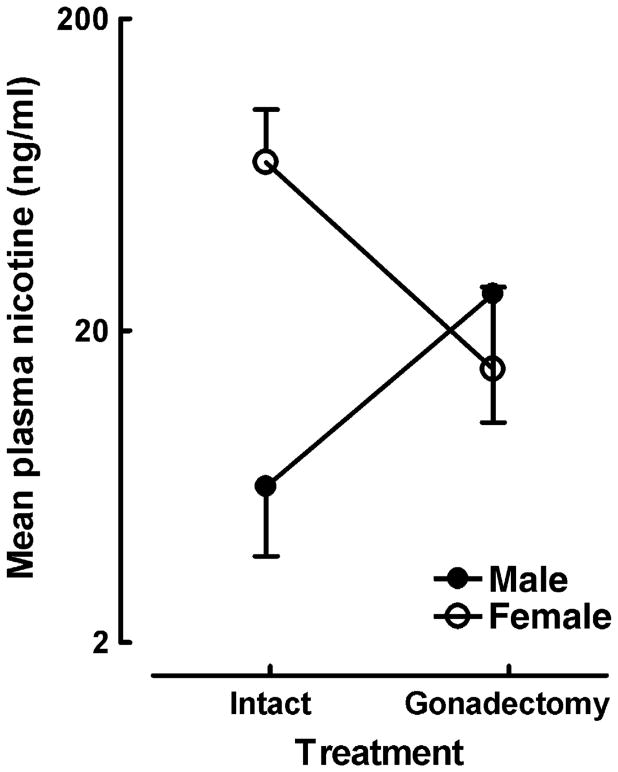
The data are illustrated in Figure 1. The specific planned comparison confirmed that the >10X increase in plasma nicotine levels exhibited in females relative to males was statistically significant [F (1, 16) = 7.67, p≤0.015]. These data demonstrate that female rats maintained higher plasma nicotine levels approximately one minute after IV nicotine injection. The presence of the significant higher order sex X gonadectomy interaction [F (1, 30) = 4.8, p<0.037], along with the failure to detect any significant difference in plasma nicotine levels between OVX and CAST rats [F (1, 14) < 1.0], suggested a clear modulatory influence of gonadal hormones on the initial distribution of nicotine.
Figure 1.
Mean (± SEM) plasma nicotine levels (ng/ml) for intact and gonadectomized male and female Sprague-Dawley rats. Trunk blood samples were taken ∼ 1 minute after the 14th IV nicotine injection (50 μg/kg/injection). Plasma nicotine levels were determined by GC. Sex X gonadectomy interaction [F (1, 30) = 5.11, p<0.05]. n =7-11 rats/group.
4. Discussion
The present experiment indicates that repeated IV nicotine injection produced a statistically significant sex difference in plasma nicotine levels: female rats exhibited higher nicotine levels than males and gonadectomy attenuated the sex difference, indicating that gonadal hormones mediate nicotine-induced sex differences on this measure. These findings further suggest that OVX and CAST rats exhibited similar plasma nicotine levels in the absence of circulating gonadal hormones.
The increased expression of plasma nicotine in females relative to male rats may be mediated by sex differences in pharmacokinetics (Kyerematan et al., 1988; Gandhi et al., 2004). The sex differences in plasma nicotine levels cannot be due to differences in absorption as the drug was experimentally delivered to the vasculature; further providing for 100% bioavailability of drug. Likewise, these findings are also not due to differences in elimination as blood was collected approximately one minute after injection; bolus IV administration of nicotine has a distribution half-life of 5.1 min (50 μg/kg/injection; Booze et al., 1999). Sex differences in the distribution and metabolism of nicotine, however, may be pharmacokinetic factors mediating the elevated plasma nicotine levels of intact females relative to males in the present study. Kyerematen et al., (1988) examined sex differences in the pharmacokinetics of an acute IV nicotine injection in Long-Evans, Sprague-Dawley, Fischer, and Wistar rats. This study demonstrated that females from all four strains exhibited a reduced rate of nicotine metabolism relative to males, and that female Sprague-Dawley rats exhibited an increased volume of distribution and a prolonged plasma nicotine half-life compared to males following IV nicotine injection. The Kyerematen et al., (1988) report suggests that the sex difference in plasma nicotine levels observed in the present experiment is due to sex differences in nicotine distribution and metabolism.
The modulation of nicotine levels by gonadectomy in the present experiment indicate that gonadal hormones play a role in nicotine plasma levels. The mechanism by which the gonadal hormones altered plasma nicotine levels cannot be determined by the present data. Estrogen-induced alterations in the expression of cytochrome P450 enzymes have been reported, and these perturbations in hepatic enzyme expression alter drug metabolism, and perhaps plasma drug levels (Gandhi et al., 2004). Although testosterone is aromatized to estrogens in brain tissue, it is also recognized to nevertheless exert unique actions, relative to 17-β-estradiol treatment. Kyerematen et al., (1988) investigated cytochrome P-450 content and the formation of nicotine metabolites from C- and N-oxidation in females and in intact and gonadectomized male rats. Females and castrates exhibited lower cytochrome P-450 content relative to intact males and decreased formation of metabolites via C- and N-oxidation of nicotine. Thus, the effects of castration and testosterone replacement on nicotine metabolism in Long Evans rats indicated an androgen-dependent mechanism in maintaining the sexual dimorphism of nicotine metabolism (Kyerematen et al., 1988).
Previous research reports sex differences in brain nicotine levels of rats (Rosecrans, 1972). In one experiment, male and female rats were injected with nicotine (0.4 mg/kg, sc) and brains were removed and frozen 5, 10, 15, 30, and 60 min later. Female brains contained more [C14]nicotine compared to males when samples were taken 15 minutes after nicotine injection. A sex difference was not reported following repeated administration (e.g., 5 injections), or when brain levels were measured at 30 minutes post injections. Notably, the present experiment differs from the Rosecrans (1972) study in numerous ways. First, nicotine was administered via IV injection and trunk blood was collected approximately 1 minute after injection in the present experiment. Thus, the sex difference in plasma nicotine levels was detected during the distribution of nicotine, which has been shown to be five minutes for the dose used in the present study (Booze et al., 1999). The Rosecrans (1972) study used the sc route, measured brain levels of nicotine, and observed sex differences in brain nicotine levels 15 minutes after sc injection, during a time point where it will be difficult to separate the processes of absorption, distribution, metabolism, and elimination of nicotine.
Research by Donny et al., 2000 did not report sex differences in arterial or brain levels following IV nicotine administration in rats that previously self-administered IV nicotine (i.e., 0.02, 0.06, or 0.09 mg/kg/injection) according to fixed ratio (FR) and progressive ratio (PR) schedules of reinforcement. Although plasma and brain nicotine levels increased as a function of dose, no sex differences in arterial plasma or brain levels of nicotine were observed. Three major differences between the current experiment and Donny et al., (2002) study may account for the contrasting findings. In the present experiment, IV nicotine was administered passively, rats received 14 nicotine injections once/day, and trunk blood was collected after a single bolus injection of nicotine. In the latter study, rats self-administered IV nicotine, had extensive experience with FR and PR responding for drug, and arterial blood samples and brains were sampled following 7 nicotine infusions. Thus, the differences in injection procedures, the number of injections, venous vs. arterial and brain samples, as well as the number of nicotine injections prior to blood/tissue sampling likely contribute to the different outcomes regarding sex differences in plasma nicotine in these two studies.
The present findings have implications for experiments investigating the neurobiological mechanisms of nicotine-induced sex differences in behavior, and more specifically, studies examining nicotine-induced behavioral sensitization. For example, Harrod et al., (2004) reported that females exhibited more behavioral sensitization than males following repeated IV nicotine. According to the current findings, although males and females received the same absolute dose of repeated IV nicotine (50 μg/kg/ injection), females may have experienced, at least initially, increased brain levels of nicotine relative to males. Whether the behavioral differences in male and female rats were mediated by sex differences in metabolic disposition or pharmacodynamics following repeated IV nicotine was not explicitly tested in the current experiment.
Collectively, these findings suggest that sex differences in nicotine pharmacokinetics is an important factor mediating sex differences in nicotine-induced behaviors, particularly IV nicotine-induced behavioral sensitization (Harrod, et al., 2004). Future work should sample arterial blood at multiple time points following IV nicotine injection to fully determine the pharmacokinetic profile in intact vs. gonadectomized male and female rats. This information will help clarify if sex differences in plasma nicotine levels are related to the distribution or metabolism of IV nicotine. Behavioral measures, which correspond to acute and repeated IV injections of nicotine, will also be a powerful way to correlate plasma nicotine levels and activity in male and female rats, as a function of gonadectomy. Understanding the relative contribution of gonadal hormones on plasma nicotine levels will help to elucidate the mechanisms that contribute to sex differences in nicotine-induced behavioral sensitization.
Acknowledgments
This research and the investigators were supported by research grants IBN-0344103, DA09160, HD043680, DA11337, DA13137 and RPS KA-2.
Non-standard abbreviations
- OVX
ovariectomy
- CAST
castration
- IV
intravenous
- DA
dopamine
- GC
gas chromatography
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Battig K. Smoking and the behavioral effects of nicotine. Trends Pharmacol Sci. 1981;2:145–147. [Google Scholar]
- Benowitz NL. Pharmacokinetic considerations in understanding nicotine dependence. Ciba Found Symp. 1990a;152:186–200. doi: 10.1002/9780470513965.ch11. [DOI] [PubMed] [Google Scholar]
- Benowitz NL. Clinical pharmacology of inhaled drugs of abuse: Implications in understanding nicotine dependence. NIDA Res Monogr. 1990b;99:12–29. [PubMed] [Google Scholar]
- Benowitz NL, Kuyt F, Jacob P., 3rd Circadian blood nicotine concentrations during cigarette smoking. Clin Pharmacol Ther. 1982;32(6):758–64. doi: 10.1038/clpt.1982.233. [DOI] [PubMed] [Google Scholar]
- Booze RM, Welch MA, Wood ML, Billings KA, Apple SR, Mactutus CF. Behavioral sensitization following repeated intravenous nicotine administration: gender differences and gonadal hormones. Pharmacol Biochem Behav. 1999;64(4):827–39. doi: 10.1016/s0091-3057(99)00169-0. [DOI] [PubMed] [Google Scholar]
- Chaudhri N, Caggiula AR, Donny EC, Booth S, Gharib MA, Craven LA, Allen SS, Sved AF, Perkins KA. Sex differences in the contribution of nicotine and nonpharmacological stimuli to nicotine self-administration in rats. Psychopharmacology. 2005a;180(2):258–66. doi: 10.1007/s00213-005-2152-3. [DOI] [PubMed] [Google Scholar]
- Chaudhri N, Caggiula AR, Donny EC, Palmatier MI, Liu X, Sved AF. Complex interactions between nicotine and nonpharmacological stimuli reveal multiple roles for nicotine in reinforcement. Psychopharmacology. 2005b;21:1–14. doi: 10.1007/s00213-005-0178-1. [DOI] [PubMed] [Google Scholar]
- Donny EC, Caggiula AR, Rowell PP, Gharib MA, Maldovan V, Booth S, Mielke MM, Hoffman A, McCallum S. Nicotine self-administration in rats: estrous cycle effects, sex differences and nicotinic receptor binding. Psychopharmacology. 2000;151(4):392–405. doi: 10.1007/s002130000497. [DOI] [PubMed] [Google Scholar]
- Downs AW, Eddy NB. The effect of repeated doses of cocaine on the rat. J Pharmacol Exp Ther. 1932;46:199–200. [Google Scholar]
- Emmett-Olglesby MW. Sensitization and tolerance to motivational and subjective effects of psychostimulants. In: Hammer RP Jr, editor. The neurobiology of cocaine: Cellular and molecular mechanisms. CRC Press; Boca Raton: 1995. pp. 31–47. [Google Scholar]
- Gandhi M, Aweeka F, Greenblatt RM, Blaschke TF. Sex differences in pharmacokinetics and pharmacodynamics. Ann Rev Pharmacol Toxicol. 2004;44:499–523. doi: 10.1146/annurev.pharmtox.44.101802.121453. [DOI] [PubMed] [Google Scholar]
- Grunberg NE, Bowden DJ, Winders SE. Effects of nicotine on body weight and food consumption in female rats. Psychopharmacology. 1986;90:101–105. doi: 10.1007/BF00172879. [DOI] [PubMed] [Google Scholar]
- Grunberg NE, Winders SE, Popp KA. Sex differences in nicotine's effects on consummatory behavior and body weight in rats. Psychopharmacology. 1987;91:221–225. doi: 10.1007/BF00217067. [DOI] [PubMed] [Google Scholar]
- Harrod SB, Mactutus CF, Bennett K, Hasselrot U, Wu G, Welch M, Booze RM. Sex differences and repeated intravenous nicotine: behavioral sensitization and dopamine receptors. Pharmacol Biochem Behav. 2004;78(3):581–92. doi: 10.1016/j.pbb.2004.04.026. [DOI] [PubMed] [Google Scholar]
- Jacob P, III, Wilson M, Benowitz NL. Improved Gas Chromatographic Method for the Determination of Nicotine and Cotinine in Biologic Fluids. Journal of Chromatography. 1981;222:61–70. doi: 10.1016/s0378-4347(00)81033-6. [DOI] [PubMed] [Google Scholar]
- Jacob P, III, Yu L, Wilson M, Benowitz NL. Selected ion Monitoring Method for Determination of Nicotine, Cotinine, and Deuterium-labeled Analogs: Absence of an Isotope Effect in the Clearance of (S)-Nicotine-3′,3′-d2 in Humans. Biological Mass Spectrometry. 1991;20:247–252. doi: 10.1002/bms.1200200503. [DOI] [PubMed] [Google Scholar]
- Kalivas PW, Weber B. Amphetamine injection into the ventral mesencephalon sensitizes rats to peripheral amphetamine and cocaine. J Pharmacol Exp Ther. 1988;46245:1991095–1102. [PubMed] [Google Scholar]
- Kanyt L, Stolerman IP, Chandler CJ, Saigusa T, Pogun S. Influence of sex and female hormones on nicotine-induced changes in locomotor activity in rats. Pharmacol Biochem Behav. 1999;62(1):179–187. doi: 10.1016/s0091-3057(98)00140-3. [DOI] [PubMed] [Google Scholar]
- Kyerematen GA, Owens GF, Chattopadhyay B, deBethizy JD, Vesell ES. Sexual dimorphism of nicotine metabolism and distribution in the rat. Studies in vivo and in vitro. Drug Metab Dispos. 1988 Nov-Dec;16(6):823–8. [PubMed] [Google Scholar]
- Le Foll B, Diaz J, Sokoloff P. Increased dopamine D3 receptor expression accompanying behavioral sensitization to nicotine in rats. Synapse. 2003;47(3):176–83. doi: 10.1002/syn.10170. [DOI] [PubMed] [Google Scholar]
- Levin ED, Morgan MM, Galvez C, Ellison GD. Chronic nicotine and withdrawal effects on body weight and food and water consumption in female rats. Physiol Behav. 1987;39:441–444. doi: 10.1016/0031-9384(87)90370-2. [DOI] [PubMed] [Google Scholar]
- Mactutus CF, Herman AS, Booze RM. Chronic intravenous model for studies of drug (ab)use in the pregnant and/or group-housed rat: An initial study with cocaine. Neurotoxicol Teratol. 1994;16:183–191. doi: 10.1016/0892-0362(94)90116-3. [DOI] [PubMed] [Google Scholar]
- Post RM. Intermittent versus continuous stimulation: Effect of time interval on the development of sensitization or tolerance. Life Sci. 1980;26:1275–1282. doi: 10.1016/0024-3205(80)90085-5. [DOI] [PubMed] [Google Scholar]
- Post RM, Contel NR. Human and animal studies of cocaine: Implications for the development of behavioral pathology. In: Creese I, editor. Stimulants: Neurochemical, behavioral and clinical perspectives. Raven Press; New York: 1983. pp. 169–203. [Google Scholar]
- Rosecrans JA. Effects of nicotine on behavioral arousal and brain 5-hydroxytryptamine function in female rats selected for differences in activity. Eur J Pharmacol. 1971;14:29–37. doi: 10.1016/0014-2999(71)90119-1. [DOI] [PubMed] [Google Scholar]
- Rosecrans JA. Brain area nicotine levels in male and female rats with different levels of spontaneous activity. Neuropharmacology. 1972;11:863–870. doi: 10.1016/0028-3908(72)90045-7. [DOI] [PubMed] [Google Scholar]
- Russell MA, Feyerabend C. Cigarette smoking: A dependence on nicotine boli. Drug Metab Rev. 1978;8:29–57. doi: 10.3109/03602537808993776. [DOI] [PubMed] [Google Scholar]
- SPSS 12.0 for Windows. SPSS Inc.; 1989-2003. [Google Scholar]
- Winer BJ. Statistical Principles in Experimental Design. McGraw-Hill; New York: 1971. [Google Scholar]
- Wonnacott S, Sidhpura N, Balfour DJ. Nicotine: from molecular mechanisms to behaviour. Curr Opin Pharmacol. 2005;5(1):53–9. doi: 10.1016/j.coph.2004.12.002. [DOI] [PubMed] [Google Scholar]
- Zahniser NR, Peris J. Neurochemicl mechanisms of cocaine-induced sensitization. In: Lakoski JM, Galloway MP, White FJ, editors. Cocaine: Pharmacology, physiology, and clinical strategies. CRC Press; Boca Raton, FL: 1992. pp. 229–260. [Google Scholar]