Abstract
Genistein, an isoflavone, has been demonstrated to promote the health of human beings by reducing the incidence of specific chronic diseases, namely, cancer and atherosclerosis. The present investigation explores a novel method of extraction of genistein from soy source which consists of a bioconversion reaction using fermentation by microorganism namely Streptomyces roseolus NRRL B-5424. In situ bioconversion of genistein glycoside to aglycone was carried out by the microbe. Such methodology has not been reported hitherto. Optimization of upstream and downstream parameters was done for maximum extraction of genistein. Genistein was isolated in a powder form by column chromatography and preparative thin layer chromatography and was characterized using massspectrometry, nuclear magnetic resonance and infrared spectroscopy and its purity determined using high performance liquid chromatography. Genistein was extracted with 91.04% purity and extraction efficiency was 67.01%.
Keywords: Betaglucosidase, extraction, fermentation, genistein, soy isoflavones
Genistein (4’,5,7-trihydroxyisoflavone) is a phytoestrogen and belongs to the category of isoflavones Isoflavones are secondary metabolites found in nature as minor constituents of soybeans and other leguminous plants. It is a common precursor in the biosynthesis of antimicrobial phytoalexins and phytoanticipins in legumes, and an important nutraceutical molecule. Most of the isoflavones are present in the glycosylated form. The aglycone derivatives of these glycosylated compounds are the active forms and show important physiological effects and are used as anticancer agents[1], in cardiovascular diseases[2], in postmenopausal problems[3]. They are tyrosine kinase inhibitors.
The aglycone derivatives can be extracted from their sources through various means such as treatment with enzyme β-glucosidase; acid treatment of soybeans, followed by solvent extraction; or the compound can be chemically synthesized[4,5]. Acid treatment is a very harsh method as concentrated inorganic acids are used. Both, enzyme treatment and chemical synthesis methods are costly. Due to this more economical processes are sought for[6–8]. One such approach consists of using fermentation to isolate genistein.
Aim of the present investigation was to extract the aglycone form of genistein (i.e. genistein) from soybeans. As genistein is present mostly in its glycoside forms; the enzyme β-glycosidase was used to convert genistein glycosides to aglycone genistein. This reaction is shown in fig. 1. β-glucosidase is an expensive enzyme, hence microorganisms, which can use soy as substrate to grow and secrete β-glucosidase, were used. Thus biotransformation of genistein glycosides to genistein aglycone using β-glucosidase secreting microorganisms was thought to be an economically feasible process, which could be adapted to large-scale production of genistein. Such use of in-situ conversion for extraction of genistein has not been reported in the literature surveyed.
Fig. 1.
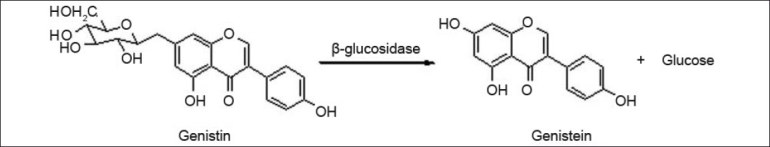
Biotransformation of genistein β-glycoside (genistin) to genistein aglycone (geninstein)[10].
MATERIALS AND METHODS
Standard genistein was a kind gift from Sahajanand Medical Technologies Pvt Ltd, Surat, India. High performance liquid chromatography (HPLC) analysis was done on Jasco HPLC system (Jasco PU-2080 Intelligent HPLC pump, Rheodyne 7725 integrator (Rheodyne, USA), Jasco UV-2075 Intelligent UV/Vis detector and Jasco ChromaPass Chromatography Data System Version 1.8.6.1). IR spectra were measured using Perkin Elmer FTIR RX-1® infrared spectrophotometer. 1H NMR spectra were obtained on Joel Eclipse® 300 MHz instrument. Mass spectra were performed on FAB mass spectra (positive mode) on micromass Q-Tof miocro (YA-105). Thin layer chromatography (TLC) was performed on preformed TLC plates (Silica Gel 60 F254 high performance plate, Merck).
Microorganisms and culture conditions:
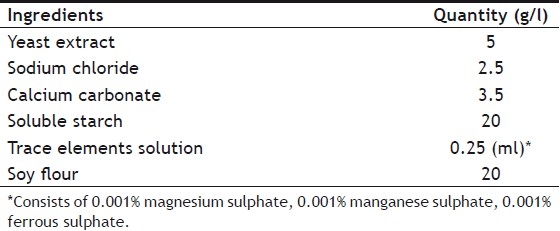
Micromonospora halophytica NRRL 2998, Micromonospora halophytica NRRL 3097 and Streptomyces roseolus NRRL B-5424 were procured as gift sample from Northern Regional Research Laboratory, Peoria, Illinois, USA. The strains were obtained as frozen vegetative spore suspension. This stock culture supply was aseptically inoculated in 250 ml Erlenmeyer flask containing 50 ml of germination broth and incubated on rotary shaker (Remi® orbital shaker CIS 24 BL, Remi Instruments Division, Vasai, India) at 180 rpm for two days. Germination broth consisted of (g/l): beef extract (3), yeast extract (5), tryptone (5), dextrose (1) and soluble starch (24)[9]. Thereafter the flasks were stored in refrigerator at 4° as the master culture. The production media was inoculated with 1 ml of suspension from culture slopes and incubated on an orbital incubator shaker at room temperature (28±2°) for 120 h. Fermentation runs were carried out in duplicate. Composition of the production media has been shown in Table 1.
TABLE 1.
PRODUCTION MEDIA SELECTED FOR OPTIMIZATION[9]
Strain selection for genistein recovery:
The best strain for genistein recovery was selected by inoculating the production media with 1 ml of 109 CFU/ml of each of the three strains namely Micromonospora halophytica NRRL 2998, Micromonospora halophytica NRRL 3097 and Streptomyces roseolus NRRL B-5424, respectively in 50 ml of autoclaved medium and subsequently studying the genistein recovery.
Optimization of fermentation medium and extraction process:
Optimization of fermentation medium and extraction process was done using one factor at-a-time method. The production media was selected for the optimization study. Flasks containing media, which was not inoculated with the microorganisms, were maintained as the controls. Also controls without the substrate were maintained to rule out the possibility of de novo synthesis of genistein. The production media was optimized with respect to the following parameters: Growth curve of microorganism, type of substrate (soy flour, soy tone and soy meal), concentration of the substrate (from 20 g/l to 220 g/l), inoculum size (1 ml to 4 ml of 109 CFU/ml), extracting solvent (1-butanol, ethyl acetate, chloroform water of pH 4 and water of pH 9.5), effect of pH on extraction (pH 1 to 13) and extraction time (1 h to 4 h). The scheme of fermentation, extraction, purification and recovery of genistein has been depicted in fig. 2. Production media (50 ml) was extracted using equal volume of ethyl acetate at room temperature on an orbital shaker for one hour at the end of 120 h of fermentation. The contents of the flask were subsequently centrifuged at 6000 rpm for 10 min on a Eltek® Cetrifuge RC 8100D Electrocraft India Pvt. Ltd. Separated ethyl acetate layer (the extract) was further concentrated by vacuum evaporation (Superfit® rotary vacuum evaporator, Superfit Continental Pvt Ltd, Mumbai, India) to 10 ml. The concentrate was stored in a refrigerator at 4° and used in HPLC analysis for optimization of fermentation and extraction processes, assay of genistein and its purification.
Fig. 2.
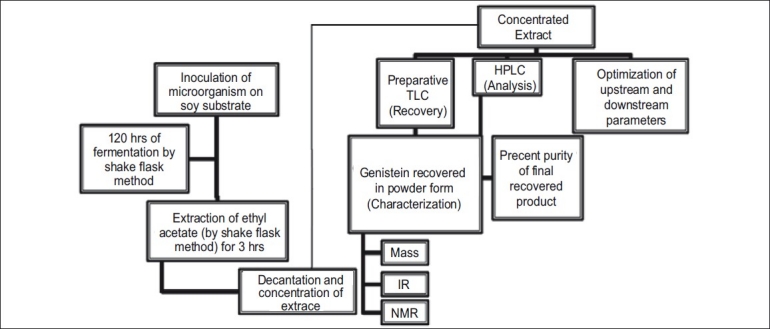
Scheme of fermentation, extraction, purification analysis and recovery of genistein.
Genistein was purified from the ethyl acetate concentrate using silica gel open column chromatography and preparative thin layer chromatography. Open column chromatography with silica gel (60-120) for purification of genistein was performed by dry packing of a pencil column with the stationary phase. Elution was done using a gradient of ethyl acetate and n-hexane. Flow rate was maintained at 1 ml/min. Eluted fractions (20 ml) were concentrated by vacuum evaporation and analyzed by spotting on preformed TLC plates (E. Merck Silica Gel 60 F254 high performance plate). Fractions, which resulted in a single spot of genistein were pooled and further concentrated by vacuum evaporation to recover genistein in powder form. Genistein was also purified using preparative thin layer chromatography where the stationary phase consisted of a mixture of silica gel and silica gel GF in the ratio 8:2 w/w. The mobile phase consisted of 1:1 v/v ratio of ethyl acetate to n-hexane. Developed TLC plate was then air-dried and the band corresponding to the band of standard genistein was scraped and extracted using ethyl acetate. Vacuum evaporation of the extract resulted in recovery of genistein in powder form. Characterization of purified genistein was done using Infrared spectroscopy (IR), mass spectrometry, and proton Nuclear Magnetic Resonance (NMR). IR spectrum, mass and 1H NMR of standard genistein was compared with that of the extracted sample to confirm the authenticity of the sample. Percent purity of extracted genistein was calculated using HPLC.
Extraction efficiency:
The extraction efficiency for genistein was determined by adding standard genistein (10 mg) to 50 ml of autoclaved and cooled media. Fermentation was not performed. The contents were consequently extracted with ethyl acetate exactly in the same manner as in case of extraction from the production medium. The ethyl acetate layer was separated and further analyzed by HPLC to determine the content of extracted genistein.
Assay of genistein by HPLC:
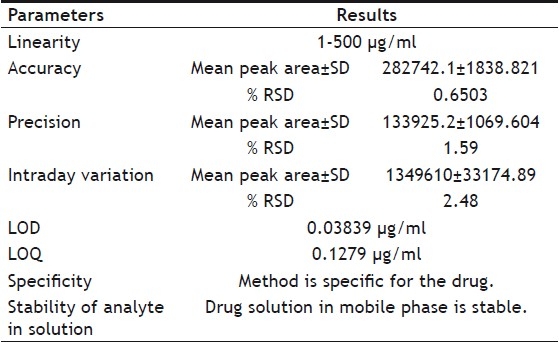
Volume of the stored extract was made up with ethyl acetate if any reduction was observed and then filtered through a 0.45 μm membrane filter (biometra®) and analyzed further for assay. Assay of genistein was performed using an optimized and validated HPLC method. HPLC was performed on a Jasco HPLC system using a reverse phase column (HiQsil C18, 250×4.6 mm ID, 10 μm). The column was set at 40°. The mobile phase composition was 25% acetonitrile (v/v), 67.5% water (v/v) and 7.5% glacial acetic acid (v/v). Flow rate was 2 ml/min and genistein was detected at 263 nm. Calculations were done using equation obtained from standard curve. The proposed analytical method was validated for linearity, accuracy, precision, limit of detection (LOD) and limit of quantification (LOQ). Results of validation are shown in Table 2.
TABLE 2.
HPLC METHOD VALIDATION
RESULTS AND DISCUSSION
Streptomyces roseolus NRRL B-5424 strain gave a higher genistein recovery (0.2993 mg/g of soy flour), when analyzed by HPLC, than Micromonospora halophytica NRRL 2998 (0.2850 mg/g of soy flour) and Micromonospora halophytica NRRL 3097 (0.1745 mg/g of soy flour); both of which have been reported by researchers earlier[9] for the production of genistein by the process of fermentation. Thus Streptomyces roseolus NRRL B-5424 was employed for all the further trials.
The soy substrates used for recovery of genistein were Soy tone, Soy meal and Soy flour. It was surprisingly found that soy tone as a substrate resulted in no product recovery. Soy flour and soy meal gave similar recovery of genistein. Soy meal gave a recovery of 0.287 mg of genistein per g of soy meal and soy flour gave a recovery of 0.268 mg of genistein per g of soy flour respectively. This might be due to the presence of oil in soy flour, which subsequently hinders the extraction of genistein. Hence, soy meal was chosen as the substrate for fermentation in all the further studies. In order to rule out the possibility of production of genistein in absence of microorganisms, un-inoculated soybean-based broths (soy flour and soy meal) were extracted in the same manner as the inoculated broths. HPLC results indicated that genistein was not present in extracts from the un-inoculated broth (fig. 3), thus fermentation was ascertained to be an essential step in the process of production of genistein from soybeans. In order to check the possibility of De Novo synthesis of genistein, Streptomyces roseolus NRRL B-5424 was inoculated in a media, which was devoid of soy source. Absence of genistein (fig. 4) in this medium confirmed that genistein was not produced by the microbe itself, but was converted from the glycone to aglycone form, during fermentation.
Fig. 3.
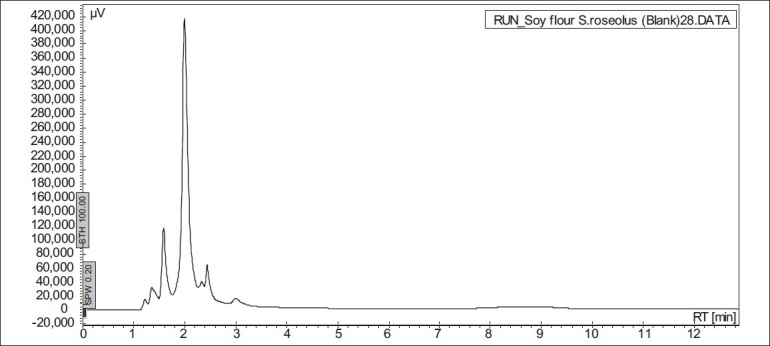
HPLC chromatogram of uninoculated soy media (retention time of genistein is 8 min).
Fig. 4.
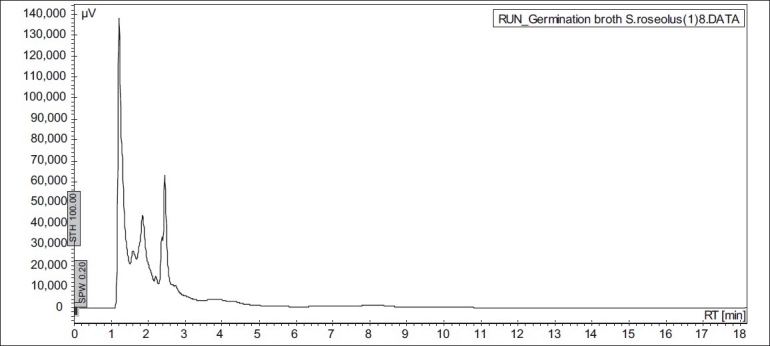
HPLC chromatogram of inoculated non-soy media (retention time of genistein is 8 min).
Genistein recovery was determined at different fermentation time points. A typical sigmoid curve characteristic for microbial growth was observed. A higher conversion of genistein glycosides to genistein aglycone was observed as the microbial population increased. The conversion of genistein glycosides to genistein started within the first 24 h of the fermentation process and reached a maximum on the fifth day of fermentation (0.298 mg/g of soy meal) with no further increase in the conversion. For all the further trials, fermentation was carried out for a period of five days.
Experiments were performed to check for genistein recovery at different concentrations of soy meal. Amount of soy meal was varied between 1 to 11 g per 50 ml of media. Recovery of genistein per gram of soy meal increased from 0.329 mg for 1 g of soy meal to 0.664 mg for 9 g of soy meal and then decreased till 11 g of soy meal in the media. Maximum genistein recovery (0.664 mg/g of soy meal) was observed when amount of substrate in the media (50 ml) was 9 g. Genistein recovery was found to decrease by 10 and 15%, for 10 and 11 g of substrate concentration, respectively when compared to 9 g substrate. Thus all the subsequent studies were carried out with soy meal concentration of 9 g/50 ml of media. Literature states that lower yields of genistein are recovered than that if complete bioconversion occurred of genistein glycosides to aglycone genistein. Bioconversion of genistein to some other structure during the fermentation is the favored hypothesis for the lower yields[6]. The pattern of genistein recovery obtained in this experiment could be explained by assuming that the bioconversion of genistein to some other product is a zero order reaction and is thus independent of concentration of soy meal.
A cake like consistency of the media was obtained after autoclaving when soy meal was used in higher concentrations in the media (from 8 g and above). Streptomyces roseolus NRRL B-5424 is an aerobic microbe and thus requires continuous aeration by shaking (around 180 rpm) for its growth in submerged fermentation. It was earlier observed that Streptomyces roseolus NRRL B-5424 did not grow in static conditions in submerged fermentation. Thus, from this observation, it may be inferred that solid-state fermentation of the soy substrate by Streptomyces roseolus NRRL B-5424 may be possible during the bioconversion of genistein glycosides to aglycone genistein.
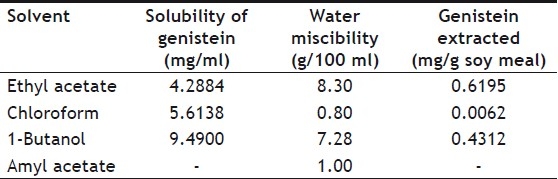
Varying volumes of inoculum from 1, 2, 3 and 4 ml of 109 CFU/ml were employed. Not much increase in recovery was seen with increase in the size of inoculum. Thus in all subsequent studies 1 ml of inoculum was used. Earlier results state that after the biotransformation, most of genistein present in the media is in the aglycone form. The aglycone form of genistein is more hydrophobic than its glycoside forms and most of the media components and other impurities in the media. Thus for maximum extraction of genistein with a minimum extraction of the impurities, a water immiscible organic solvent should be used. Literature states use of ethyl acetate for extraction of erythromycin along with genistein[9]. Various organic solvents such as ethyl acetate, amyl acetate, chloroform and 1-butanol were employed based on their solubilizing ability for the active constituent. Genistein solubility and recovery in these solvents is listed in the Table 3. It also gives the miscibility of these solvents in water. It can be seen that genistein exhibited the least solubility in ethyl acetate and the highest solubility in 1-butanol. However maximum genistein extraction was observed in ethyl acetate though solubility of genistein in this solvent was less as compared to chloroform and 1-butanol. This might be attributed to the surface tension and viscosity of the solvents, which in turn are responsible for the area of contact between the solvent and aqueous media during extraction. Out of the solvents listed in the Table 3, amyl acetate was not used due to its higher boiling point (149°), which in turn would hinder the further concentration of the extract. The extraction efficiency 1-butanol was found to be comparable to that of ethyl acetate. However it was avoided due to its high viscosity (3 cP at 25°) and boiling point (117.73°); which would render it difficult to handle in further extraction process. Hence ethyl acetate was chosen as the extracting solvent in all subsequent studies. Maximum genistein recovered was 0.6195 mg/g of soy meal using ethyl acetate as extracting solvent. It was also observed that solvents having more water miscibility exhibited better extraction capacity than those with lower water miscibility.
TABLE 3.
SOLUBILITY OF GENISTEIN IN DIFFERENT ORGANIC SOLVENTS, WATER MISCIBILITY OF VARIOUS ORGANIC SOLVENTS ALONG WITH THEIR GENISTEIN RECOVERY
Study of the effect of pH on extraction of genistein by Streptomyces roseolus from the production media was done by adjusting the pH of the production media from pH 1 to pH 13, after 120 h of fermentation. Subsequently, the media were subjected to genistein extraction. Maximum recovery of genistein was seen when the production media was extracted at pH 1. Genistein is chemically, 5,7-dihydroxy-3-(4-hydroxyphenyl)-4H-1-benzopyran-4-one, or 4’,5,7-trihydroxyisoflavone (fig. 5). It has three hydroxyl groups; 5-OH, 7-OH and 4’-OH, respectively in its structure which show the following sequence of deprotonation; 7-OH>4’-OH>5-OH. The first step of deprotonation takes place at the 7-OH group at about pH 7.2. The 7-monoanion in more basic solution undergoes another deprotonation reaction from the 4’-OH group to give corresponding 7,4’-dianion at pH 10 and all the three hydroxyls are ionized at pH 13.1. Thus genistein has 3 pKa; 7.2, 10 and 13.1. In the extraction process, ethyl acetate is used as the extracting solvent. In this liquid-liquid extraction, more a substance is in non ionized form, more it will partition in the ethyl acetate (organic) phase and vice versa. A sharp decrease in genistein recovery after pH 10 was seen which is supported by the fact that at pH more than 10, genistein is in the 7,4’-dianion form and its solubility in ethyl acetate is decreased.
Fig. 5.
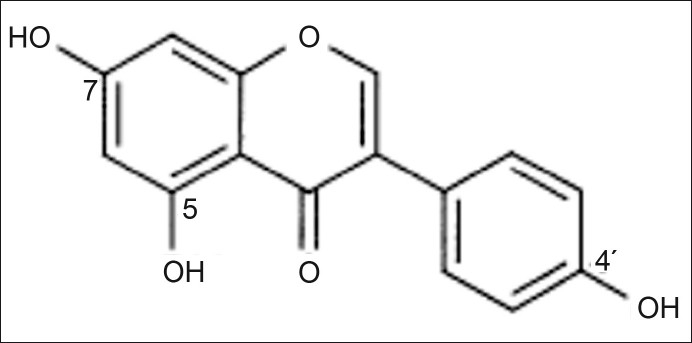
Chemical structure of genistein.
Study of the effect of different extracting times on genistein recovery by Streptomyces roseolus was done by extracting the production media for 1, 2, 3 and 4 h. It could be seen that not much difference in recovery of genistein was seen by varying the time of extraction. The best recovery of genistein was observed at the end of 3 h and hence in all subsequent studies, 3 h of extraction time was used. Results of optimization of fermentation medium using one factor at-a-time method are as summarized in Table 4.
TABLE 4.
RESULTS OF OPTIMIZED FERMENTATION MEDIUM BY ONE FACTOR AT-A-TIME METHOD
Elution was done using a gradient of ethyl acetate and n-hexane. Ethyl acetate concentration was increased gradually from 5% v/v to 50% v/v. 45% v/v ethyl acetate fractions gave a single spot of genistein. These fractions were pooled together and concentrated under vacuum to recover genistein in powder form. In preparative TLC, the plate was run with a mobile phase composition of 1:1 ratio (v/v) of ethyl acetate to n-hexane. The band of genistein was second from the mobile front and was well separated from other bands (of impurities). Genistein band was extracted in ethyl acetate and extract was concentrated and air-dried. After evaporation of the solvent, genistein was recovered in powder form. The recovered sample was analyzed by mass spectroscopy and its percent purity was calculated by HPLC.
Characterization of extracted genistein was done using Infrared spectroscopy, 1H NMR spectroscopy and mass spectrometry. IR spectra were measured using Perkin Elmer FTIR RX-1® infrared spectrophotometer. IR spectra of the standard compound and that of the sample (extracted) compound are as shown in fig. 6. IR spectra of standard show a broad band at 3422.1 cm-1 corresponding to the phenolic-OH group and carbonyl stretching vibrations in region 1640-1660 cm-1. The carbonyl peak of the ketone group has shown absorption at longer wavelength. This is because of the conjugation with the C=C causing delocalization of the electrons of C=O group and reducing the double bond character of carbon to oxygen bond, causing absorption at longer wavelength. O-C stretching band was seen at 1015.2 cm-1. Aromatic C=C stretching bands were observed in the range of 1600-1400 cm-1. IR spectra of the sample compound showa broad band at 3403.8 cm-1 corresponding to the phenolic-OH group. The compound showed carbonyl stretching vibrations at 1645.2 cm-1. The compound showed O-C stretching band at 1010.4 cm-1. Aromatic C=C stretching bands were observed in the range of 1600-1400 cm-1.
Fig. 6.
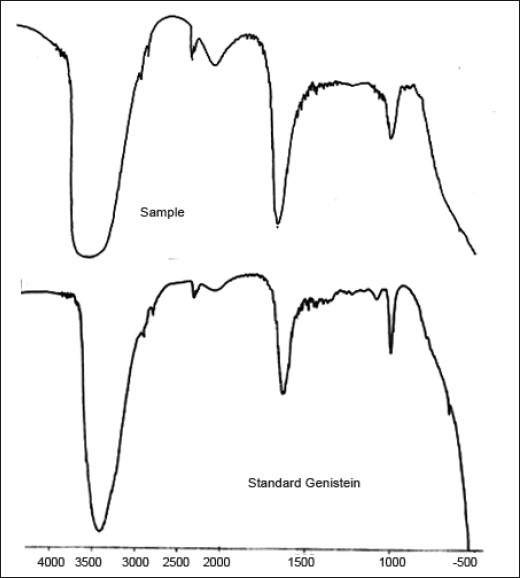
Infrared spectrum of standard genistein and that of extracted sample.
1H NMR spectrum was obtained for standard and purified genistein on JOEL Eclipse® 300 MHz instrument. The 1H NMR spectrum was recorded using DMSO-d6 as the solvent. Spectrums for both standard and purified genistein were identical and showed the following details. The resonance peaks for aromatic protons and phenolic protons were found in the region of δ 6.0–8.5 and δ 9.0–13.0, respectively. In addition, most of the peaks were in the low field region except for peak at δ 3.34 for H2O and peak at δ 2.5 for dimethyl sulfoxide (DMSO). The detailed data for 1H NMR (DMSO-d6) δ: 12.97 (C5-OH), 10.91 (C7-OH), 9.61 (C4’-OH), 8.33 (s, 1H, C2-H), 7.38 (d, 2H, C2’,6’-H), 6.82 (d, 2H, C3’,5’-H), 6.39 (s, 1H, C8-H), 6.23 (s, 1H, C6-H). 1H NMR results further confirmed that the extracted compound is genistein.
Full scan electrospray ionization (ESI) mass spectra was performed on FAB mass spectra (positive mode) on micromass Q-Tof miocro (YA-105). The mass scanning range (m/z) was from 70 to 400. Molecular weight of genistein is 270. The mass spectrum of standard genistein and that of the extracted sample have been depicted in fig. 7. The spectra of the standard genistein and sample exactly match as seen from fig. 7 with peaks in the same region. It is observed from the spectrums that the mass peak of 271 corresponding to M+1 for genistein was found in the standard and also in the sample spectra. The base peak was found to be different than the predicted mass of the compound. But this peak was found both in the standard and the sample. The expected peak of M+1 was 75% intense as compared to the base peak. Thus it was confirmed that the extracted sample was genistein.
Fig. 7.
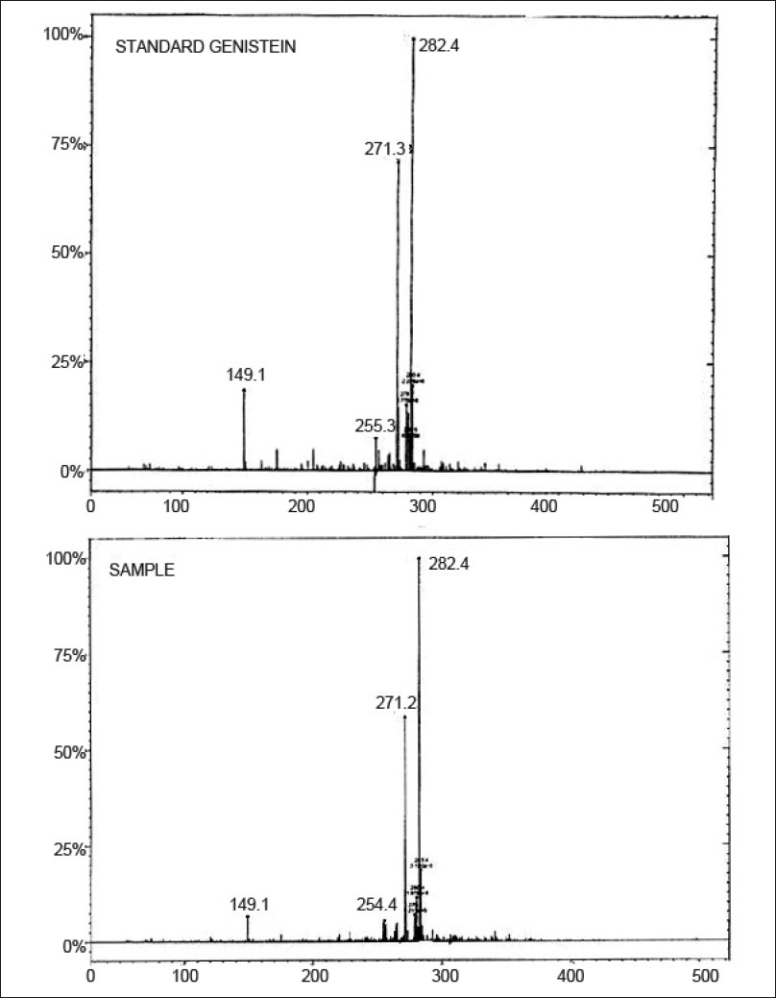
Mass spectra
Standard genistein (top) and that of the extracted sample (bottom).
Percent purity of extracted genistein was determined using HPLC. An impurity peak was observed at retention time (Rt) of 4 min and another at the Rt of 13 min. Percent purity was found to be 91.04%. The extraction efficiency was determined by comparing the HPLC peak area of the recovered standard genistein with that of standard genistein. Extraction efficiency was calculated to be 67.01%.
Genistein was extracted from soy meal by fermentation of a novel microbe Streptomyces roseolus NRRL B-5424. Results revealed Streptomyces roseolus NRRL B-5424 biotransformed genistein glycosides in soy meal to aglycone genistein by secreting enzyme β-glucosidase extracellularly. Optimum recovery of genistein was observed in solvent ethyl acetate. Genistein was purified by column chromatography and preparative TLC and characterized by Mass spectrometry, Infrared spectra and Nuclear magnetic resonance spectrometry. Percent purity of recovered genistein was calculated by HPLC and was found out to be 91.04% pure. Extraction efficiency was found to be 67.01%.[10]
Thus at lab scale, genistein was extracted from a soy source by a biotransformation reaction using microorganisms. This method showed potential of extraction of genistein on a large scale, which would drastically reduce the price tag of genistein in the market.
Footnotes
Pandit and Patravale: A Novel Method for Extraction of Genistein
REFERENCES
- 1.Lee HP, Gourley L, Duffy SW, Esteve J, Lee J, Day NE. Dietary effects on breast-cancer risk in Singapore. Lancet. 1991;337:1197–200. doi: 10.1016/0140-6736(91)92867-2. [DOI] [PubMed] [Google Scholar]
- 2.Merz-Demlow BE, Duncan AM, Wangen KE, Xu X, Carr T, Phipps WR, et al. Soy isoflavones improve plasma lipids in normocholesterolemic, premenopausal women. Am J Clin Nutr. 2000;71:1462–9. doi: 10.1093/ajcn/71.6.1462. [DOI] [PubMed] [Google Scholar]
- 3.Alekel DL, StGermain A, Peterson CT, Hanson KB, Stewart JW, Toda T. Isoflavone-rich soy protein isolate attenuates bone loss in the lumbar spine of perimenopausal women. Am J Clin Nutr. 2000;72:844–52. doi: 10.1093/ajcn/72.3.844. [DOI] [PubMed] [Google Scholar]
- 4.Baker W, Robinson R. Synthetical experiments in the isofavone group. Part III. A synthesis of genistein. J Chem Soc. 1928;131:3115–8. [Google Scholar]
- 5.Prakash O, Tanwar MP. Hypervalent iodine oxidation of favanones: Convenient and useful syntheses of favones and isofavones. J Chem Res. 1995;26:1429–47. [Google Scholar]
- 6.Fleury Y, Magnolato D. Process for obtaining genistin malonate and daidzin malonate. US Patent 5141746. 1992 [Google Scholar]
- 7.Ogawara H, Akiyama T, Ishida J, Watanabe S, Suzuki K. A specific inhibitor for tyrosine protein kinase from Pseudomonas. J Antibiot. 1986;39:606–8. doi: 10.7164/antibiotics.39.606. [DOI] [PubMed] [Google Scholar]
- 8.Shen JL. Aglucone isofavone enriched vegetable protein fiber. US Patent 5352384. 1994 [Google Scholar]
- 9.Weber MJ, Constantinou A, Hessler P. Process for preparing genistein. US Patent 5554519. 1996 [Google Scholar]
- 10.Hessler PE, Larsen PE, Constantinou AI, Schram KH, Weber JM. Isolation of isofavones from soy-based fermentations of the erythromycin-producing bacterium Saccharopolyspora erythraea. Appl Microbiol Biotechnol. 1997;47:398–04. doi: 10.1007/s002530050947. [DOI] [PubMed] [Google Scholar]


