Abstract
The aim of the current research was to evaluate the immunomodulatory potential of methanol extract of Aegle marmelos in an experimental animal model of cellular and humoral immunity. Administration of methanol extract of Aegle marmelos (500 and 1000 mg/kg, p.o.) and Ocimum sanctum (100 mg/kg, p.o.), produced significant increase in adhesion of neutrophils and an increase in phagocytic index in carbon clearance assay. Both doses of Aegle marmelos prevented the mortality induced by bovine Pasteurella multocida in mice. Moreover, all treated groups demonstrated significant elevation in circulating antibody titre in the indirect haemagglunation test. From the above results, it can be concluded that methanol extract of Aegle marmelos possess immunomodulatory potential by stimulating cellular and humoral immune mechanisms. However, low dose of methanol extract of Aegle marmelos was more effective for augmenting cellular immunity, whereas, high dose was more inclined towards humoral immunity.
Keywords: Aegle marmelos, carbon clearance, cellular immunity, haemagglunation test, humoral immunity, mice lethality, neutrophil adhesion
Irreversible unwanted and intolerable effects of conventional drugs and therapies might the underlying reason for an intense research in alternative systems of medicine. Medicinal plants and its products have been explored for variety of acute and chronic diseases across the globe. A number of ayurvedic formulations containing one or more medicinal plants have been exploited for modulation of immune system. Aegle marmelos (Rutaceae) is commonly called Bael, found in the dry deciduous forests of Himalayas[1]. Traditionally, various parts of the plant are used to treat abdomen pain, palpitation of the heart and urinary troubles. According to Bhumkas (local healers) of Patalkot valley in Chhindwara district of Madhya Pradesh, it acts as laxative and febrifuge when taken fresh; it cleans and tones up the intestines. Root and bark cures intermittent fever. An infusion of Bael leaves is regarded as an effective remedy for peptic ulcer[2]. The plant is reported to possess antiinflammatory, antipyretic and analgesic [3,4], antidiabetic[5–7], antidiarrhoeal[8], antihyperlipidemic[9], antifungal [10], antimicrobial, antiparasitic[11], anticancer[12], antimalarial[13] and hepatoproctective activities[14]. It has been reported that a furanocoumarin marmesinin isolated from Aegle marmelose exerted the protective effect against the damage caused by experimental myocardial injury[15].
Environmental pollutants and dietary habits are reported to influence the activity of immune system and diet containing micronutrients and antioxidants are known to enhance the immune system[16]. From earlier studies it is evident that, the leaf extract of Aegle marmelos, by its free-radical scavenging activity possess the radioprotective effect in mice[17]. Literature study revealed the presence of many functional and bioactive compounds such as carotenoids, phenolics, alkaloids, coumarins, flavonoids, terpenoids, and other antioxidants in the leaf extract of Aegle marmelos[18]. Components such as polysaccharides, lectins, proteins and peptide present in plants like Viscum album, Panax ginseng, Tinospora cardifolia, Aspargus racemosus, have been shown to stimulate the immune system[19]. Therefore, the chemical profile may suggest that Aegle marmelos would be a good source of immunomodulatory agent. However, as of now, no biological study is performed demonstrating the immunostimulatory role of the plant. Hence, present research work was designed to study the status of immune system in animals subjected to Aegle marmelos leaves extract using models of cellular and humoral immunity in animals.
Laboratory bred Wistar rats (180-200 g) and albino mice (20-25 g) of either sex were housed in polypropylene cages, maintained under standardized condition (12 h light/dark cycles, 28±2°) with paddy husk bedding at the central animal house, Krupanidhi College of Pharmacy, Bangalore, provided with standard pellet food and had free access to purified drinking water ad libitum. The guidelines of the committee for the purpose of control and supervision of experiments on animals (CPCSEA) were followed and prior permission was sought from the institutional animal ethics committee for conducting the present study (KCP/IAEC-25/2008-09).
Aegle marmelos leaves were collected from the fields of Mandya, Karnataka, India. The plant were identified and authenticated by Regional Research Institute (RRCBI-Mus/06, Bangalore, India). The leaves were given to Phytotech Extracts Pvt. Ltd. (Bangalore, India) to get methanol leaf extract of Aegle marmelos (LEAM). Percent yield of the extract was 17% w/w. The extract was subjected to preliminary phytochemical analysis. The ethanol extract of Ocimum sanctum (Natural remedies) was used as standard immunomodulatory agent.
Leishmann's stain and gluteraldehyde were bought from Merck (Mumbai, India). Indian ink was procured from Hi-Media (Mumbai, India), whereas, WBC diluting fluid and EDTA were purchased from Nice Chemicals (Cochin, India). Cyclophosphamide (Endoxan German Remedies (Mumbai, India). Pasteurella multocida of bovine origin and its vaccine were obtained from Institute of Animal Health and Veterinary Biologicals (Bangalore, India).
Fresh sheep blood was collected from the local slaughter house. Sheep red blood cells (SRBCs) were washed three times in large volumes of pyrogen free 0.9% normal saline and adjusted to a concentration of 0.5×10 9cells/ml for immunization and challenge.
The acute toxicity study was carried out according to the up and down or stair case test described in the fundamentals of experimental pharmacology[20]. The animals were administered test dose of 50 mg/kg orally and observed for a period of 24 h for mortality; subsequent dose was increased by 1.5 factors. The extract was found to be safe upto a dose of 10 g/kg; p.o. According to OPPTS guidelines[21], 1/10th and 1/20th of the maximum safe dose corresponding to 1000 mg/kg and 500 mg/kg were selected as high and low doses respectively.
The drug solutions were prepared in distilled water for oral administration. Evaluation of immunomodulatory effect was carried out by the following models of cellular and humoral immunity. The animals were distributed into four groups consisting of six animals each. The first group served as control (vehicle 1 ml/100 g, p.o.), second group, received the ethanol extract of Ocimum sanctum (OSE) at a dose of (100 mg/kg, p.o.)[22], the third and fourth groups were administered low (500 mg/kg, oral) and high dose (1000 mg/kg, oral) of LEAM, respectively. However, in mice lethality, an additional negative control group was also present.
The rats were treated orally with vehicle or extracts for 14 days[23,24]. On day 14, blood samples were withdrawn from the retro-orbital plexus into heparinized vials and analyzed for differential leukocyte count (DLC). After the initial counts, blood samples were incubated with 80 mg/ml of nylon fibres for 15 min at 37°. Once the incubation was complete, blood samples were again analyzed for TLC and DLC, respectively to get neutrophil index of blood samples. The percent neutrophil adhesion was calculated using the following formula: Neutrophil adhesion (%) = (NIu-NIt)/NIu×100, where NIu is the neutrophil index of untreated blood samples and NIt is the neutrophil index of treated blood samples.
Swiss albino mice were treated with extract orally for 10 days [25,26]. After 48 h of the last dose, all the animals of the different groups were given an intravenous injection of (0.3 ml per 30 g) Indian ink via the tail vein. Blood samples were collected from each animal by retro-orbital plexus at an interval of 0 and 15 min after the injection. A 50-μl blood sample was added into the tube containing 4 ml of 0.1% sodium carbonate solution and the absorbance of this solution was determined at 660 nm. The phagocytic index K was calculated using the following formula: K= (Loge OD1-Loge OD2)/15 where OD1 and OD2 are the optical densities at 0 and 15 min, respectively.
Albino mice were orally pretreated with drugs for 21 days in their respective groups[27]. On the 7thand 17th day of the treatment, the animals were immunized with haemorrhagic septicaemic vaccine (HS vaccine) through subcutaneous route. On the 21st day, the animals were challenged subcutaneously with 0.2 ml of lethal dose (25×LD50) of Pasteurella multocida (bovine origin) containing 107cells per ml. The animals were observed for a period of 72 h and the mortality percentage was determined as follows: percent mortality = 100×(number of animals dead)/total number of animals.
Animals of all groups were pretreated with the drugs for 14 days and all animals of each group were immunized with 0.5×10 9 sheep red blood cells (SRBCs) intraperitoneally in their respective group[22]. The day of immunization was considered as day 0. The treatment was continued for 14 more days and blood samples were collected from rat at the end of the drug treatment and the titre value was estimated by titrating serum dilutions of SRBC (0.025×109 cells) in microtitre plates. The plates were incubated at room temperature for 2 h and examined visually for agglutination. The minimum volume of serum showing heamagglutination was expressed as heamagglutination (HA) titre. The statistical significance was assessed using one-way analysis of variance (ANOVA) followed by Bonferroni's comparison test. The values are expressed as mean±SEM and P<0.05 was considered significant.
Incubation of neutrophils with nylon fibres (NF) showed a decline in the neutrophil counts due to adhesion of neutrophils to the fibres. Both doses of LEAM and OSE showed significant increase in the neutrophil adhesion when compared to control. The low dose of LEAM was found to be more effective than high dose of LEAM. There was also rise in neutrophil count in untreated blood of all treatment groups (Table 1).
TABLE 1.
EFFECT OF LEAF EXTRACT OF AEGLE MARMELOS ON NEUTROPHIL ADHESION TEST
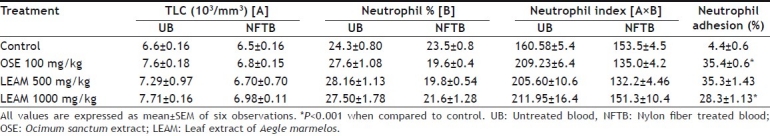
Both the doses of Aegle marmelos extract and OSE showed significant increase in the phagocytic index when compared to control thus result suggest that there was increase in the clearance of colloidal carbon from the blood after administration of these drugs. However, the clearance was best with low dose of LEAM and OSE(Table 2).
TABLE 2.
EFFECT OF LEAF EXTRACT OF AEGLE MARMELOS ON PHAGOCYTIC INDEX AND HAEMAGGLUTINATION TITRE
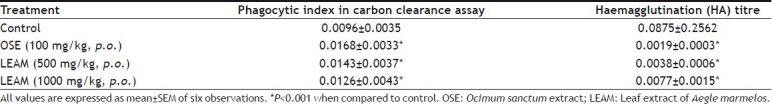
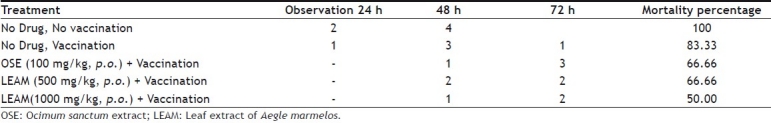
Mortality was found to be 100% within 72 h in control group upon administration of Pasteurella multocida. There was 83.33% mortality in vaccinated group without any prior treatment of drug. The low dose of LEAM reduced the mortality percentage to 66.66% and high dose posses 50.00% mortality with survival of three animals out of six (Table 3). The haemmagglutinating antibody (HA) titre value was significantly increased in animals that received vaccination along with low or high dose of LEAM or OSE compared to animals that received vaccination alone (Table 2).
TABLE 3.
EFFECT OF LEAF EXTRACT OF AEGLE MARMELOS ON MICE LETHALITY TEST
In this paper we report that methanol extract of Aegle marmelos possessed immunomodulatory effect in experimental models of cellular and humoral immunity in animals. The extract was found to be most effective at low dose (500 mg/kg, p.o.), whereas, high dose (1000 mg/kg, p.o.) of LEAM was moderately effective in modulating cellular immune system. On the contrary, high dose of (1000 mg/kg, p.o.) Aegle marmelos (LEAM) demonstrated better immunostimulating property than the low dose (500 mg/kg, p.o.) in humoral mediated immunity. The study was carried out using four models of immunity highlighting the different stages of immunity.
The marginations of polymorphonuclear lymphocyte in the vasculature as well as transmigration of neutrophils to the site of inflammation are described by neutrophil adhesion test[24]. Both low and high doses of LEAM (500 and 1000 mg/kg, p.o.) showed a substantial rise in the neutrophil adhesion to nylon fibres. This could be attributed to upregulation of β2 integrins, situated on the membrane of the neutrophils by that they adhere firmly to the nylon fibres[28]. Hence, it was inferred that LEAM causes stimulation of neutrophils towards the site of inflammation.
The influence of drugs on reticuloendothelial system (RES) was assessed by carbon clearance test. The reticuloendothelial system (RES) is a diffuse system consisting of phagocytic cells. Cells of the RES have a direct influence on the clearance of particles from the bloodstream. When colloidal carbon particles in the form of ink are injected directly into the systemic circulation, the rate of clearance of carbon from the blood by macrophage is governed by an exponential equation[26]. Both doses of LEAM as well as OSE showed remarkable augmentation in the phagocytic index due to their ability to increase the activity of the reticuloendothelial system.
The mouse lethality test is one of the commonly employed tests to assess serological responses in animals immunized with vaccines. Pasteurella multocida is pathogenic to mice. The mouse lethality test involves injecting mice with the vaccine before administration of the bacterial culture and determining the mortality percentage[29]. The vaccination induces humoral immunization. The survival of animals is dependent on the ability of drug to produce adequate number of antibodies, which can counter the pathogen. The low dose of LEAM prevented the death of 33.33% of animals at the end of 72 h, and OSE showed only 33.33% mortality, whereas high dose of LEAM resulted in survival of 50% of animals. Hence it is speculated that LEAM in low dose and OSE carries moderate immunopotential in humoral immunity in increasing the number of survival, while the LEAM in high dose is remarkably effective in preventing the mortality.
The confirmation of potency of LEAM on antibody-mediated immune response was done by indirect haemagglutination test. It is mainly composed of interacting B cell with antigens and subsequently proliferating and differentiating into antibody producing cells. Antibody works by binding with antigens and neutralizing it or facilitating its elimination by cross linking to form latex that is more readily ingested by phagocytic cells[25]. The results showed that levels of circulating antibodies are increased if the test animals are pretreated with Aegle marmelos extract or OSE.
In conclusion, both low dose (500 mg/kg, p.o) as well as high dose (1000 mg/kg, p.o) of Aegle marmelos stimulates immune system by acting through cellular and humoral immunity in experimental models of immunity in animals. However, low dose was found to be most effective in cell mediated immune response, whereas, in humoral immunity, high dose was best effective.
ACKNOWLEDGEMENTS
The authors are thankful to Prof. Suresh Nagpal, Chairman, Krupanidhi Educational Trust, Prof. Sunil Dhaminigi, Secretary, Krupanidhi Educational Trust and Dr. Amit Kumar Das, Principal, Krupanidhi College of Pharmacy for providing facilities to carry out the work.
Footnotes
Govinda and Asdaq: Immunomodulatory activity of Aegle marmelos
REFERENCES
- 1. [Last accessed on 2008 Nov 23]. Available from: http://www.divineremedies.com/angle_marmelos .
- 2.Acharya D, Shrivastava A. Jaipur: Aavishkar Publishers Distributors; 2008. Indigenous Herbal Medicines: Tribal Formulations and Traditional Herbal Practices; pp. 252–7. [Google Scholar]
- 3.Arul V, Miyazaki S, Dhananjayan R. Studies on the anti-inflammatory, antipyretic and analgesic properties of the leaves of Aegle marmelos. J Ethnopharmacol. 2005;96:159–63. doi: 10.1016/j.jep.2004.09.013. [DOI] [PubMed] [Google Scholar]
- 4.Shankarananth V, Balakrishnan N, Suresh D, Sureshpandian G, Edwin E, Sheej E. Analgesic activity of methanol extract of Aegle marmelos leaves. Fitoterapia. 2007;78:258–9. doi: 10.1016/j.fitote.2006.12.004. [DOI] [PubMed] [Google Scholar]
- 5.Kamalakkannan N, Stanely P, Prince M. Hypoglycaemic effect of water extracts of Aegle marmelos fruits in streptozotocin diabetic rats. J Ethnopharmacol. 2003;87:207–10. doi: 10.1016/s0378-8741(03)00148-x. [DOI] [PubMed] [Google Scholar]
- 6.Arumugam S, Kavimani S, Kadalmani B, Ahmed AB, Akbarsha MA, Rao MV. Antidiabetic activity of leaf and callus extracts of Aegle marmelos in rabbit. Sci Asia. 2008;34:317–21. [Google Scholar]
- 7.Kesari AN, Gupta RK, Singh SK, Diwakar S, Watal G. Hypoglycemic and antihyperglycemic activity of Aegle marmelos seed extract in normal and diabetic rats. J Ethnopharmacol. 2006;107:374–9. doi: 10.1016/j.jep.2006.03.042. [DOI] [PubMed] [Google Scholar]
- 8.Shoba FG, Thomas M. Study of antidiarrhoeal activity of four medicinal plants in castor-oil induced diarrhea. J Ethnopharmacol. 2001;76:73–6. doi: 10.1016/s0378-8741(00)00379-2. [DOI] [PubMed] [Google Scholar]
- 9.Vijaya C, Ramanathan M, Suresh B. Lipid lowering activity ethanolic extractof leaves Aegle marmelos in hyperlipidaemic models Wistar albino rats. Indian J Exp Biol. 2009;47:182–5. [PubMed] [Google Scholar]
- 10.Rana BK, Singh UP, Taneja V. Antifungal activity and kinetics of inhibition by essential oil isolated from leaves Aegle marmelos. J Ethnopharmacol. 1997;57:29–34. doi: 10.1016/s0378-8741(97)00044-5. [DOI] [PubMed] [Google Scholar]
- 11.Ulahannan RK, Thomas T, Sadasivan C. Antibacterial Action of Leaves of Aegle marmelos. Int J Sci. 2008;2:134–8. [Google Scholar]
- 12.Gangadevi V, Muthumary J. Taxol, an anticancer drug produced by an endophytic fungus Bartalinia robillardoides Tassi, isolated from a medicinal plant, Aegle marmelos Correa ex Roxb. World J Microbiol Biotechnol. 2008;24:717–24. [Google Scholar]
- 13.Elango G, Abdul Rahuman A, Bagavan A, Kamaraj C, Abduz Zahir A, Venkatesan C. Laboratory study on larvicidal activity of indigenous plant extracts against Anopheles subpictus and Culex tritaeniorhynchus. Parasitol Res. 2009;104:1381–8. doi: 10.1007/s00436-009-1339-7. [DOI] [PubMed] [Google Scholar]
- 14.Singh R, Rao HS. Hepatoprotective effect of the pulp/seed of Aegle marmelos Correa ex Roxb against carbon tetrachloride induced liver damage in rats. Int J Green Pharmacy. 2008;2:232–4. [Google Scholar]
- 15.Vimal V, Devaki T. Linear furanocoumarin protects rat myocardium against lipidperoxidation and membrane damage during experimental myocardial injury. Biomed Pharmacother. 2004;58:393–400. doi: 10.1016/j.biopha.2003.12.007. [DOI] [PubMed] [Google Scholar]
- 16. [Last accessed on 2007 Sep 21]. Available from: http://www.clevelandclinic.org/health/healthinfo/docs/0900/0955.asp?index=5429 .
- 17.Jagetia GC, Venkatesh P, Baliga MS. Evaluation of radioprotective effect of bael leaf (Aegle marmelos) extract in mice. Int J Radiat Biolnone. 2004;80:281–90. doi: 10.1080/09553000410001679776. [DOI] [PubMed] [Google Scholar]
- 18.Bioactive compounds and volatile compounds of Thai bael fruit (Aegle marmelos (L.) Correa) as a valuable source for functional food ingredients. [Last accessed on 2008 Nov 22]. Available from: http://www.ifrj.upm.edu.my/galley%20proof/Suvimol.pdf .
- 19.Oladunmoye MK. Immunomodulatory Effects of Ethanolic Extract of Tridax procumbens on Swiss Albino Rats Orogastrically Dosed with Pseudomonas aeruginosa (NCIB 950) Trends Med Res. 2006;1:122–6. [Google Scholar]
- 20.Ghosh MN. 2nd. Kolkata: J. Sinha Publishers; 1984. Fundamentals of experimental pharmacology. [Google Scholar]
- 21. [Last accessed on 2009 Nov 11]. Available from: http://www.epa.gov/oppts/
- 22.Sharma M, Kishore K, Gupta SK, Joshi S, Arya DS. Cardioprotective potential of Ocimum sanctum in rats. Mol Cell Biochemnone. 2001;225:75–83. doi: 10.1023/a:1012220908636. [DOI] [PubMed] [Google Scholar]
- 23.Fulzele SV, Satturwar PM, Joshi SB, Dorle AK. Study of the immunomodulatory activity of Haridradi ghrita in rats. Indian J Pharmacol. 2003;35:51–4. [PubMed] [Google Scholar]
- 24.Shinde UA, Phadke AS, Nair AM, Mungantiwar AA, Dikshit VJ, Saraf MN. Preliminary studies on the immunomodulatory activity of Cedrus deodara wood oil. Fitoterapia. 1999;70:333–9. [Google Scholar]
- 25.Jayathirtha MG, Mishra SH. Preliminary immunomodulatory activities of methanol extracts of Eclipta alba and Centella asiatica. Phytomedicine. 2004;11:361–5. doi: 10.1078/0944711041495236. [DOI] [PubMed] [Google Scholar]
- 26.Gokhale AB, Damre AS, Saraf MN. Investigations into the immunomodulatory activity of Argyreia speciosa. J Ethnopharmacol. 2003;84:109–14. doi: 10.1016/s0378-8741(02)00168-x. [DOI] [PubMed] [Google Scholar]
- 27.Ramanatha KR, Lakshminaryana R, Gopal T. Potency test of Duck Pasteurella vaccine in mice. Mysore J Agric Sci. 1995;29:155–7. [Google Scholar]
- 28.Srikumar R, Narayanaperumal JP, Rathisamy SD. Immunomodulatory activity of Triphala over neutrophil functions. Biol Pharm Bull. 2005;28:139–403. doi: 10.1248/bpb.28.1398. [DOI] [PubMed] [Google Scholar]
- 29.Finco Kent DL, Galvin JE, Suiter BT, Huether MJ. Pasteurella multocida toxin type D serological assay as an alternative to the toxin neutralisation lethality test in mice. Biologicals. 2001;29:7–10. doi: 10.1006/biol.2001.0268. [DOI] [PubMed] [Google Scholar]


