Abstract
Solid dispersions of mefanamic acid with a water-soluble polymer polyvinyl pyrrolidine and a super disintegrant, primojel were prepared by common solvent and solvent evaporation methods employing methanol as the solvent. The dissolution rate and dissolution efficiency of the prepared solid dispersions were evaluated in comparison to the corresponding pure drug. Solid dispersions of mefenamic acid showed a marked enhancement in dissolution rate and dissolution efficiency. At 1:4 ratio of mefenamic acid-primojel a 2.61 fold increase in the dissolution rate of mefenamic acid was observed with solid dispersion. The solid dispersions in combined carriers gave much higher rates of dissolution than super disintegrants alone. Mefanamic acid-primojel-polyvinyl pyrrolidine (1:3.2:0.8) solid dispersion gave a 4.11 fold increase in the dissolution rate of mefenamic acid. Super disintegrants alone or in combination with polyvinyl pyrrolidine could be used to enhance the dissolution rate of mefenamic acid.
Keywords: Dissolution rate, mefenamic acid, polyvinyl pyrrolidone, solid dispersions
Mefenamic acid (MA), an anthranilic acid derivative, is a non-steroidal antiinflammatory drig (NSAID) [1] and short (2 h) plasma half-life drug. It is used as antipyretic analgesic[2], and antirheumatic[3] for the treatment of headache, dental pain, postoperative and postpartum pain, dysmenorrhea and osteoarthritis. The usual dose orally is 500 mg three times daily. Mefenamic acid is absorbed from the gastrointestinal tract. Peak plasma concentrations occur about 2 to 4 h after ingestion. Most of the NSAIDs belong to class II category under biopharmaceutical classification system, since they are inherently highly permeable through biological membranes, but exhibit low aqueous solubility. Rate of absorption and/or extent of bioavailability for such hydrophobic drugs are controlled by rate of dissolution in gastrointestinal fluids[4]. Solid dispersions are one of the most promising strategies to improve the oral bioavailability of poorly water-soluble drugs. By reducing drug particle size to the absolute minimum, and hence improving drug wettability, bioavailability may be significantly improved[5]. The present study aims at enhancing the dissolution rate and bioavailability of MA with solid dispersions[6] by employing common solvent and solvent evaporation methods. Water dispersible super disintegrants, a new class of tablet excipients were evaluated as carriers, alone and in combination with polyvinyl pyrrolidine (PVP), for enhancing the dissolution rate and bioavailability of MA.
MA was a gift sample from M/s. Sigma Laboratories, Mumbai, India, methanol (Qualigens) and polyvinyl pyrrolidone (PVP K30) was a gift sample from M/s. Sun Pharma Ind. Ltd., Mumbai, India. All other materials used were of pharmacopoeial grade and were procured from commercial sources.
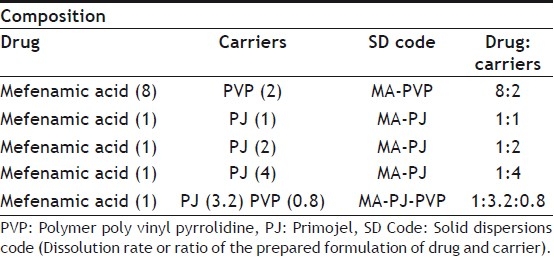
Solid Dispersions of mefenamic Acid were prepared by common solvent method employing methanol as solvent. The required quantities of MA and PVP were weighed and dissolved in the corresponding solvent in a round bottom flask to get a clear solution. The solvent was then removed by evaporation under reduced pressure (vacuum) at 60° with constant mixing. The mass obtained was crushed, pulverized and shifted through mesh no. 100. In each case solid dispersions were prepared in the ratio of drug carrier namely 8:2.
Solid dispersions of MA in superdisintegrate primojel were prepared by solvent evaporation method. The required quantities of MA were dissolved in methanol to get a clear solution in a dry mortar. The superdisintegrate primojel (PJ, passed through 120 No. mesh) was then added to clear drug solution and dispersed. The solvent was removed by continuous titration until a dry mass was obtained. The mass obtained was further dried at 50° for 4 h in an oven. The product was crushed, pulverized and shifted through mesh no. 100. In each case solid dispersions in the superdisintegrate primojel were prepared at three different ratios of MA, PJ namely 1:1, 1:2 and 1:4, respectively.
The required quantities of MA and water-soluble carrier (PVP) were dissolved in the solvent to get a clear solution in a dry mortar. The superdisintegrate (PJ) was then added to the drug solution and dispersed. The solvent was then evaporated by continuous titration until a dry mass was obtained. The mass obtained was further dried at 50° for 4 h in an oven. The product was crushed, pulverized and shifted through mesh no. 100. Various solid dispersions prepared with their composition are listed in Table 1.
TABLE 1.
COMPOSITION OF VARIOUS SOLID DISPERSIONS PREPARED
A spectrophotmetric method based on the measurement of absorbance at 279 nm in phosphate buffer pH 7.4 was used in the present study for the estimation of mefenamic acid. The method was validated for reproducibility, accuracy, precision and linearity by analyzing six individually weighed samples of MA. The stock solution of MA was subsequently diluted to a series of dilution containing 5, 10, 15 and 20 μg/ml of solution, using phosphate buffer of pH 7.4. The absorbance of these solutions was measured in UV/Vis spectrophotometer (ELICO SL-159). The method obeyed Beer's law in the concentration of 0-20 μg/ml.
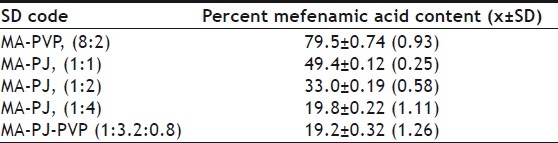
From each batch, 4 samples of 50 mg each were taken and analyzed for the drug mefenamic acid. Fifty milligrans of dispersions were weighed into a 100 ml volumetric flask. Methanol was added and the contents were mixed thoroughly to dissolve the drug from the dispersion. The solution was then filtered and collected carefully into another 100 ml volumetric flask. The solution was made up to volume with the solvent. The solution was suitably diluted with phosphate buffer of pH 7.4 and assayed at 279 nm for MA. The results are given in Table 2.
TABLE 2.
MEFENAMIC ACID CONTENT OF VARIOUS SOLID DISPERSIONS PREPARED
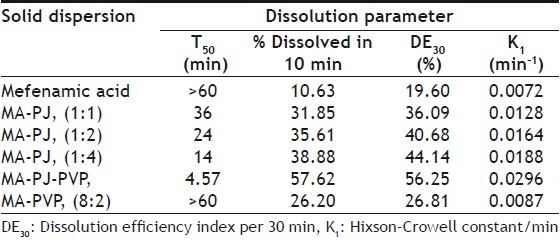
Dissolution rate of MA was studied using an USP XXIII six station dissolution rate test apparatus (Electro Lab). Paddle stirrer at a speed of 50 rpm and temperature of 37±1° were used in each test. Drug or solid dispersion of drug equivalent to 100 mg of MA was used in each dissolution rate test. Samples of dissolution medium i.e., phosphate buffer pH 7.4 (5 ml) were withdrawn through a filter (0.45 μ) at different time intervals, suitably diluted, and assayed for MA. The dissolution experiments were conducted in triplicate. The results are given in Table 3 and fig 1. Dissolution rates of MA and its solid dispersions followed first order kinetics (Table 4 and fig 2). Dissolution parameters such as T50, DE30, K1, Percent of MA dissolved in 10 minutes are given in Table 5.
TABLE 3.
DISSOLUTION PROFILES OF MEFENAMIC ACID SOLID DISPERSIONS
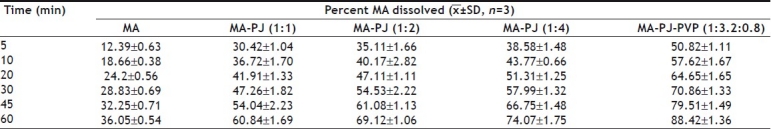
Fig. 1.
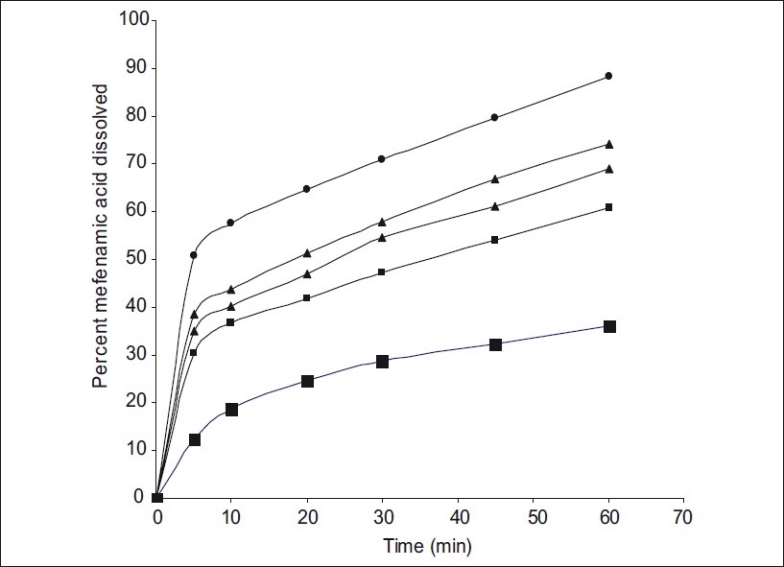
Dissolution profiles of mefenamic acid and its solid dispersions
Mefenamic Acid (MA) -■-; Mefenamic Acid: Primojel (MA-PJ-1:1) -■-; Mefenamic Acid: Primojel (MA-PJ-1:2) -▲-; Mefenamic Acid: Primojel (MA-PJ-1:4) -▲-; Mefenamic Acid: Primojel: Polymer poly Vinyl Pyrrolidine -●-
TABLE 4.
THE CORRELATION COEFFICIENT (R) VALUES IN THE ANALYSIS OF DISSOLUTION DATA OF MEFENAMIC ACID SOLID DISPERSIONS AS PER ZERO ORDER, FIRST ORDER AND HIXSON CROWELL CUBE ROOT MODELS
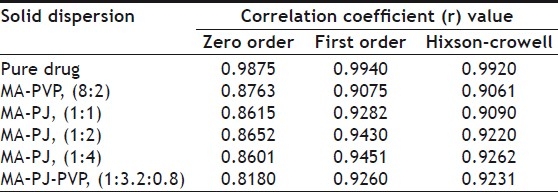
Fig. 2.
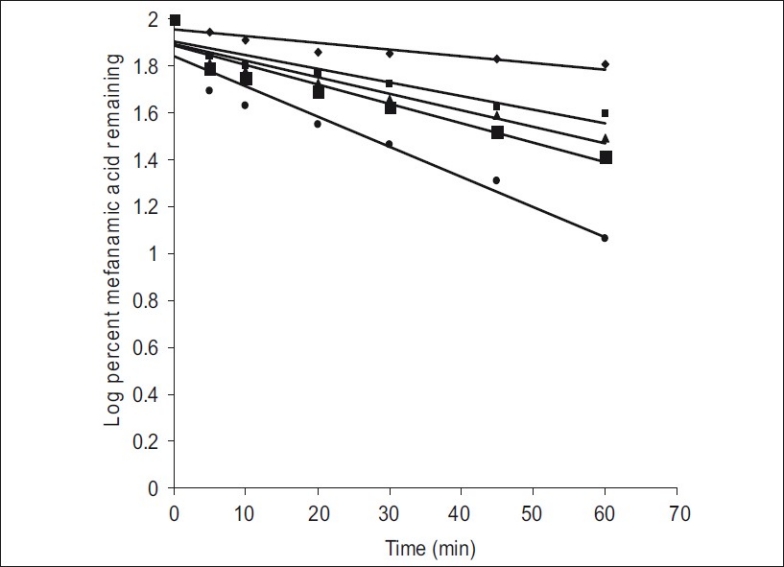
First order dissolution plots of mefenamic acid and its solid dispersions
Mefenamic acid (MA, -♦-); Mefenamic acid: Primojel (MAPJ- 1:1,-▲-); Mefenamic acid: primojel (MA-PJ-1:2, -■-); Mefenamic acid: Primojel (MA-PJ-1:4, -■-); Mefenamic acid:Primojel: Poly vinyl pyrrolidine (MA-PJ-PVP-1:41:3.2:0.8, -●-)
TABLE 5.
DISSOLUTION PARAMETERS OF MEFENAMIC ACID AND ITS SOLID DISPERSIONS IN SUPERDISINTEGRANTS
The dissolution data of MA and their solid dispersions were also analyzed as per Hixson-Crowell's [7] cube root equation. Hixson-Crowell introduced the concept of changing surface area during dissolution and derived the “cube-root law” to nullify the effect of changing surface area and to linearize the dissolution curves. Hixson-Crowell's cube root law is given by the following equation. (Wo)1/3-(Wt)1/3= Kt, where Wois initial mass and W
t is the mass remained at time 't'. The cube root equation is applicable to the dissolution of monodisperse powder consisting of uniform sized particles. A plot of (Wo)1/3-(Wt)1/3 versus time will be linear when dissolution occurs from monodisperse particles of uniform size. Hixson-Crowell plots of the dissolution data were found to be linear (fig 3) with all solid dispersions. This observation indicated the drug dissolution from all the solid dispersions is occurring from discretely suspended or deposited (monodisperse) particles. This might have also contributed to the enhanced dissolution rate of the solid dispersions. The correlation coefficient (r) values of the first order release model are found to be (0.9075 to 0.9940) slightly higher when compared to the Hixson-Crowell's cube root model. Hence the release of drug from the preparations followed predominantly first order kinetics compared to Hixson-Crowell cube root law. Correlation coefficient values in the analysis of dissolution data as per zero order, first order and Hixson-Crowell cube root are given in Table 3. Another parameter suitable for evaluation of in vitro dissolution has been suggested by Khan[8] is, dissolution efficiency (DE). DE is defined as the area under the dissolution curve up to a certain time 't' expressed as percentage of the area of the rectangle described by 100% dissolution in the same time. Dissolution efficiency (DE) = 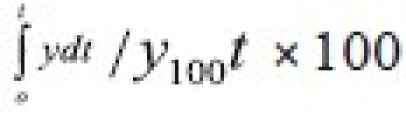 and the index DE30 would relate to the dissolution of drug from a particular formulation after 30 min and could be compared with DE30 of other formulations. Summation of the large dissolution data into a single figure DE enables ready comparison to be made between a large numbers of formulations.
and the index DE30 would relate to the dissolution of drug from a particular formulation after 30 min and could be compared with DE30 of other formulations. Summation of the large dissolution data into a single figure DE enables ready comparison to be made between a large numbers of formulations.
Fig. 3.
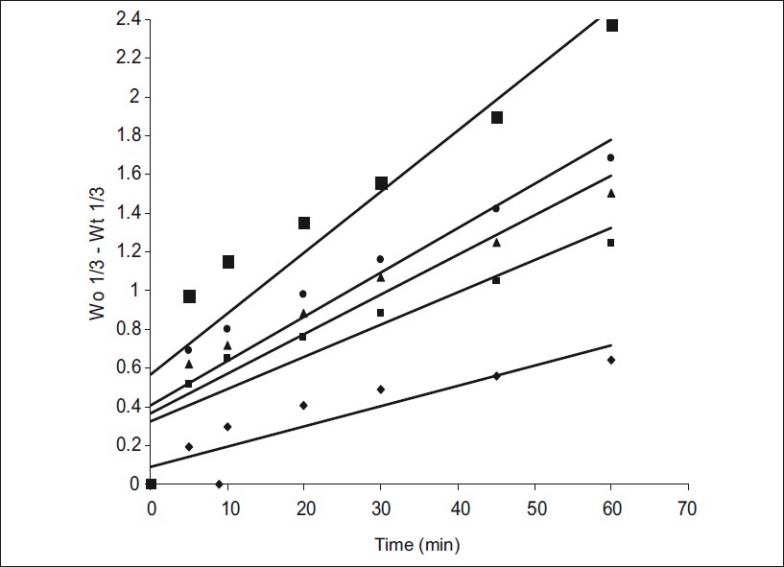
Hixson-crowell dissolution plots of mefenamic acid and its solid dispersions
Mefenamic acid (MA, -♦-); Mefenamic acid: Primojel (MA-PJ-1:1, -■-); Mefenamic acid: primojel (MA-PJ-1:2, -▲-); Mefenamic acid: Primojel (MA-PJ-1:4, -●-); Mefenamic acid:Primojel: Poly vinyl pyrrolidine (MA-PJ-PVP-1:41:3.2:0.8, -■-)
All the dissolution parameters given in Table 2 indicated rapid and higher dissolution of MA from all solid dispersions when compared to MA pure drug. MA-PVP (8:2) solid dispersion gave rapid and higher dissolution than the pure drug. A 1.21 fold increase in the dissolution rate of MA was obtained with this solid dispersion when compared to pure drug. Water dispersible superdisintegrants gave much higher enhancement in the dissolution rate of mefenamic acid than water-soluble carriers. Solid dispersions of superdisintegrants gave rapid and higher dissolution of MA when compared to pure drug as well as its solid dispersion in water soluble PVP. In each case, the K1 and DE30 values were increased as the concentration of carrier (superdisintegrant) in the solid dispersion was increased. At 1:4 ratio of MA:PJ, the order of increasing dissolution rate with various superdisintegrants was 1:4>1:2>1:1. A 2.61 fold increase in the dissolution rate of MA was observed with MA-PJ (1:4) solid dispersion. All the solid dispersions in combined carriers gave much higher rates of dissolution, several times higher than the dissolution rate of pure drug. PVP combined super disintegrants gave higher dissolution rates than superdisintegrants alone. MA-PJ-PVP solid dispersion gave a 4.11 fold increase in the dissolution rate of MA whereas solid dispersion of MA in PJ lone (MA-PJ 14 solid dispersion) gave only 2.61 fold increase. Thus combination of superdisintegrants with water soluble carrier PVP resulted in a greater enhancement in the dissolution rate of MA. Hladon et al.[9], the inclusion complex of MA with b-cyclodextrin was obtained by the method of coprecipitation from diethyl ether. The product was identified by the thermogravimetric and X-ray methods. The complex stability constants were determined by the potentiometric method. The effect of β-CD on the solubility and stability of MA was analysed. Thus superdisintegrant, PJ was found to be useful as a carrier in MA solid dispersions alone and in combination with PVP to enhance the solubility, dissolution rate and dissolution efficiency.
ACKNOWLEDGMENTS
The authors would like to express sincere thanks to the Management of D. C. R. M. Pharmacy College for their encouragement and providing necessary facilities in carryout this research work. The authors would also express sincere thanks to M/s. Sigma Laboratories, Mumbai for generous gift of mefenamic acid samples.
Footnotes
Rao, et al.: In vitro Dissolution Studies
REFERENCES
- 1.Sweetmann SC. London: The Pharmaceutical Press; 2005. Martindale- The Extra Pharmacopoeia. [Google Scholar]
- 2.Kato F, Otsuka M, Matsuda Y. Kinetic study of the transformation of mefenamic acid polymorphs in various solvents and under high humidity conditions. Int J Pharm. 2006;321:18–26. doi: 10.1016/j.ijpharm.2006.04.020. [DOI] [PubMed] [Google Scholar]
- 3.Fang L, Numajiri S, Kobayashi D, Ueda H, Nakayama K, Miyamae H, et al. Physicochemical and crystallographic characterization of mefenamic acid complexes with alkanolamines. J Pharm Sci. 2004;93:144–54. doi: 10.1002/jps.10468. [DOI] [PubMed] [Google Scholar]
- 4.Guirguis M, Jammali F. Disease-drug interaction: Reduced response to propranolol despite increased concentration in the rat with inflammation. J Pharm Pharm Sci. 2001;4:1077–84. doi: 10.1002/jps.10381. [DOI] [PubMed] [Google Scholar]
- 5.Vasconcelos T, Sarmento B, Costa P. Solid dispersions as strategy to improve oral bioavailability of poor water soluble drugs. Drug Discov Today. 2007;12:1068–75. doi: 10.1016/j.drudis.2007.09.005. [DOI] [PubMed] [Google Scholar]
- 6.Sekiguchi K, Obi N. Studies on absorption of eutectic mixture-I. Chem Pharm Bull. 1961;9:866–72. doi: 10.1248/cpb.12.134. [DOI] [PubMed] [Google Scholar]
- 7.Hixon AW, Crowell JH. Dependence of reaction velocity upon surface and agitation: I-theoretical consideration. Ind Eng Chem. 1931;23:923–31. [Google Scholar]
- 8.Khan KA, Rhodes CT. Effect of compaction pressure on the dissolution efficiency of direct compression systems. Pharm Acta Helv. 1972;47:594–607. [PubMed] [Google Scholar]
- 9.Hładoń T, Pawlaczyk J, Szafran B. Stability of mefenamic acid in the inclusion complex with β-cyclodextrin in the solid phase. J Inclusion Phenomena Macrocyclic Chem. 1999;35:497–506. [Google Scholar]


