Abstract
Though still in infancy, the field of psychiatric genetics holds great potential to contribute to the development of new diagnostic and therapeutic options to treat these disorders. Among a large number of existing neurotransmitter systems, the serotonin system dysfunction has been implicated in many psychiatric disorders and therapeutic efficacy of many drugs is also thought to be based on modulation of serotonin. Serotonin transporter gene polymorphism is one of the most extensively studied polymorphisms in psychiatric behavioral genetics. In this article, we review the status of evidence for association between the serotonin gene polymorphism and some common mental disorders like affective disorders, post-traumatic stress disorder, obsessive-compulsive disorder, suicide, autism, and other anxiety and personality disorders. Going beyond traditional association studies, gene-environment interaction, currently gaining momentum, is also discussed in the review. While the existing information of psychiatric genetics is inadequate for putting into practice genetic testing in the diagnostic work-up of the psychiatric patient, if consistent in future research attempts, such results can be of great help to improve the clinical care of a vast majority of patients suffering from such disorders.
Keywords: Affective disorders, gene-environment interaction, obsessive-compulsive disorder, polymorphism, post-traumatic stress disorder, serotonin transporter gene
INTRODUCTION
Global research efforts to underpin factors relevant to precise etiology of various psychiatric disorders continue to be confronted with ambiguity.[1,2] Major impediments in appropriate, evidence-based treatment of mental disorders are lack of biological markers that could play an important role in the early identification and management of many of them. In recent years, attempts to investigate the genetic influence for development of these disorders have emerged as one of the prominent areas of medical molecular biology.[3–7]
Specific mental disorders are believed to be related to different sets of genes.[8] Understanding these correlates and susceptibility loci linked to respective familial predisposition has substantially increased our knowledge of the neurobiological foundations of complex psychiatric disorders during the previous decade.[9] Among the large number of existing neurotransmitters, serotonin [5-hydroxytryptamine (5-HT)] modulates a wide variety of physiological processes including sleep, appetite, thermoregulation, pain perception, respiration, and proper functioning of bowel.[10] Serotonergic system dysfunction has been implicated in common psychiatric syndromes, and the therapeutic efficacy of several psychopharmological agents is thought to be based on modulation of serotonin. Identification of possible candidate genes and polymorphisms for the serotoninergic system may therefore be important, as this system is known to influence mood, emotion, and cognition.[11,12] Subsequent to its vesicular release, the level of serotonin is maintained by the serotonin transporter (5-HTT) that actively clears it from the synapse, returning it to the presynaptic neuron where it can be degraded or released at a later time.[10,13,14] The serotonin transporter is encoded by a single gene [the solute carrier family 6, member 4 (SLC6A4) gene] [Figure 1] located on chromosome 17q11.1-17q12 and spans 31 kb.[15] It consists of 14 exons[15] and belongs to a large gene family of neurotransmitter transporters, which have a sodium- and chloride-dependant transport activity, and the transporter contains 12 transmembrane domains.[15,16] The transcription of this gene is modulated by two primary polymorphisms in its upstream regulatory region. The first polymorphism is a 44-bp insertion deletion located in the promoter region (5-HTTLPR), creating the long and the short variants of the gene. The long variant (L) indicates the presence of an insertion, resulting in a 528-bp allele having 20-23 bp long, GC-rich 16 repeat elements, whereas the absence of this insertion yields a short variant (S) of 484 bp allele having 14 repeat elements.[13,17] The L allele confers a threefold increase in transcriptional efficacy and activity than the S allele.[13,17,18] Serotonin transporter-linked polymorphic region (5-HTTLPR) is one of the most extensively studied polymorphisms in psychiatric behavioral genetics.[19] Two functionally distinct subtypes of long allele, LA and LG,[16,20] have been described recently. Type “A” has been shown to be associated with high levels of 5-HTT messenger ribonucleic acid expression.[20,21] Another polymorphism existing in the intron 2 of the serotonin transporter gene called variable number of tandem repeats (VNTR) has also been described to be associated with susceptibility to psychiatric disorders. The three alleles of VNTR region in intron 2 are denoted as STin2.9, STin2.10, and STin 2.12 having 9, 10, and 12 repeats, respectively.[22–24]
Figure 1.
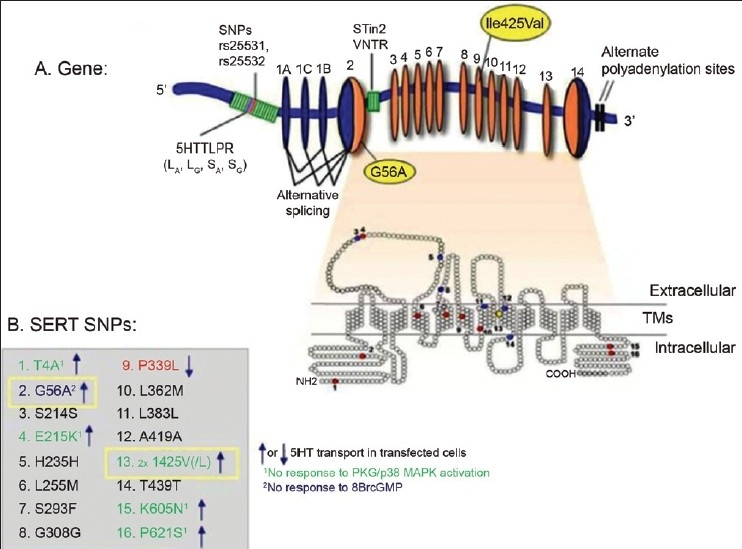
Human SERT gene organization, with multiple functional variants. Reprinted from “How the Serotonin story is being rewritten by new gene-based discoveries principally related to SLC6A4, the Serotonin transporter gene, which functions to influence all cellular Serotonin systems”, Volume 55: 6; Dennis L. Murphy, Meredith A. Fox, Kiara R. Timpano, Pablo Moya, Renee Ren-Patterson, Anne M. Andrews, Andrew Holmes, Klaus-Peter Lesch, and Jens R. Wendland; 2008 with permission from Elsevier
In the light of available data, we briefly seek to ascertain the status of evidence for the association between the 5-HTTLPR polymorphism and some common mental disorders like affective disorders, post-traumatic stress disorder (PTSD), obsessive-compulsive disorder (OCD), suicide, autism, and other anxiety and personality disorders.
AFFECTIVE DISORDERS
The last few years have seen a considerable increase in gene-environment (G×E) interaction research. Studies looking into the involvement of 5-HT in mental disorders have not been consistent.[25,26] Evidence exists for the association of S allele and S/S genotype with major depressive disorder, unipolar or bipolar depression.[27,28] Additionally, significant association of the S allele with seasonal affective disorder (SAD) has also been reported.[29] One family-based study of bipolar probands and their parents used the transmission disequilibrium test (TDT) to find a preferential transmission of the S allele[30] and observed evidence of an association with bipolar disorder. Recent findings from structural imaging indicate that patients with the S allele of 5-HTTLPR, suffering from major depression, exhibit smaller hippocampal volumes only when they had a history of emotional neglect in childhood, compared to patients with only the genetic or the environmental risk factor. The long L allele appeared to confer protective effects with larger prefrontal cortex (PFC) volumes in patients carrying this allele.[31]
There are also a number of reports supporting a lack of association between 5-HTTLPR polymorphism and depressive disorders.[32–35] A recent study conducted on Croatian patients reported that the frequency distribution of the L and S alleles and genotypes of the 5-HTTLPR were not statistically different between major depressive disorder (MDD) patients and control participants.[36] Two family-based studies found no linkage of the 5-HTTLPR and bipolar disorder.[37,38] One study reported that the higher activity genotypes are associated with increased incidence of major depressive disorder in the presence of environmental trauma.[39] Laucht et al.[40] investigated gene × environment interaction between 5-HTTLPR and exposure to environmental adversity, using different phenotypic and genotypic characterizations as well as different types of adversity within a prospective study design. Individuals with the L/L genotype exposed to high family adversity displayed significantly higher rates of depressive or anxiety disorders and had more depressive symptoms than those without either condition. Another study showed similar results reporting that the individuals with the L/L genotype and exposed to high family adversity or other negative life events displayed significantly higher rates of depressive or anxiety disorders and could be susceptible to MDD.[41]
Efforts to identify an association of the 5-HTT intron 2 polymorphism with affective disorder have yielded mixed results. One group has found an increase of the rare 9-repeat allele in unipolar depression in Scottish populations, supporting its contribution to affective disorder in only a small subset of patients.[18,42] However, there are reports that have failed to replicate this finding in bipolar depression or MDD.[34,43,44] An association of the 12-repeat allele with depression has also been reported.[35] A meta-analysis that included the results of the studies described above found no involvement of the intron 2 VNTR in unipolar or bipolar depression.[45] An analysis of haplotype distribution of the two polymorphisms showed significant differences between cases and controls, reporting an increase in of S.STin2.10 haplotype in patients with MDD.[27] A study investigating the association of STin2 polymorphism and cognitive dysfunction in major depression suggested that the presence of STin2.10 and an absence of STin2.12 allele may be related to a possible genetic endophyte for characteristic cognitive dysfunctions detected in MDD.[39] A recent meta-analysis of 24 independent samples concluded that there was a strong evidence of significant association between the 5-HTTLPR genotype and unipolar depression.[46] However, this report, as pointed out by Munafς et al.[47] did not include data from the gene × environment interaction studies, and only included studies published up to 2006.
Association between variations in the serotonin transporter gene and the development of depression in response to life stress was first elucidated by Caspi and colleagues in 2003.[48] Today the gene-environment interaction of 5-HTTLPR and stress has become one of the most replicated findings in psychiatric genetics using different populations and different designs. A series of studies have shown that the 5-HTTLPR consistently interacts with childhood maltreatment and medical illness to predict depression.[49–54] On the contrary, when stressful life events (SLE) are chosen as the environmental variable, this yields controversial results, possibly reflecting the less stringent definition of the included stressors with a wide range in quantity and quality, duration, and timing.[48,49,55] Recent negative meta-analyses raised attention to crucial aspects of study design in gene × environment interactions. Contrary to the results of the smaller two earlier meta-analyses by Risch et al.[56] and Munafò et al.[47] the most recent large meta-analysis by Karg and colleagues[57] found strong evidence that the studies published to date support the hypothesis that 5-HTTLPR moderates the relationship between stress and depression. They argue that even the larger one of the two meta-analyses included only 14 of the 56 studies that had assessed the relationship between 5-HTTLPR, stress, and depression. They suggest that the difference in results between their own meta-analyses and the earlier two was due to the different set of included studies rather than the meta-analytic technique.[57] It has now become evident that the evaluation of the environmental stressors has to be as detailed as possible for the genetic polymorphisms. Though the biological/molecular mechanism behind gene-environment interactions has not yet been determined, a plausible model implies allele-specific moderation of experience-dependent alteration of epigenetic marks, such as deoxyribonucleic acid methylation or histone modification.[49] In addition, epigenetic memory of environmental exposure can be allele specific,[58] and may thus serve to integrate genetic vulnerability and environmental exposure to define behavioral phenotypes.[49]
Evidence suggests that marked inter-individual variation in antidepressant treatment response is likely to be related to biological distinction in phenotypically similar patients. The search for markers that define patho-physiologically similar patients who will respond to similar treatment strategies is therefore significantly important in the goal to optimize antidepressant treatment response by more personalized treatment algorithms.[49,59,60–63] The serotonin transporter gene promoter polymorphism (5-HTTLPR) has been repeatedly associated with antidepressant response in mood disorder patients, but findings are not consistent across studies. This effect is quite robust to ethnic differences although a significant heterogeneity is present in Asian samples.[64]
In one of the first allelic variation and common psychiatric disorder studies among Indian population, Mukherji et al.[65] observed that serotonin transporter allele frequencies in the Indian population differed from those in the other populations. Genomic analysis of clinical response to sertraline across two ethnicities for the long and short allele variants of the 5-HTTLPR polymorphism showed a significant ethnic difference for distributions of alleles and genotypes between Japanese and Caucasian populations.[66] Higher rates of L/L genotype among Caucasian subjects were observed while Chinese subjects had higher frequencies of S/L and S/S genotypes. Comparison of the treatment response of 5-HTTLPR S/S genotype subjects with L/L and S/L subgroups showed no significant differences in the Hamilton depression rating scale scores, clinical global impressions (CGI) scores, response rates, adverse effects, and sertraline plasma concentrations with sertraline treatment at week 6.[66] The negative correlation could also be the result of a methodological fallacy of comparing the L/L and S/L genotypes together, with S/S genotype subgroup allele.[67]
Robust scientific data have suggested that attenuated promoter segment of S allele, whether single or in pair, is associated with reduced transcription and functional capacity of serotonin transporter relative to the L allele. The S allele has in general been reported to be associated with a poorer response to various antidepressant treatments.[68–73] A double-blind, randomized study investigating the influences of 5-HTTLPR polymorphism on the efficacy and tolerability of mirtazapine and paroxetine has reported that the S allele is associated with a poor outcome after treatment with selective serotonin reuptake inhibitors.[74] A pilot study by Margoob et al.[67] to test the association between selective serotonin reuptake inhibitor (SSRI) treatment response in major depressive disorders and the allelic variation of the serotonin transporter gene also supports a relationship between serotonin transporter gene polymorphism and the efficacy of SSRIs. Fifty-seven consecutive patients with unipolar depressive episode, genotyped for the 5-HTT gene polymorphism and treated with a fixed-dose, 20 mg/day escitalopram showed a poorer response to SSRI treatment among short (S/S) variant, as well as heterozygous LS genotype at 6 weeks.
On the contrary, the studies undertaken among Japanese and Korean populations have reported that the S allele is associated with more favorable treatment outcomes.[75–77] Smits et al.[78] conducted a systematic review of the literature on the influence of polymorphisms in the serotonin transporter gene (5-HTTLPR and STin2) on SSRI response and reported a less favorable response to SSRI treatment among Caucasian patients with the 5-HTTLPR S/S genotype and among (Asian) patients with the STin2 10/12 genotype. A meta-analysis performed on 15 studies by Serretti et al. (2007)[64] observed a significant association of the S/S variant of 5-HTTLPR with remission rate and of both S/S and S/L variants with response rate. A report by Keers et al.[79] suggests that patients with a history of SLE and carrying the L allele of 5-HTTLPR might benefit from treatment with SSRIs to a greater extent than from Tri-cyclic antidepressants (TCAs). Individuals carrying the S allele of this polymorphism and exposed to child abuse might especially profit from psychotherapy.[80]
POST-TRAUMATIC STRESS DISORDER
Lesch et al.[13] reported that the individuals possessing one or two copies of the S allele, 5-HTTLPR allele, had higher levels of depression and suicidality in the context of recent life stressors. Caspi and colleagues’[81] population-based study of 549 male and female twins with a mean age at participation of 34.9 years suggested that individuals with two short (S) alleles at the 5-HTT locus were more sensitive to the depressogenic effects of all SLE than those with one or two long (L) alleles. When the level of SLE-associated threat was examined, the interaction between genotype and SLE resulted from an increased sensitivity of SS individuals to the depressogenic effects of common low-threat events.[82] A study conducted in a Korean sample reported an excess of S/S genotypes in Korean PTSD patients compared with normal controls.[83] Our own study has also shown an association of the S allele with PTSD.[84]
Kilpatrick et al.[85] found that the S allele increased the risk of post-hurricane PTSD in a sample of persons exposed to the 2004 Florida hurricanes only under the conditions of high hurricane exposure and low social support. Of high risk (n=27) were those with the S/S genotype, low social support, and high hurricane exposure. Medium risk individuals (n=54) were those with the S/L genotype with low social support and high hurricane exposure. All others (n=498) were of low risk. There was a strong association between risk group and prevalence of PTSD. High-risk individuals had 4.5 times the risk of developing PTSD as compared to low-risk individuals. A study examining whether the features of the social environment (county-level crime rate and unemployment) modified the association between 5-HTTLPR variants and risk of current PTSD in a sample of 590 participants indicated that the S allele of the 5-HTTLPR polymorphism was associated with decreased risk of PTSD in low-risk environments, but increased the risk of PTSD in high-risk environments.[86]
In a new line of research, both the serotonin and serotonin transporter gene have been found to impact brain structure and function. The evidence for serotonin involvement in hippocampal changes comes from studies of psychosocial stress in rats, in which remodeling of dendrites is accompanied by the downregulation of 5-HTT expression in the hippocampus, indicating either a reduced density of serotonin terminals or a reduced expression of the 5-HTT.[87] Moreover, repeated restraint stress and psychosocial stress in rats suppresses the expression of the inhibitory 5-HT1A receptor in the hippocampus.[87,88] These animal studies convincingly demonstrate bidirectional interactions between the hypothalamic-pituitary-adrenal (HPA) axis and the serotonin system. The finding that individuals with the S allele are at greater risk for depression following stress than are L allele homozygotes has been replicated by other groups.[89,90]
The hypothesis that variation in 5-HTT gene function increases vulnerability to environmental stress finds strong support from research using animal models. Rhesus macaques carrying an ortholog of the 5-HTTLPR S allele have been found to exhibit exaggerated behavioral and neuroendocrine responses to stress and abnormalities in 5-HT metabolism, but only when reared in a stressful environment.[91,92] This involvement of 5-HT in stress coping is supported by complex interactions between 5-HT and the neuroendocrine stress system.[93] Acute stress increases 5-HT neurotransmission[94,95] which promotes stress adaptation by mediating negative feedback control of cortisol on the HPA axis.[96,97] In accordance with the current findings, S allele carriers are thought to be 5-HT vulnerable and susceptible to depression symptoms particularly in the face of stress.[81,96,98] Recent studies have begun to shed light on how the relationship between the 5-HTTLPR S allele and stress reactivity is mediated at the level of the neural pathways regulating emotion. In functional neuroimaging, there is increased amygdala activation in response to emotionally negative stimuli in the individuals with an S/S or S/L genotype, which is consistent with the structural findings. Numerous studies have replicated the findings of increased amygdala reactivity in both healthy and depressed adults. Using the sensitivity of non-invasive neuroimaging such as functional magnetic resonance imaging (fMRI), Hariri and colleagues[99] assessed neural activation in a relatively small number of S allele carriers during perceptual processing of fearful and angry human facial expressions. During the task, S allele carriers exhibited nearly fivefold greater amygdala activity than L homozygotes, a difference accounting for 20% of the total variance in the amygdala response to fearful and angry faces. A single study, using a measure of biased attention, found increased vigilance for threat-related words in S allele carriers among a psychiatric inpatient group and an evidence for a strong positive bias in the L/L group such that vigilance for positive material was observed among those homozygous for L allele in addition to a clear avoidance of negative material.[100] This protective pattern was completely absent in the S allele carriers.[100] Canli et al.[101] reported that resting activation in the amygdala and hippocampus increases with increasing life stress for S/S and S/L, but decreases with increasing life stress in the L/L group. Likewise, findings from other neuroimaging studies indicate the neural correlates of the relation found between 5-HTTLPR and attentional bias for threat. It has been recently shown that the short allele of the 5-HTTLPR is related to a deficient interplay between prefrontal sites and amygdaloid nuclei.[99] This effect of the 5-HTTLPR is probably bounded to the effects of serotonin on neuro-developmental shaping.[102,103] The examination of the serotonin transporter polymorphism on functional neuroimaging may contribute to the eventual development of a “phenotypic assay” for vulnerability to PTSD. Dhuha et al.[104] have conducted what we believe to be the first study of the influence of 5-HTTLPR polymorphisms on PTSD outcomes following treatment with an SSRI (the only drug approved by Food and drug association for treatment of PTSD). Association between 5-HTTLPR genotype (S/S vs. S/L vs. L/L) and sertraline treatment outcome in PTSD was examined in 226 Indian patients. Relative to the S/S and S/L 5-HTTLPR genotypes, the L/L genotype was associated with greater responsiveness to sertraline (100 mg/day) and with lower dropout due to adverse events, suggesting that 5-HTTLPR genotyping may have the potential to predict SSRI treatment outcomes in patients with PTSD.
OBSESSIVE-COMPULSIVE DISORDER
OCD is characterized by repetitive, intrusive thoughts, images, and impulses and by repetitive, ritualistic physical or mental acts performed to reduce the attendant anxiety. It has been suggested since long that serotoninergic system is involved in the pathophysiology of OCD.[105–107] Mc Dougle et al.[108] used a family-controlled TDT with a set of 34 European American family trios of OCD and found the association and linkage disequilibrium between the 5-HTTLPR L allele and OCD. They also reported that a poor response to SSRIs might be related with the L allele. Bengel et al.[109] recruited 75 Caucasian OCD patients and 397 ethnically matched individuals from a non-patient control group and found that the patients with OCD were more likely than controls to carry two copies of the L allele. However, Kinner et al.[110] did not find any significant association between the distribution of the 5-HTTLPR genotypes and OCD in 54 OCD patients of Afrikaner descent and 82 ethnically matched controls. Camarena et al.[111] analyzed the 5-HTTLPR polymorphic system in 115 Mexican OCD patients and 136 controls and could not find a significant association between the 5-HTTLPR polymorphism and OCD in both cases and controls. A study conducted on German and French population also did not find a significant association between 5-HTTLPR polymorphism and OCD.[112] In Korea, there has been only one study of the association between 5-HTTLPR polymorphism and OCD. This study genotyped 124 OCD patients and 171 normal controls, but could not find any association between 5-HTTLPR polymorphism and the development of OCD.[113] A study by Perez et al.[114] revealed that there were higher frequencies of the S/S genotype among OCD group when compared to the controls. A recent meta-analysis examining the association between the 5-HTTLPR polymorphism and OCD in case-control studies also found a positive association between S allele homozygosity and susceptibility to OCD and an inverse association between heterozygosity at the 5-HTTLPR locus and OCD susceptibility.[115] One very recent study conducted on Indian population found no association of OCD with the 5-HTTLPR or the STin.2 polymorphism and no significant association of OCD with the 5-HTTLPR genotype even on inclusion of the LA and LG alleles.[116]
SUICIDE
Arato et al.[117] were the first to produce evidence of localized 5-HTT binding changes in suicide victims compared to normal subjects. They reported that suicide victims had significantly higher 5-HTT binding in the left frontal hemisphere compared to the right, whereas normal controls had higher binding in the right compared to the left. There were, however, no hemispheric differences when suicides were compared to the control group. Others have failed to replicate this finding of reversed laterality in the frontal cortex of suicide completers.[118,119] Additional evidence has recently emerged, suggesting that 5-HTT binding alterations in suicide victims may be distinct from depressed patients. In an elegant study, Arango et al.[120] demonstrated that 5-HTT binding in suicide completers is relatively localized to the ventrolateral aspect of the PFC when compared to normals. Ohara et al.[121] conducted a study in Asian population and failed to demonstrate any link between suicidal behavior and serotonin transporter gene genotype or 5-HTTLPR allele frequency. One study demonstrated a higher frequency of the L allele in depressed suicide victims compared to non-suicidal controls.[122] This finding was supported by another group which found that subjects with the L/L genotype had significantly higher scores on Beck's Hopelessness Scale and Beck's Scale for Suicide Ideation than subjects with either the L/S or S/S genotype.[123] A study by Chong et al.[124] examined the association of suicidal behavior in Chinese schizophrenic patients and reported no significant genotypic or allelic association of the 5-HTTLPR polymorphism with history of attempted suicide. These results were supported by several other studies that did not find any association of the 5-HTTLPR with suicide.[125–127] A pilot study by Baca-Garcia et al.[128] recruited 180 suicide attempters and 212 controls from Spanish population to test the gender specificity of the association between suicide attempts and a polymorphism in the promoter area of the serotonin transporter with two allelic variants, L variant and S variant. They found that the S allele was significantly overrepresented in female attempters when compared with female controls and male attempters. Lethality appeared to have a significant influence on the effects of the genotype in suicide since S females were overrepresented among non-lethal female attempters. Hranilovic et al.[23] investigated a sample of 135 suicide victims and 299 healthy control subjects of Croatian/southern Slavic origin for a possible association of 5-HTTLPR and STin.2 polymorphism with suicidal behavior. No significant differences in genotype and allele frequency distributions of both the polymorphisms were found between suicide victims and healthy control subjects. However, a tendency toward an increase of L allele and STin2.10allele was observed in the suicide group. Further analysis of distribution of estimated haplotype frequencies revealed differences between suicide victims and control subjects, with an excess of haplotype L10 among suicide victims. A recent study performed the analysis of the promoter 5-HTTLPR and intron 2 polymorphisms and haplotypes of the serotonin transporter gene in Russian suicide attempters separately in men and women and indicated the contribution of the 5-HTT gene to susceptibility for suicidal behavior in women, but not in men and observed that the L/L genotype and L10 haplotype were associated with suicide in women only.[129] Roy et al.[130] examined whether the 5-HTTLPR polymorphism alone or interacting with childhood trauma was predictive of suicidal behavior in 306 abstinent male African-American substance-dependent patients, a clinical population that is at high risk of suicide, as well as childhood trauma and other stress. The distribution of 5-HTTLPR genotypes did not differ between patients and controls, or between suicide attempters and non-attempters.
AUTISM
Cook and co-workers[131] were the first to identify linkage of the S allele of the 5-HTTLPR gene polymorphism in autism. However, other family-based TDT studies, including subgroup analyses, support preferential transmission of the L allele.[132,133] Betancur et al.[134] performed a meta-analysis of 5-HTTLPR and autism with data from four family-based association studies plus their own sample. They found no association of either the short or long allele with autistic disorder. Conroy et al.[135] demonstrated that the short allele was associated with autism in Irish population. Devlin et al.[136] summarized 12 published family-based studies of the transmission of 5-HTTLPR alleles to autistic individuals. Seven of the 12 studies reported significant transmission bias of 5-HTTLPR alleles, with an over transmission of the S allele in 4 and of the L allele in 3 studies. Koishi et al.[137] summarized 13 family-based studies investigating transmission of 5-HTTLPR alleles in autistic individuals, of which 10 studies overlapped with those examined by Devlin et al.[136] They reported more non-significant than significant associations (7 vs. 6). Among the six studies reporting significant associations, S and L alleles were equally reported as over transmitted. Guhathakurta et al.[138] failed to establish any association or linkage of 5-HTTLPR with autism in the Indian population by case-control studies and family-based approaches; however, when a meta-analysis of all the available data, inclusive of the present study, was carried out, a highly significant preferential transmission of the S allele from parents to the affected offspring was observed. Huang and Susan[139] conducted a systematic review and meta-analysis to test for an association between autism and either or both of the 5-HTTLPR and STin2 VNTR polymorphisms and observed that the S allele of 5-HTTLPR and/or the STin2 12 allele of the VNTR are the specific risk alleles for autism. A recent study explored whether variants of two functional polymorphisms of 5-HTT gene were related to behavioral characteristics measured by the Autism Diagnostic Interview-Revised and Autism Diagnostic Observation Schedule.[140] An evidence of genotype-phenotype interactions on the Autism Diagnostic Interview-Revised was found with the S/L or S/S genotypes being rated as more severe on the subdomain “failure to use nonverbal communication to regulate social interaction,” and the L/L genotype being rated as more severe on the subdomain “stereotyped and repetitive motor mannerisms” and on an aggression measure. In contrast, on the Autism Diagnostic Observation Schedule, the long group was associated with greater severity on directed facial expressions and unusual sensory interests. However, no significant relationships were found between the intron 2 VNTR genotypes and subdomains or domains of symptoms on both the scales.[140]
ALCOHOL DEPENDENCE
Turker et al.[141] found that high alcohol tolerance is associated with the S allele, although only among individuals younger than 26 years. Tan et al.[142] examined alleles of an intronic VNTR polymorphism among heroin-dependent individuals of Chinese descent who lived in Singapore. These investigators found a significant difference in allele and genotype frequencies in relation to phenotype. A study showed a significantly higher frequency of the S allele only in early-onset Finnish alcoholics who were also habitually violent.[143] In contrast, a study by Ishiguro et al.[144] showed a lower frequency of the S allele among the subgroup of Japanese alcoholics with a past history of antisocial behavior while drinking. In another study, Schuckit et al.[145] found that individuals with low response to alcohol had a lower frequency of the S allele. Another study hypothesized an association of subtype of alchoholism with alleles of 5-HTTLPR and reported that early-onset alcoholics are more likely to have the L/L genotype, which results in increased 5-HTT number and function and reduced levels of intrasynaptic 5-HT.[146] Consistent with the finding of this study, Javors et al.[147] observed that early-onset alcoholics have increased uptake of 5-HT into platelets. A study in Japanese showed no association of 5-HTTLPR polymorphism with alcohol dependence, but a greater frequency of the S allele was found in alcoholics who binge drink compared with alcoholics who do not binge drink.[148] A study conducted in alcoholics showed a nonsignificant excess of S alleles compared with that in controls, although the small sample size limits interpretation of those findings.[149] Feinn et al.[150] conducted a meta-analysis of data from 17 published studies including 3489 alcoholics and 2325 controls and reported that the frequency of the short allele at 5-HTTLPR was significantly associated with alcohol dependence. Bleich et al.[151] investigated an association of this polymorphism with obsessive-compulsive alcohol craving in 124 male patients admitted for alcohol detoxification treatment and suggested that the long variant of the 5-HTTLPR polymorphism is associated with higher compulsive alcohol craving at the beginning of alcohol withdrawal. A recent study investigating the putative link between short allele and relapse showed that the S allele was significantly associated with relapse while no other factor that was measured played a significant role.[152] Mokrovic et al.[153] also tested polymorphisms of genes involved in 5-HT transport and turnover for their association with alcohol dependence in a case group of 59 males with type 2 alcoholism and a control group of 282 healthy males, both of Croatian origin, and found an increase in the frequencies of 10-repeat allele and 10/10 genotype in alcoholic patients.
OTHER DISORDERS
A twin study has reported that a considerable part of the human personality is governed by the genetic factors.[154] Lesch et al.[13] reported an association between the 5-HTTLPR and anxiety-related traits, neuroticism, and harm avoidance, and found the involvement of S allele with neuroticisms. Thereafter, the polymorphism of the serotonin transporter gene was extensively explored in the studies of depression and its trait marker, neuroticism.[155]
Various attempts have been made to confirm the association of 5-HTTLPR with anxiety-related traits. Although these studies have yielded conflicting results, S/S genotypes of the 5-HTT gene have been associated with higher scores on the neuroticism extraversion openess to experience (NEO) personality inventory (NEO-PI-R) on the neuroticism scale, which is based on anxiety and depressive symptoms, and estimates harm avoidance, based on anxiety symptoms.[156,157] However, another study reported an association of the 5-HTTLPR with anxiety-related traits but not with neuroticism.[105] Willis-Owen et al.[158] also did not find any association between 5-HTTLPR and neuroticism, which was further supported by many other studies.[159–161] Various meta-analyses conducted on the associations of 5-HTTLPR and personality traits also revealed conflicting results. Some identified a positive association with neuroticism but not with measures of harm avoidance.[162,163] Another found an opposite association, reporting a positive association of 5-HTTLPR with harm avoidance but not with neuroticism.[164]
Most recent research has started exploring the difference in brain circuitry involved with emotion processing and deficits associated with various psychopathological conditions vis-à-vis serotonin transporter gene polymorphism.[165–168] Essentially enormous work still needs to be done to understand how common genetic variants influence brain and behavior.
CONCLUSION
The results presented in this review demonstrate that though the role of 5-HT gene polymorphism has not been conclusively linked to any particular mental disorder, strong evidence does exist for its implication in many common mental disorders like mood disorders, PTSD, suicide, OCD, autism, etc. Though many studies suggest a relationship between 5-HTTLPR and psychiatric morbidity, a significant minority of research results are not consistent with such conclusions. Besides ethnogenetic variations in the sample of different studies, other confounding results that could contribute to the possible discrepancies in the result need to be considered before coming to any conclusion about the relationship of these allelic variants in the serotonin transporter gene to various mental disorders. To rule out a coincidental association, different phenotypes not only need to be measured but also need to be measured repeatedly as has also very recently been suggested by some other researchers.[164] The conflicting results from various studies may also partly be due to methodological differences. The most reliable way of obtaining reproducible results is through large well-designed primary studies as stated by Munafo et al.[164] Two functionally distinct subtypes of long allele A and G identified very recently, if included in such future functional polymorphisim investigations, can also help to clarify inconsistent findings of the 5-HTTLPR polymorphisim association studies. The long A allele subtype has been shown to be associated with high levels of the serotonin transporter Ribonucleic acid expression. This is consistent with the findings reporting that the long allele has threefold higher basal activity compared to S allele, which needs further exploration and duplication in future research endeavors. Findings suggesting genotype (5-HTTLPR polymorphism) related alterations in anatomy and function of an amygdala-cingulate feedback circuit to genetic susceptibility for depression are also quite interesting and need to be explored further. If consistent, such results not only may increase our understanding of the genetic and biological risk factors important for the manifestation of mental disorders like depression, anxiety, PTSD, alcohol dependence, etc., but also can be of immense help to improve the clinical care of a vast majority of patients suffering from such disorders. This may prove to be a significant step forward in the direction of tailoring treatment according to patient's specific current pathophysiology, as well as selectively and individually target the disturbed pathways. Due to the scarcity and heterogeneity of the studies, current information is insufficiently reliable as a basis for puting into practice genetic testing in the diagnostic work-up of the psychiatric patient.
ACKNOWLEDGMENTS
Our sincere thanks to Elsevier publishers for granting permission to republish the figure in our review article. The authors and the source of the material is cited below the figure legend.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared
REFERENCES
- 1.Trivedi JK, Jilani AQ. Pathway of psychiatric care. Indian J Psychiatry. 2011;53:97–8. doi: 10.4103/0019-5545.82530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rao TS, Ramesh BN, Vasudevaraju P, Rao K. Molecular biology research in neuropsychiatry: India's contribution. Indian J Psychiatry. 2010;52:120–7. doi: 10.4103/0019-5545.69223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Grover S, Dutt A, Avasthi A. An overview of Indian research in depression. Indian J Psychiatry. 2010;52:178–88. doi: 10.4103/0019-5545.69231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shrivastava A. Initiatives in biological research in Indian psychiatry. Indian J Psychiatry. 2010;52:110–9. doi: 10.4103/0019-5545.69222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Avasthi A, Grover S, Aggarwal M. Research on antidepressants in India. Indian J Psychiatry. 2010;52:341–54. doi: 10.4103/0019-5545.69263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rao TSS, Rao KSJ, Asha MR. Drooping genes v/s dancing genes. Indian J Psychiatry. 2009;51:167–8. doi: 10.4103/0019-5545.55080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bruno P, Guiard BP, Mostafa El Mansari ME, Dennis L, Murphy DL, Blier P. Altered response to the selective serotonin reuptake inhibitor escitalopram in mice heterozygous for the serotonin transporter: An electrophysiological and neurochemical study. Int J Neuropsychopharmacol. 2011;25:1–13. doi: 10.1017/S1461145711000484. [DOI] [PubMed] [Google Scholar]
- 8.Davies W, Isles AR, Wilkinson AS. Imprinted genes and mental dysfunction. Ann Med. 2001;33:428–36. doi: 10.3109/07853890108995956. [DOI] [PubMed] [Google Scholar]
- 9.Lewis M. Putting mind over matter: Rethink inking current strategies for unmasking the genetics of mental illness. Clin Gene. 2004;66:177–82. [Google Scholar]
- 10.Ozsarc N, Santha E, Hoffman BJ. Alternate non-coding exons support serotonin transporter mRNA expression in the brain and gut. J Neurochem. 2002;82:336–44. doi: 10.1046/j.1471-4159.2002.00964.x. [DOI] [PubMed] [Google Scholar]
- 11.Lasky-Su JA, Faraone SV, Stephen J, Glatt SJ, Tsuang MT. Meta-analysis of the association between two polymorphisms in the serotonin transporter gene and affective disorders. Am J Med Genet Part B(Neuropsychiatric Genetics) 2005;133:110–5. doi: 10.1002/ajmg.b.30104. [DOI] [PubMed] [Google Scholar]
- 12.Heils A, Mobner R, Lesch KP. The human serotonin transporter basic research and clinical implications. Neural Transm. 1997;104:1005–14. doi: 10.1007/BF01273314. [DOI] [PubMed] [Google Scholar]
- 13.Lesch KP, Bengel D, Heils A, Sabol SZ, Greenberg BD, Petri S, et al. Association of anxiety related traits with a polymorphism in the serotonin transporter gene regulatory region. Science. 1996;274:1527–31. doi: 10.1126/science.274.5292.1527. [DOI] [PubMed] [Google Scholar]
- 14.Karg K, Burmeister M, Shedden K, Sen S. The Serotonin Transporter Promoter Variant (5-HTTLPR), stress, and depression meta-analysis revisited: Evidence of genetic moderation. Arch Gen Psychiatry. 2011;68:444–54. doi: 10.1001/archgenpsychiatry.2010.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ramamoorthy S, Bauman Al, Moore KR, Han H, Yang-Feng T, Chang AS, et al. Antidepressant and cocaine sensitive human transporter-molecular cloning, expression and chromosomal localization. Proc Natl Acad Sci USA. 1993;90:2542–6. doi: 10.1073/pnas.90.6.2542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lesch KP, Woolzin BL, Murphy DL, Riederer P. Primary structure of human platelet serotonin (5HT) uptake site-identity with the brain 5-HT transporter. J Neurochem. 1993;60:2319–22. doi: 10.1111/j.1471-4159.1993.tb03522.x. [DOI] [PubMed] [Google Scholar]
- 17.Heils A, Teufel A, Petri S, Stober G, Riederer P, Bengel D, et al. The importance of the trends in this study cannot be allelic variation of human serotonin transporter gene expression. J Neurochem. 1996;66:2621–4. doi: 10.1046/j.1471-4159.1996.66062621.x. [DOI] [PubMed] [Google Scholar]
- 18.Nakamura M, Uneo S, Sano A, Tanabe H. The human serotonin transporter linked polymorphisim (5HTTLPR) shows ten novel allelic variants. Mol Psychiatr. 2000;5:32–8. doi: 10.1038/sj.mp.4000698. [DOI] [PubMed] [Google Scholar]
- 19.Gillihan JS, Rao H, Wang J, Detre AJ, Breland J, Sankoorika VM, et al. Serotonin transporter genotype modulates amygdala activity during mood regulation. Soc Cogn Affect Neurosci. 2010;5:1–10. doi: 10.1093/scan/nsp035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Luca VD, Tharmalingam S, King N, Straussy J, Bulgin N, Kennedy JL. Association study of a novel functional polymorphisim of the serotonin transporter gene in bipolar disorder and suicidal behaviour. Psychopharmacology. 2005;182:128–31. doi: 10.1007/s00213-005-0046-z. [DOI] [PubMed] [Google Scholar]
- 21.Goldman D, Hu X, Zhu G, Lipsky R, Murphy D. The serotonin transporter: New alleles, function and phenotype. In: 3rd Annual Pharmacogenetics in Psychiatry Meeting. 1994:16–7. [Google Scholar]
- 22.Ogilvie AD, Batterby S, Bubb VJ, Fink G, Harmar AJ, Goodwin GM, et al. Polymorphism in the serotonin transporter gene associated with susceptibility to major depression. Lancet. 1996;347:731–3. doi: 10.1016/s0140-6736(96)90079-3. [DOI] [PubMed] [Google Scholar]
- 23.Hranilovic D, Stefulji J, Furac I, Kubal M, Balija M, Jernej B. Serotonin transporter gene promoter (5-HTTLPR) and intron 2(VNTR) polymorhisim in Croation suicide victims. Biol Psychiatry. 2003;54:884–9. doi: 10.1016/s0006-3223(03)00179-3. [DOI] [PubMed] [Google Scholar]
- 24.Zalsman G, Huang Y, Oquendo MA, Burke AK, Hu X, Brent DA, et al. Association of a Triallelic Serotonin Promoter Region (5-HTTLPR) polymorphism with stressful life events and severity of depression. Am J Psychiatry. 2006;163:1588–93. doi: 10.1176/ajp.2006.163.9.1588. [DOI] [PubMed] [Google Scholar]
- 25.Thomas AJ, Hendrickson M, Pigot M, Ferriec IN, Perry E, Ince P, et al. A study of the serotonin transporter in the prefrontal cortex in late life depression and Alzheimer disease with and without depression. Neuropathol Appl Neurobiol. 2006;32:296–303. doi: 10.1111/j.1365-2990.2006.00728.x. [DOI] [PubMed] [Google Scholar]
- 26.Fergusson MD, Horwood LJ, Miller AL, Kenned AM. Life stress, 5-HTTLPR and mental disorder: Findings from a 30-year longitudinal study. Br J Psychiatry. 2011;198:129–35. doi: 10.1192/bjp.bp.110.085993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gutierrez B, Pintor L, Gasto C, Rosa A, Bertranpetit J, Vieta E, et al. Variability in the serotonin transporter gene and increased risk for major depression with melancholia. Hum Genet. 1998;103:319–22. doi: 10.1007/s004390050823. [DOI] [PubMed] [Google Scholar]
- 28.Collier DA, Arranz MJ, Sham P, Battersby S, Vallada H, Gill, et al. The serotonin transporter is a potential susceptibility factor for bipolar affective disorder. Neuro Rep. 1996;7:1675–9. doi: 10.1097/00001756-199607080-00030. [DOI] [PubMed] [Google Scholar]
- 29.Rosenthal NE, Mazzanti CM, Barnett RL, Hardin TA, Turner EH, Lam GK, et al. Role of serotonin transporter promoter repeat length polymorphism (5-HTTLPR) in seasonality and seasonal affective disorder. Mol Psychiatry. 1998;3:175–7. doi: 10.1038/sj.mp.4000360. [DOI] [PubMed] [Google Scholar]
- 30.Mynett-Johnson L, Kealey C, Claffey E, Curtis D, Bouchier-Hayes L, Powell C, et al. Multimarkerhaplotypes within the serotonin transporter gene suggest evidence of an association with bipolar disorder. Am J Med Genet. 2000;96:845–9. doi: 10.1002/1096-8628(20001204)96:6<845::aid-ajmg30>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- 31.Frodl T, Reinhold E, Koutsouleris N, Donohoe G, Bondy B, Reiser M, et al. Childhood stress, serotonin transporter gene and brain structures in major depression. Neuropsychopharmacology. 2010;35:1383–90. doi: 10.1038/npp.2010.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Minov C, Baghai TC, Schule C, Zwanzger P, Schwarz MJ, Zill P, et al. Serotonin - 2A receptor and transporter polymorphisms: Lack of association in patients with major depression. Neurosci Lett. 2001;303:119–22. doi: 10.1016/s0304-3940(01)01704-9. [DOI] [PubMed] [Google Scholar]
- 33.Seretti A, Cusin C, Lattuada E, Di Bella D, Catalano M, Smeraldi E. Serotonin transporter gene (5-HTTLPR) is not associated with depressive symptomatology in mood disorders. Mol Psychiatry. 1999;4:280–3. doi: 10.1038/sj.mp.4000485. [DOI] [PubMed] [Google Scholar]
- 34.Hoehe MR, Wendel B, Grunewald I, Chiaroni P, Levy N, Morris-Rosendahl DM, et al. Serotonin transporter (5-HTT) gene polymorphisms are not associated with susceptibility to mood disorders. Am J Med Genet. 1998;81:1–3. doi: 10.1002/(sici)1096-8628(19980207)81:1<1::aid-ajmg1>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- 35.Kunugi H, Hattori M, Kato T, Tatsumi M, Sakai T, Sasaki T, et al. Serotonin transporter gene polymorphisms: Ethnic difference and possible association with bipolar affective disorder. Mol Psychiatry. 1997;2:457–62. doi: 10.1038/sj.mp.4000334. [DOI] [PubMed] [Google Scholar]
- 36.Bozina N, Mihaljević-Peles A, Sagud M, Jakovljević M, Sertić J. Serotonin transporter polymorphism in Croatian patients with major depressive disorder. Psychiatr Danub. 2006;18:83–9. [PubMed] [Google Scholar]
- 37.Mundo E, Walker M, Tims H, Macciardi F, Kennedy JL. Lack of linkage disequilibrium between serotonin transporter protein gene (SLC6A4) and bipolar disorder. Am J Med. 2000;96:379–83. doi: 10.1002/1096-8628(20000612)96:3<379::aid-ajmg27>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 38.Esterling LE, Yoshikawa T, Turner G, Badner JA, Bengel D, Gershon E. Serotonin transporter (5-HTT) gene and bipolar affective disorder. Am J Med Genet. 1998;81:37–40. [PubMed] [Google Scholar]
- 39.Sarosi A, Gonda X, Balogh G, Domotor E, Szekely A, Hejjas K, et al. Association of the STin2 polymorphism of the serotonin transporter gene with a neurocognitive endophenotype in major depressive disorder. Prog Neuropsychopharmacol Biol Psychiatry. 2008;32:1667–72. doi: 10.1016/j.pnpbp.2008.06.014. [DOI] [PubMed] [Google Scholar]
- 40.Laucht M, Treutlein J, Blomeyer D, Buchmann AF, Schmid B, Becker K, et al. Interaction between the 5-HTTLPR serotonin transporter polymorphism and environmental adversity for mood and anxiety psychopathology: Evidence from a high-risk community sample of young adults. Int J Neuropsychopharmacol. 2009;20:1–11. doi: 10.1017/S1461145708009875. [DOI] [PubMed] [Google Scholar]
- 41.Zhang K, Xu Q, Xu Y, Yang H, Luo J, Sun Y, et al. The combined effects of the 5-HTTLPR and 5-HTR1A genes modulates the relationship between negative life events and major depressive disorder in a Chinese population. J Affect Disord. 2009;114:224–31. doi: 10.1016/j.jad.2008.07.012. [DOI] [PubMed] [Google Scholar]
- 42.Harmar AJ, Ogilvie AD, Battersby S, Smith CA, Blackwood DH, Muir WJ, et al. The serotonin transporter gene and affective disorder. Cold Spring Harb Symp Quant Biol. 1996;61:791–5. [PubMed] [Google Scholar]
- 43.Saleem Q, Ganesh S, Vijaykumar M, Reddy YC, Brahmachari SK, Jain S. Association analysis of 5HT transporter gene in bipolar disorder in the Indian population. Am J Med Genet. 2000;96:170–2. [PubMed] [Google Scholar]
- 44.Stober G, Heils A, Lesch KP. Serotonin transporter gene polymorphism and affective disorder. Lancet. 1996;347:1340–1. [PubMed] [Google Scholar]
- 45.Furlong RA, Ho L, Walsh C, Rubinsztein JS, Jain S, Paykel ES, et al. Analysis and meta-analysis of two serotonin transporter gene polymorphisms in bipolar and unipolar affective disorders. Am J Med Genet. 1998;81:58–63. [PubMed] [Google Scholar]
- 46.Lopez-Leon S, Janssens AC, Gonzalez-Zuloeta Ladd AM, Del-Favero J, Claes SJ, Oostra BA, et al. Meta-analyses of genetic studies on major depressive disorder. Mole Psychiatry. 2008;13:772–85. doi: 10.1038/sj.mp.4002088. [DOI] [PubMed] [Google Scholar]
- 47.Munafò MR, Freimer NB, Ng W, Ophoff R, Veijola J, Miettunen J, et al. 5-HTTLPR genotype and anxiety-related personality traits: A meta-analysis and new data. Am J Med Genet Part B Neuropsychiatr Genet. 2009;150:271–81. doi: 10.1002/ajmg.b.30808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Caspi A, Hariri AR, Holmes A, Uher R, Moffitt TE. Genetic sensitivity to the environment: The case of the serotonin transporter gene and its implications for studying complex diseases and traits. Am J Psychiatry. 2010;16:509–27. doi: 10.1176/appi.ajp.2010.09101452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Klengel T, Elisabeth B, Binder EB. Gene-environment interactions in mood disorders: Focus on unipolar depressio. Person Med. 2011;8:23–34. doi: 10.2217/pme.10.73. [DOI] [PubMed] [Google Scholar]
- 50.Brown GW, Harris TO. Depression and the serotonin transporter 5-HTTLPR polymorphism: A review and a hypothesis concerning gene-environment interaction. J Affect Disord. 2008;111:1–12. doi: 10.1016/j.jad.2008.04.009. [DOI] [PubMed] [Google Scholar]
- 51.Antypa N, van der Does AJ. Serotonin transporter gene, childhood emotional abuse and cognitive vulnerability to depression. Genes Brain Behav. 2010;9:615–20. doi: 10.1111/j.1601-183X.2010.00593.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ritchie K, Jaussent I, Stewart R, Dupuy AM, Courtet P, Ancelin ML, et al. Association of adverse childhood environment and 5-HTTLPR genotype with late-life depression. J Clin Psychiatry. 2009;70:1281–8. doi: 10.4088/JCP.08m04510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kaufman J, Yang BZ, Douglas-Palumberi H, Houshyar S, Lipschitz D, Krystal JH, et al. Social supports and serotonin transporter gene moderate depression in maltreated children. Proc Natl Acad Sci USA. 2004;10:17316–21. doi: 10.1073/pnas.0404376101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Uher R, McGuffin P. The moderation by the serotonin transporter gene of environmental adversity in the etiology of depression: Update. Mol Psychiatry. 2009;15:18–22. doi: 10.1038/mp.2009.123. [DOI] [PubMed] [Google Scholar]
- 55.Rutter M. Gene-environment interplay. Depress Anxiety. 2010;27:1–4. doi: 10.1002/da.20641. [DOI] [PubMed] [Google Scholar]
- 56.Risch N, Herrell R, Lehner T, Liang KY, Eaves L, Hoh J, et al. Interaction between the serotonin transporter gene (5-HTTLPR), stressful life events, and risk of depression: A meta-analysis. J Am Med Assoc. 2009;301:2462–71. doi: 10.1001/jama.2009.878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Karg K, Burmeister, Shedden K, Sen S. The serotonin transporter promoter variant (5-HTTLPR), stress, and depression meta-analysis revisited:evidence of genetic moderation. Arch Gen Psychiatry. 2011;68:444–54. doi: 10.1001/archgenpsychiatry.2010.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Meaburn EL, Schalkwyk LC, Mill J. Allele-specific methylation in the human genome Implications for genetic studies of complex diseases. Epigenetics. 2010;5:578–82. doi: 10.4161/epi.5.7.12960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Holsboer F. How can we realize the promise of personalized antidepressant medicines? Nat Rev Neurosci. 2008;9:638–46. doi: 10.1038/nrn2453. [DOI] [PubMed] [Google Scholar]
- 60.Binder EB, Holsboer F. Pharmacogenomics. Hand Exp Pharmacol. 2005;169:527–46. doi: 10.1007/3-540-28082-0_19. [DOI] [PubMed] [Google Scholar]
- 61.Malhotra AK, Murphy GM, Jr, Kennedy JL. Pharmacogenetics of psychotropic drug response. Am J Psychiatry. 2004;161:780–96. doi: 10.1176/appi.ajp.161.5.780. [DOI] [PubMed] [Google Scholar]
- 62.Kato M, Serretti A. Review and meta-analysis of antidepressant pharmacogenetic findings in major depressive disorder. Mol Psychiatry. 2010;15:473–500. doi: 10.1038/mp.2008.116. [DOI] [PubMed] [Google Scholar]
- 63.Horstmann S, Binder EB. Pharmacogenomics of antidepressant drugs. Pharmacol Ther. 2009;124:57–73. doi: 10.1016/j.pharmthera.2009.06.007. [DOI] [PubMed] [Google Scholar]
- 64.Serretti A, Kato M, De Ronchi D, Kinoshita T. Meta-analysis of serotonin transporter gene promoter polymorphism (5-HTTLPR) association with selective serotonin reuptake inhibitor efficacy in depressed patients 5-HTTLPR and SSRI meta-analysis. Mole Psychiatry. 2007;12:247–57. doi: 10.1038/sj.mp.4001926. [DOI] [PubMed] [Google Scholar]
- 65.Mukherjee O, Saleem Q, Purushottam M, Anand A, Brahmachari SK, Jain S. Common psychiatric diseases and human genetic variation. Commun Genet. 2002;5:171–7. doi: 10.1159/000066332. [DOI] [PubMed] [Google Scholar]
- 66.Ng HC, Easteal S, Tan S, Schweitzer I, Brian Kong Wai Ho KB, Aziz S. Serotonin transporter polymorphisms and clinical response to sertraline across ethnicities. Prog Neuropsychopharmacol Biol Psychiatry. 2006;30:953–7. doi: 10.1016/j.pnpbp.2006.02.015. [DOI] [PubMed] [Google Scholar]
- 67.Margoob MA, Mushtaq D, Murtza I, Mushtaq H, Ali A. Serotonin transporter gene polymorphism and treatment response to serotonin reuptake inhibitor (escitalopram) in depression: An open pilot study. Indian J Psychiatry. 2008;50:47–50. doi: 10.4103/0019-5545.39759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Pollock BG, Ferrell RE, Mulsant BH, Mazumdar S, Miller M, Sweet RA, et al. Allelic variation in the serotonin transporter promoter affects onset of paroxetine treatment response in late-life depression. Neuropsychopharmacology. 2000;23:587–90. doi: 10.1016/S0893-133X(00)00132-9. [DOI] [PubMed] [Google Scholar]
- 69.Zanardi R, Benedetti F, Di Bella D, Catalano M, Smeraldi E. Efficacy of paroxetine in depression is influenced by a functional polymorphism within the promoter of the serotonin transporter gene. J Clin Psychopharmacol. 2000;20:105–7. doi: 10.1097/00004714-200002000-00021. [DOI] [PubMed] [Google Scholar]
- 70.Zanardi R, Serretti A, Rossini D, Franchini L, Cusin C, Lattuada E, et al. Factors affecting fluvoxamine antidepressant activity: Influence of pindolol and 5-HTTLPR in delusional and nondelusional depression. Biol Psychiatry. 2001;50:323–3. doi: 10.1016/s0006-3223(01)01118-0. [DOI] [PubMed] [Google Scholar]
- 71.Arias B, Catalán R, Gastó C, Gutierrez B, Fañanás L. 5-HTTLPR polymorphism of the serotonin transporter gene predicts non-remission in major depression patients treated with citalopram in a 12-weeks follow up study. J Clin Psychopharmacol. 2003;23:563–7. doi: 10.1097/01.jcp.0000095350.32154.73. [DOI] [PubMed] [Google Scholar]
- 72.Rausch JL, Johnson ME, Fei YJ, Li JQ, Shendarkar N, Hobby HM, Ganapathy V, Leibach FH. Initial conditions of serotonin transporter kinetics and genotype: Influence on SSRI treatment trial outcome. Biol Psychiatry. 2002;51:723–32. doi: 10.1016/s0006-3223(01)01283-5. Rausch et al, 2002. [DOI] [PubMed] [Google Scholar]
- 73.Mandelli L, Marino E, Pirovano A, Calati R, Zanardi R, Colombo C, et al. Interaction between SERTPR and stressful life events on response to antidepressant treatment. Eur Neuropsychopharmacol. 2009;19:64–7. doi: 10.1016/j.euroneuro.2008.08.005. [DOI] [PubMed] [Google Scholar]
- 74.Murphy GM, Hollander BS, Rodrigues HE, Kremer C, Schatzberg F. A effects of the serotonin transporter gene promoter polymorphism on mirtazapine and paroxetine efficacy and adverse events in geriatric major depression. Arch Gen Psychiatry. 2004;61:1163–9. doi: 10.1001/archpsyc.61.11.1163. [DOI] [PubMed] [Google Scholar]
- 75.Yoshida K, Ito K, Sato K, Takahashi H, Kamata M, Higuchi H, et al. Influence of the serotonin transporter gene linked polymorphic region on the antidepressant response to fluvoxamine in Japanese depressed patients. Prog Neuropsychopharmacol Biol Psychiatry. 2002;26:383–6. doi: 10.1016/s0278-5846(01)00287-1. [DOI] [PubMed] [Google Scholar]
- 76.Kim DK, Lim SW, Lee S, Sohn SE, Kim S, Hahn CG, et al. Serotonin transporter gene polymorphisim and anti depressant response. Neuro Rep. 2000;11:215–9. doi: 10.1097/00001756-200001170-00042. [DOI] [PubMed] [Google Scholar]
- 77.Kang RH, Wong ML, Choi MJ, Paik JW, Lee MS. Association study of the serotonin transporter promoter polymorphism and mirtazapine antidepressant response in major depressive disorder. Prog Neuropsychopharmacol Biol Psychiatry. 2007;31:1317–21. doi: 10.1016/j.pnpbp.2007.05.018. [DOI] [PubMed] [Google Scholar]
- 78.Smits KM, Smits LJ, Schouten JS, Stelma FF, Nelemans P, Prins MH. Influence of SERTPR and STin2 in the serotonin transporter gene on the effect of selective serotonin reuptake inhibitors in depression: A systematic review. Mol Psychiatry. 2004;9:433–41. doi: 10.1038/sj.mp.4001488. [DOI] [PubMed] [Google Scholar]
- 79.Keers R, Uher R, Huezo-Diaz P, Smith R, Jaffee S, Rietschel M, et al. Interaction between serotonin transporter gene variants and life events predicts response to antidepressants in the GENDEP project. Pharmacogenomics J. 2011;11:138–45. doi: 10.1038/tpj.2010.14. [DOI] [PubMed] [Google Scholar]
- 80.Nemeroff CB, Heim CM, Thase ME, Klein DN, Rush AJ, Schatzberg AF, et al. Differential responses to psychotherapy versus pharmacotherapy in patients with chronic forms of major depression and childhood trauma. Proc Natl Acad Sci USA. 2003;100:14293–6. doi: 10.1073/pnas.2336126100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Caspi A, Sugden K, Moffitt TE, Taylor A, Craig I, Harrington H, et al. Influence of life stress on depression: Moderation by a polymorphismin the 5-HTT gene. Science. 2003;301:386–9. doi: 10.1126/science.1083968. [DOI] [PubMed] [Google Scholar]
- 82.Kendler KS, Kuhn JW, Vittum J, Prescott CA, Riley B. The interaction of stressful life events and a serotonin transporter polymorphism in the prediction of episodes of major depression: A replication. Arch Gen Psychiatry. 2005;62:529–35. doi: 10.1001/archpsyc.62.5.529. [DOI] [PubMed] [Google Scholar]
- 83.Lee HJ, Lee MS, Kang RH, Kim H, Kim SD, Kee BS, et al. Influence of the serotonin transporter promoter gene polymorphism on susceptibility to posttraumatic stress disorder. Depress Anxiety. 2005;21:135–9. doi: 10.1002/da.20064. [DOI] [PubMed] [Google Scholar]
- 84.Murtaza I, Margoob MA, Dhuha M, Huda M, Ahmad I, Ali A. A preliminary investigation on serotonin transporter gene regulatory region polymorphism in posttraumatic stress disorders. JK Practit. 2006;13:S66–8. [Google Scholar]
- 85.Kilpatrick DG, Koenen KC, Ruggiero KJ. The serotonin transporter genotype and social support and moderation of posttraumatic stress disorder and depression in hurricane exposed adults. Am J Psychiatry. 2007;164:1693–9. doi: 10.1176/appi.ajp.2007.06122007. [DOI] [PubMed] [Google Scholar]
- 86.Koenen KC, Stellman SD, Dohrenwend BP, Sommer JF, Jr, Stellman JM. The consistency of combat exposure reporting and course of PTSD in Vietnam War veterans. J Trauma Stress. 2007;20:3–13. doi: 10.1002/jts.20191. [DOI] [PubMed] [Google Scholar]
- 87.McKittrick CR, Magarinos AM, Blanchard DC, Blanchard RJ, McEwen BS, Sakai RR. Chronic social stress reduces dendritic arbors in CA3 of hippocampus and decreases binding to serotonin transporter sites. Synapse. 2000;36:85–94. doi: 10.1002/(SICI)1098-2396(200005)36:2<85::AID-SYN1>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- 88.Flugge G. Dynamics of central nervous 5-HT1A-receptors under psychosocial stress. J Neurosci. 1995;15:7132–40. doi: 10.1523/JNEUROSCI.15-11-07132.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Surtees PG, Wainright NW, Willis Owen SA, Luben R, Day NE, Flint J. Social diversity, the serotonin transporter (5HTTLPR) Polymorhisim and Major depressive disorder. Biol Psychiatry. 2006;61:59224–9. doi: 10.1016/j.biopsych.2005.07.014. [DOI] [PubMed] [Google Scholar]
- 90.Eley TC, Sugden K, Corsico A, Gregory AM, Sham P, McGuffin P, et al. Gene-environment interaction analysis of serotonin system markers with adolescent depression. Mol Psychiatry. 2004;9:908–15. doi: 10.1038/sj.mp.4001546. [DOI] [PubMed] [Google Scholar]
- 91.Barr CS, Newman KL, Schwandt M, Shannon C, Dvoskin RL, Lindell SG, et al. Sexual dichotomy of an interaction between early adversity and the serotonin transporter gene promoter variant in rhesus macaques. Proc Natl Acad Sci U S A. 2004;101:12358–63. doi: 10.1073/pnas.0403763101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Champoux M, Bennett A, Shannon C, Higley JD, Lesch KP, Suomi SJ. Serotonin transporter gene polymorphism, differential early rearing, and behavior in rhesus monkey neonates. Mol Psychiatry. 2002;7:1058–63. doi: 10.1038/sj.mp.4001157. [DOI] [PubMed] [Google Scholar]
- 93.Porter RJ, Gallagher P, Watson S, Young AH. Corticosteroidserotonin interactions in depression: A review of the human evidence. Psychopharmacology (Berl) 2004;173:1–17. doi: 10.1007/s00213-004-1774-1. [DOI] [PubMed] [Google Scholar]
- 94.Davis S, Heal DJ, Stanford SC. Long-lasting effects of an acute stress on the neurochemistry and function of 5-hydroxytryptaminergic neurones in the mouse brain. Psychopharmacology (Berl) 1995;118:267–72. doi: 10.1007/BF02245954. [DOI] [PubMed] [Google Scholar]
- 95.De Kloet ER, Versteeg DH, Kovacs GL. Aldosterone blocks the response to corticosterone in the raphe-hippocampal serotonin system. Brain Res. 1983;264:323–7. doi: 10.1016/0006-8993(83)90834-x. [DOI] [PubMed] [Google Scholar]
- 96.Van Praag HM. Can stress cause depression? Prog Neuropsychopharmacol Biol Psychiatry. 2004;28:891–907. doi: 10.1016/j.pnpbp.2004.05.031. [DOI] [PubMed] [Google Scholar]
- 97.Nuller JL, Ostroumova MN. Resistance to inhibiting effect of dexamethasone in patients with endogenous depression. Acta Psychiatr Scand. 1980;61:169–77. doi: 10.1111/j.1600-0447.1980.tb00576.x. [DOI] [PubMed] [Google Scholar]
- 98.Firk C, Markus CR. Review: Serotonin by stress interaction: A susceptibility factor for the development of depression? J Psychopharmacol. 2007;21:538–44. doi: 10.1177/0269881106075588. [DOI] [PubMed] [Google Scholar]
- 99.Hariri AR, Mattay VS, Tessitore A, Kolachana B, Fera F, Goldman D, et al. Serotonin transporter genetic variation and the response of the human amygdala. Science. 2002;297:400–3. doi: 10.1126/science.1071829. [DOI] [PubMed] [Google Scholar]
- 100.Beevers CG, Gibb BE, McGeary JE, Miller IW. Serotonin transporter genetic variation and biased attention for emotional word stimuli among psychiatric inpatients. J Abnorm Psychol. 2007;116:208–12. doi: 10.1037/0021-843X.116.1.208. [DOI] [PubMed] [Google Scholar]
- 101.Canli T. Neural correlates of epigenesis. Proc Natl Acad Sci U S A. 2006;103:16033–8. doi: 10.1073/pnas.0601674103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Hariri AR, Mattay VS, Tessitore A, Kolachana B, Fera F, Goldman D, et al. Serotonin transporter genetic variation and the response of the human amygdala. Science. 2002;297:400–3. doi: 10.1126/science.1071829. [DOI] [PubMed] [Google Scholar]
- 103.Lesch KP. Variation of serotonergic gene expression: Neurodevelopment and the complexity of response to psychopharmacologic drugs. Eur Neuropsychopharmacol. 2001;11:457–74. doi: 10.1016/s0924-977x(01)00123-7. [DOI] [PubMed] [Google Scholar]
- 104.Mushtaq D, Ali A, Margoob MA, Murtaza I, Andrade C. Association between serotonin transporter promoter polymorphism and sertraline treatment response in post traumatic stress disorder. J Affect Disord. 2011 doi: 10.1016/j.jad.2011.08.033. in press. [DOI] [PubMed] [Google Scholar]
- 105.Mazzanti CM, Lappalainen J, Long JC, Bengel D, Naukkarinen H, Eggert M, et al. Role of the serotonin transporter promoter polymorphism in anxiety-related traits. Arch Gen Psychiatry. 1998;55:936–40. doi: 10.1001/archpsyc.55.10.936. [DOI] [PubMed] [Google Scholar]
- 106.Pauls DL, Alsobrook J, Goodman WK, Rasmussen SA, Leckman J. A family study of obsessive-compulsive disorder. Am J Psychiatry. 1995;152:76–84. doi: 10.1176/ajp.152.1.76. [DOI] [PubMed] [Google Scholar]
- 107.Bastani B, Arora RC, Meltzer HY. Serotonin uptake and imipramine binding in the blood platelets of obsessive compulsive disorder patients. Biol Psychiatry. 1991;30:131–9. doi: 10.1016/0006-3223(91)90166-j. [DOI] [PubMed] [Google Scholar]
- 108.McDougle C, Naylor S, Cohen D, Volkmar F, Heninger G, Price L. A double blind, placebo-controlled study of Fluvoxamine in adults with autistic disorder. Arch Gen Psychiatr. 1996;53:1001–8. doi: 10.1001/archpsyc.1996.01830110037005. [DOI] [PubMed] [Google Scholar]
- 109.Bengel D, Greenberg BD, Cora-Locatelli G, Altemus M, Heils A, Li Q, et al. Association of the serotonin transporter promoter regulatory region polymorphism and obsessive-compulsive disorder. Mol Psychiatry. 1999;4:463–6. doi: 10.1038/sj.mp.4000550. [DOI] [PubMed] [Google Scholar]
- 110.Kinnear CJ, Niehaus DJ, Smook JC, Toit PL, Krandenberg JV, Weyers JB, et al. Obssesive compulsive disorder and the promoter region polymorphisim (5HTTLPR) in the serotonin transporter gene (SLC6A4): A negative association study in the Afrikaner population. Int J Neuropsychopharmacol. 2002;3:327–31. doi: 10.1017/S1461145700002054. [DOI] [PubMed] [Google Scholar]
- 111.Camarena B, Rinetti G, Cruz C, Hernandez S, de la Fuente JR, Nicolini H. Association study of the serotonin transporter gene polymorphism in obsessive-compulsive disorder. Int J Neuropsychopharmacol. 2001;4:269–72. doi: 10.1017/S1461145701002516. [DOI] [PubMed] [Google Scholar]
- 112.Chabane N, Millet B, Delorme R, Lichtermann D, Mathieu F, Laplanche JL, et al. Lack of evidence for association between serotonin transporter gene (5-HTTLPR) and obsessive-compulsive disorder by case control and family association study in humans. Neurosci Lett. 2004;363:154–6. doi: 10.1016/j.neulet.2004.03.065. [DOI] [PubMed] [Google Scholar]
- 113.Kim SJ, Lee HS, Kim CH. Obsessive-compulsive disorder, factor analyzed symptom dimensions and serotonin transporter polymorphism. Neuropsychobiol. 2005;4:176–82. doi: 10.1159/000088860. [DOI] [PubMed] [Google Scholar]
- 114.Perez M, Brown JS, Vrshek-Schallhorn S, Johnson F, Joiner TE., Jr Differentiation of obsessive-compulsive-, panic-, obsessive-compulsive personality-, and non-disordered individuals by variation in the promoter region of the serotonin transporter gene. J Anxiety Disord. 2006;20:794–806. doi: 10.1016/j.janxdis.2005.09.001. [DOI] [PubMed] [Google Scholar]
- 115.Lin PY. Meta analysis of the association of serotonin transporter gene polymorphism with OCD realated disorders. Prog Neuropsychopharmacol Biol Psychiatry. 2007;31:683–9. doi: 10.1016/j.pnpbp.2006.12.024. [DOI] [PubMed] [Google Scholar]
- 116.Tibrewal P, Kumar HB, Shubha GN, Subhashree D, Purushottam M, Thennarasu K, et al. Association of serotonin transporter gene polymorphisms with obsessive-compulsive disorder (OCD) in a south Indian population. Indian J Med Res. 2010;132:690–5. [PMC free article] [PubMed] [Google Scholar]
- 117.Arato M, Tekes K, Tothfalusi L, Magyar K, Palkovits M, Demeter E, et al. Serotonergic split brain and suicide. Psychiatry Res. 1987;21:355–6. doi: 10.1016/0165-1781(87)90019-9. [DOI] [PubMed] [Google Scholar]
- 118.Arora RC, Meltzer HY. Laterality and 3H-imipramine binding: Studies in the frontal cortex of normal controls and suicide victims. Biol Psychiatry. 1991;29:1016–22. doi: 10.1016/0006-3223(91)90358-s. [DOI] [PubMed] [Google Scholar]
- 119.Lawrence KM, De Paermentier F, Cheetham SC, Crompton MR, Katona CL, Horton RW. Symmetrical hemispheric distribution of 3H-paroxetine binding sites in post-mortem human brain from controls and suicides. Biol Psychiatry. 1990;28:544–6. doi: 10.1016/0006-3223(90)90492-k. [DOI] [PubMed] [Google Scholar]
- 120.Arango V, Huang YY, Underwood MD, Mann JJ. Genetics of the serotonergic system in suicidal behavior. J Psychiatr Res. 2003;37:375–86. doi: 10.1016/s0022-3956(03)00048-7. [DOI] [PubMed] [Google Scholar]
- 121.Ohara K, Nagai M, Tsukamoto T, Tani K, Suzuki Y. Functional polymorphism in the serotonin transporter promoter at the SLC6A4 locus and mood disorders. Biol Psychiatry. 1998;44:550–4. doi: 10.1016/s0006-3223(98)00112-7. [DOI] [PubMed] [Google Scholar]
- 122.Du L, Faludi G, Palkovits M, Demeter E, Bakish D, Lappierre YD. Frequency of long allele in serotonin transporter gene is increased in depressed suicide victims. Biol Psychiatry. 1999;46:196–201. doi: 10.1016/s0006-3223(98)00376-x. [DOI] [PubMed] [Google Scholar]
- 123.Russ MJ, Lachman HM, Kashdan T, Saito T, Bajmakovic-Kacila S. Analysis of catechol-O-methyltransferase and 5-hydroxytryptamine transporter polymorphisms in patients at risk for suicide. Psychiatry Res. 2000;93:73–8. doi: 10.1016/s0165-1781(00)00128-1. [DOI] [PubMed] [Google Scholar]
- 124.Chong SA, Lee WL, Tan CH, Tay AH, Chan AO, Tan EC. Attempted suicide and polymorphism of the serotonin transporter gene in Chinese patients with schizophrenia. Psychiatry Res. 2000;97:101–6. doi: 10.1016/s0165-1781(00)00229-8. [DOI] [PubMed] [Google Scholar]
- 125.Fitch D, Lesage A, Seguin M, Trousignant M, Bankelfat C, Rouleau GA, et al. Suicide and the serotonin transporter gene. Mol. Psychiatry. 2001;6:127–8. doi: 10.1038/sj.mp.4000833. [DOI] [PubMed] [Google Scholar]
- 126.Mann JJ, Haung Y, Underwood MD, Kassir SA, Oppenheim S, Kelly TM, et al. A serotonin transporter gene promoter polymorphism (5HTTLPR) and prefrontal cortical binding in major depression and suicide. Arch Gen Psychiatry. 2000;57:729–38. doi: 10.1001/archpsyc.57.8.729. [DOI] [PubMed] [Google Scholar]
- 127.Geijer T, Frisch A, Pearson ML, Wasserman D, Rockoh R, Michaelovsky, et al. Search for association between suicide attempt and serotonergic polymorphisms. Psychiatry Genet. 2000;10:19–2. doi: 10.1097/00041444-200010010-00004. [DOI] [PubMed] [Google Scholar]
- 128.Bacca-Garcia E, Vaquero C, Diaz-Sastre C, Saiz-Ruiz J, De leon J. A gender specific association between the serotonin transporter gene and suicide attempts. Neuropsypharmacol. 2000;26:692–5. doi: 10.1016/S0893-133X(01)00394-3. [DOI] [PubMed] [Google Scholar]
- 129.Gaysina D, Zainullina A, Gabdulhakov R, Khusnutdinova E. The serotonin transporter gene: Polymorphism and haplotype analysis in Russian suicide attempters. Neuropsychobiology. 2006;54:70–4. doi: 10.1159/000096041. [DOI] [PubMed] [Google Scholar]
- 130.Roy A, Hu XZ, Janal MN, Goldman D. Interaction between childhood trauma and serotonin transporter gene variation in suicide. Neuropsychopharmacology. 2007;32:2046–52. doi: 10.1038/sj.npp.1301331. [DOI] [PubMed] [Google Scholar]
- 131.Cook EH, Jr, Courchesne R, Lord C, Cox NJ, Yan S, Lincoln A, et al. Evidence of linkage between the serotonin transporter and autistic disorder. Mol Psychiatry. 1997;2:247–50. doi: 10.1038/sj.mp.4000266. [DOI] [PubMed] [Google Scholar]
- 132.Yirmiya N, Pilowsky T, Nemanov L, Arbelle S, Feinsilver T, Fried I. Evidence for an association with the serotonin transporter promoter region polymorphism and autism. Am J Med Genet. 2001;105:381–6. doi: 10.1002/ajmg.1365. [DOI] [PubMed] [Google Scholar]
- 133.Klauck SM, Poustka F, Benner A, Lesch KP, Poustka A. Serotonin transporter (5-HTT) gene variants associated with autism. Hum Mol Genet. 1997;13:2233–8. doi: 10.1093/hmg/6.13.2233. [DOI] [PubMed] [Google Scholar]
- 134.Betancur C, Corbex M, Spielewoy C, Philippe A, Laplanche JL, Launay JM, et al. Serotonin transporter gene polymorphisms and hyperserotonemia in autistic disorder. Mol Psychiatry. 2002;7:67–71. doi: 10.1038/sj.mp.4001923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Conroy J, Meally E, Kearney G, Fitzgerald M, Gill M, Gallagher L. Serotonin transporter gene and autism: A haplotype analysis in an Irish autistic population. Mol Psychiatry. 2004;9:587–93. doi: 10.1038/sj.mp.4001459. [DOI] [PubMed] [Google Scholar]
- 136.Devlin B, Cook EH, Jr, Coon H, Dawson G, Grigorenko EL, McMahon W, et al. Autism and the serotonin transporter: The long and short of it. Mol Psychiatry. 2005;10:1110–6. doi: 10.1038/sj.mp.4001724. [DOI] [PubMed] [Google Scholar]
- 137.Koishi S, Yamamoto K, Matsumoto H, Koishi S, Enseki Y, Oya A, et al. Serotonin transporter gene promoter polymorphism and autism: A family-based genetic association study in Japanese Population. Brain Dev. 2006;28:257–60. doi: 10.1016/j.braindev.2005.09.003. [DOI] [PubMed] [Google Scholar]
- 138.Guhathakurta S, Ghosh S, Sinha S, Chatterjee A, Ahmed S, Chowdhury SR, et al. Serotonin transporter promoter variants: Analysis in Indian autistic and control population. Brain Res. 2006;1092:28–35. doi: 10.1016/j.brainres.2006.03.078. [DOI] [PubMed] [Google Scholar]
- 139.Huang C, Susan S. Autism and serotonin transporter gene polymorphisms: A systematic review and meta-analysis. Am J Med Genet Part B Neuropsychiatr Genet. 2003;147:903–13. doi: 10.1002/ajmg.b.30720. [DOI] [PubMed] [Google Scholar]
- 140.Brune CW, Kim SJ, Salt J, Leventhal BL, Lord C, Cook EH., Jr 5-HTTLPR genotype-specific phenotype in children and adolescents with autism. Am J Psychiatry. 2006;163:2148–56. doi: 10.1176/ajp.2006.163.12.2148. [DOI] [PubMed] [Google Scholar]
- 141.Turker T, Sodmann R, Goebel U, Jatzke S, Knapp M, Lesch KP, et al. High ethanol tolerance in young adults is associated with the low-activity variant of the promoter of the human serotonin transporter gene. Neurosci Lett. 1998;248:147–50. doi: 10.1016/s0304-3940(98)00347-4. [DOI] [PubMed] [Google Scholar]
- 142.Tan EC, Yeo BK, Ho BK, Tay AH, Tan CH. Evidence for an association between heroin dependence and a VNTR polymorphism at the serotonin transporter locus. Mol Psychiatry. 1999;4:215–7. doi: 10.1038/sj.mp.4000541. [DOI] [PubMed] [Google Scholar]
- 143.Hallikainen T, Saito T, Lachman HM, Volavka J, Pohjalainen T, Ryynanen OP. Association between low activity serotonin transporter promoter genotype and early onset alcoholism with habitual impulsive violent behavior. Mol Psychiatry. 1999;4:385–8. doi: 10.1038/sj.mp.4000526. [DOI] [PubMed] [Google Scholar]
- 144.Ishiguro H, Saito T, Akazawa S, Mitushio H, Tada K, Enomoto M, et al. Association between drinking related antisocial behaviour and a polymorphism in the serotonin transporter gene in a Japanese population. Alcohol Clin Exp Res. 1999;23:1281–4. [PubMed] [Google Scholar]
- 145.Schuckit MA, Mazzanti C, Smith TL, Ahmad U, Radel M, Iwata N, et al. Selective genotyping for the role of 5HT2A, 5HT2C, and GABA alpha 6 receptors and Serotonin transporter in the level of response to alcohol: A pilot study. Biol Psychiatry. 1999;45:647–51. doi: 10.1016/s0006-3223(98)00248-0. [DOI] [PubMed] [Google Scholar]
- 146.Johnson BA. Serotonergic agents and alcoholism treatment: Rebirth of the subtype concept: An hypothesis. Alcohol Exp Res. 2000;24:1597–601. [PubMed] [Google Scholar]
- 147.Javors M, Tiouririne M, Prihoda T. Platelet serotonin is higher in early-onset than in late-onset alcoholics. Alcohol. 2000;35:390–3. doi: 10.1093/alcalc/35.4.390. [DOI] [PubMed] [Google Scholar]
- 148.Matsushita S, Yoshino A, Murayama M, Kimura M, Muramatsu T, Higuchi S. Association study of serotonin transporter gene regulatory region polymorphism and alcoholism. Am J Med Genet. 2001;105:446–50. doi: 10.1002/ajmg.1405. [DOI] [PubMed] [Google Scholar]
- 149.Stoltenberg SF, Twitchell GR, Hanna GL, Cook EH, Fitzgerald HE, Zucker RA, et al. Serotonin transporter promoter polymorphism, peripheral indexes of serotonin function, and personality measures in families with alcoholism. Am J Med Genet. 2002;114:230–4. doi: 10.1002/ajmg.10187. [DOI] [PubMed] [Google Scholar]
- 150.Feinn R, Nellissery M, Kranzler HR. Meta-analysis of the association of a functional serotonin transporter promoter polymorphism with alcohol dependence. Am J Med Genet B Neuropsychiatr Genet. 2005;133:79–84. doi: 10.1002/ajmg.b.30132. [DOI] [PubMed] [Google Scholar]
- 151.Bleich S, Bönsch D, Rauh J, Bayerlein K, Fiszer R, Frieling H, et al. Association of the long allele of the 5-HTTLPR polymorphism with compulsive craving in alcohol dependence. Alcohol. 2007;42:509–12. doi: 10.1093/alcalc/agm068. [DOI] [PubMed] [Google Scholar]
- 152.Pinto E, Reggers J, Gorwood P, Boni C, Scantamburlo G, Pitchot W, et al. The short allele of the serotonin transporter promoter polymorphism influences relapse in alcohol dependence. Alcohol. 2008;4:398–400. doi: 10.1093/alcalc/agn015. [DOI] [PubMed] [Google Scholar]
- 153.Mokrović G, Matosić A, Hranilović D, Stefulj J, Novokmet M, Oresković D, et al. Alcohol dependence and polymorphisms of serotonin-related genes: Association studies. Coll Antropol. 2008;32(Suppl 1):127–3. [PubMed] [Google Scholar]
- 154.Loehlin JC. Nature, nurture and conservatism in the Australian twin. Behav Genet. 1993;23:287–90. doi: 10.1007/BF01082468. [DOI] [PubMed] [Google Scholar]
- 155.Middledrop CM, de Geus EJ, Beem AL, Lakenberg N, Hottenga J, Slagboom PE, et al. Family based association analyses between the serotonin transporter gene polymorphism (5-HTTLPR) and neuroticism, anxiety and depression. Behav Genet. 2007;37:294–301. doi: 10.1007/s10519-006-9139-7. [DOI] [PubMed] [Google Scholar]
- 156.Sen S, Burmeister M, Ghosh D. Meta-analysis of the association between a serotonin transporter promoter polymorphism (5-HTTLPR) and anxiety-related personality traits. Am J Med Genet Part B Neuropsychiatr Genet. 2004;127:85–9. doi: 10.1002/ajmg.b.20158. [DOI] [PubMed] [Google Scholar]
- 157.Nakamura T, Muramatsu T, Ono Y, Matsushita S, Higuchi S, Mizushima H, et al. Serotonin transporter gene regulatory region polymorphism and anxiety-related traits in the Japanese. Am J Med Genet. 1997;74:544–5. doi: 10.1002/(sici)1096-8628(19970919)74:5<544::aid-ajmg18>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- 158.Willis-Owen SA, Turri MG, Munafò MR, Surtees PG, Wainwright NW, Brixey RD, et al. The serotonin transporter length polymorphism, neuroticism, and depression: A comprehensive assessment of association. Biol Psychiatry. 2005;58:451–6. doi: 10.1016/j.biopsych.2005.04.050. [DOI] [PubMed] [Google Scholar]
- 159.Deary IJ, Battersby S, Whiteman MC, Connor JM, Fowkes FG, Harmar A. Neuroticism and polymorphisms in the serotonin transporter gene. Psychol Med. 1999;29:735–9. doi: 10.1017/s0033291798007557. [DOI] [PubMed] [Google Scholar]
- 160.Jorm AF, Henderson AS, Jacomb PA, Christensen H, Korten AE, Rodgers B, et al. An association study of a functional polymorphism of the serotonin transporter gene with personality and psychiatric symptoms. Mol Psychiatry. 1998;3:449–51. doi: 10.1038/sj.mp.4000424. [DOI] [PubMed] [Google Scholar]
- 161.Ball D, Hill L, Freeman B, Eley TC, Strelau J, Riemann R. The serotonin transporter gene and peer-rated neuroticism. Neuroreport. 1997;8:1301–4. doi: 10.1097/00001756-199703240-00048. [DOI] [PubMed] [Google Scholar]
- 162.Sen S, Burmeister M, Ghosh D. 5-HTTLPR and anxiety related personality traits meta-analysis revisited: Response to Munafo and colleagues. Mol Psychiatry. 2005;10:893–5. [Google Scholar]
- 163.Schinka JA, Busch RM, Robichaux-Keene N. A meta analysis of the association between the serotonin transporter gene polymorphism (5-HTTLPR) and trait anxiety. Mol Psychiatry. 2004;9:197–202. doi: 10.1038/sj.mp.4001405. [DOI] [PubMed] [Google Scholar]
- 164.Munafo MR, Clark T, Flint J. Promise and pitfalls in the meta-analysis of genetic association studies: A response to Sen and Schinka. Mol Psychiatry. 2005;10:895–7. [Google Scholar]
- 165.Murrough WJ, Charney SD. The serotonin transporter and emotionality: Risk, resilience, and new therapeutic opportunities. Biol Psychiatry. 2011;69:510–2. doi: 10.1016/j.biopsych.2011.01.019. [DOI] [PubMed] [Google Scholar]
- 166.Homberg RJ, Lesch KP. Looking on the bright side of serotonin transporter gene variation. Biol Psychiatry. 2011;69:513–9. doi: 10.1016/j.biopsych.2010.09.024. [DOI] [PubMed] [Google Scholar]
- 167.Pluess M, Belsky J. Prenatal programming of postnatal plasticity? Dev Psychopathol. 2011;23:29–38. doi: 10.1017/S0954579410000623. [DOI] [PubMed] [Google Scholar]
- 168.Morey RA, Hariri AR, Gold AL, Hauser MA, Munger HJ, Dolcos F, et al. Serotonin transporter gene polymorphisms and brain function during emotional distraction from cognitive processing in posttraumatic stress disorder. BMC Psychiatry. 2011;11:76. doi: 10.1186/1471-244X-11-76. [DOI] [PMC free article] [PubMed] [Google Scholar]


