Abstract
In nearly all animals, mitochondrial DNA (mtDNA) consists of a single circular molecule that encodes several subunits of the protein complexes involved in oxidative phosphorylation as well as part of the machinery for their expression. By contrast, mtDNA in species belonging to Medusozoa (one of the two major lineages in the phylum Cnidaria) comprises one to several linear molecules. Many questions remain on the ubiquity of linear mtDNA in medusozoans and the mechanisms responsible for its evolution, replication, and transcription. To address some of these questions, we determined the sequences of nearly complete linear mtDNA from 24 species representing all four medusozoan classes: Cubozoa, Hydrozoa, Scyphozoa, and Staurozoa. All newly determined medusozoan mitochondrial genomes harbor the 17 genes typical for cnidarians and map as linear molecules with a high degree of gene order conservation relative to the anthozoans. In addition, two open reading frames (ORFs), polB and ORF314, are identified in cubozoan, schyphozoan, staurozoan, and trachyline hydrozoan mtDNA. polB belongs to the B-type DNA polymerase gene family, while the product of ORF314 may act as a terminal protein that binds telomeres. We posit that these two ORFs are remnants of a linear plasmid that invaded the mitochondrial genomes of the last common ancestor of Medusozoa and are responsible for its linearity. Hydroidolinan hydrozoans have lost the two ORFs and instead have duplicated cox1 at each end of their mitochondrial chromosome(s). Fragmentation of mtDNA occurred independently in Cubozoa and Hydridae (Hydrozoa, Hydroidolina). Our broad sampling allows us to reconstruct the evolutionary history of linear mtDNA in medusozoans.
Keywords: linear mtDNA, medusozoa, cnidaria, ORF314, polB
Introduction
The mitochondrial genome is one of the most commonly used molecular markers in animal phylogenetics, with informative characters at the nucleotide, amino acid and gene organization levels (Moret and Warnow 2005; Lavrov 2007). As a consequence, the metazoan mitogenomic data set has benefited from a wide sampling effort across the animal kingdom. In most animals, mitochondrial DNA (mtDNA) is a single small (usually <20 kbp) circular molecule that contains a nearly constant set of genes. With a few exceptions, these genes are orthologous across animals (Wolstenholme 1992; Boore 1999). Most animal mitochondrial genomes encode 12–14 protein genes and up to 28 RNA genes (Gissi et al. 2008). Although complete or nearly complete mitochondrial genomes for more than 2,400 animal species are available on the Organelle Genome Resources database in GenBank (ncbi.nlm.nih.gov/genomes/OrganelleResource.cgi?taxid=33208 [cited 2011 October]), only 97 represent nonbilaterians: 5 for Placozoa, 42 for Porifera, 49 for Cnidaria, and 1 for Ctenophora. This paucity of completely sequenced nonbilaterian mtDNAs is unfortunate because nonbilaterian mitochondrial genomes represent a large portion of the variability in both sequence and genome organization among animals (Lavrov 2007; Gissi et al. 2008).
The phylum Cnidaria represents a “hot spot” of animal mitochondrial genomic diversity, with the mtDNA displaying variation in both the gene content and the genome organization. Variation in the gene content is illustrated by the loss of all but one or two tRNA genes, a feature also found in some demosponges and in all chaetognaths (Helfenbein et al. 2004; Papillon et al. 2004; Faure and Casanova 2006; Wang and Lavrov 2008; Miyamoto et al. 2010). In addition, introns (e.g., Beagley et al., 1998; Chen et al. 2008), duplicated genes (Kayal and Lavrov 2008; Voigt et al. 2008), and extra protein genes (mutS in octocorals, polB in Scyphozoa, and unidentified open reading frames (ORFs) in several hexacorals) have been found in several cnidarian classes (Pont-Kingdon et al. 1998; Medina et al. 2006; Shao et al. 2006). Furthermore, the structure of the cnidarian mtDNA is also variable: a single circular molecule is found in Anthozoa (hexa- and octocorals), whereas the medusozoan mtDNA consists of one or several exclusively linear molecules (Warrior and Gall 1985; Bridge et al. 1992; Ender and Schierwater 2003). Working with linear mtDNA presents unique challenges for both polymerase chain reaction (PCR) amplification and sequencing. As a potential reflection of these challenges, only six medusozoan linear mitochondrial genomes have been determined to date, those of the jellyfishes Aurelia aurita and Chrysaora quinquecirrha (Scyphozoa) and the three hydra species Hydra magnipapillata, Hydra oligactis, and Hydra vulgaris (Hydrozoa) (Shao et al. 2006; Kayal and Lavrov 2008; Voigt et al. 2008; Park, Hwang, et al. 2011), while 43 complete circular mitochondrial genomes are available for the anthozoans.
Linear mtDNA is common outside the animals and has been extensively studied in fungi, plants, and several groups of unicellular eukaryotes (Bendich 1993; Burger et al. 2003; Nosek and Tomáska 2003; Hikosaka et al. 2010; Valach et al. 2011). The linear form of these molecules is thought to affect their stability, mechanisms of DNA replication, and expression of the genes they harbor. Unlike circular DNA molecules, linear chromosomes are less stable due to their higher susceptibility to exonuclease activity. In addition, replication of linear genomes has to overcome what is known as “the challenge of the ends,” namely the shortening of the linear molecule after each replication cycle (Nosek et al. 1998). In the nucleus, the ends of the linear chromosomes are capped by repetitive sequences known as telomeres that both protect the molecule from degradation and allow its replication in a faithful manner (i.e., without the loss of essential sequences). Similarly, linear mtDNAs in algae and fungi have overcome linear instability with an array of telomere structures (Nosek et al. 1998). Recent studies of several yeast species from the genus Candida have suggested the concomitant involvement of the product of 1) a B-type DNA polymerase gene encoded by the mtDNA for 5′ end DNA extension and 2) terminal proteins (TPs) that bind the single stranded end of the linear molecules (Fricova et al. 2010 and reference therein). Thus far, the small number of available linear mitochondrial genomes for medusozoans has hindered attempts to assign any of the available models for the maintenance of chromosome ends proposed for the nonanimal groups (Nosek et al. 1998; Nosek and Tomaska 2002) or to reconstruct the evolutionary history of linear mtDNA in medusozoans.
Here, we present a description of 24 nearly complete mitochondrial genomes from a phylogenetically diverse set of species representing each of the four medusozoan classes, as well as their primary lineages: Cubozoa (Carybdeida and Chirodropida), Hydrozoa (Hydroidolina and Trachylina), Scyphozoa (Coronatae and Discomedusae), and Staurozoa (Cleistocarpida and Eleutherocarpida). Our study represents the most in-depth exploration of the linear mtDNA in Cnidaria, and includes the first mitochondrial genomes from the classes Cubozoa and Staurozoa.
Materials and Methods
Animal Sampling, DNA Extractions, and PCR Amplification
We collected several specimens of Cassiopea andromeda (Scyphozoa) and Millepora platyphylla (Hydrozoa) on the site of Tiahura in Moorea (French Polynesia). We collected specimens of Carybdea xaymacana (Cubozoa), Cassiopea frondosa and Chrysaora sp. (Scyphozoa), and Cubaia aphrodite (Hydrozoa) at Bocas del Toro (Panama). The staurozoan Craterolophus convolvulus was collected off the island of Helgoland (Germany) and Haliclystus “sanjuanensis” was collected off the coast of Washington state. The cubozoans Carukia barnesi and Chironex fleckeri were collected at Cairns and Weipa, respectively (Queensland, Australia); Alatina moseri was collected in Waikiki (Hawaii), and Chiropsalmus quadrumanus was collected on the east coast of the United States, near the border between North and South Carolina. We purchased specimens of Cyanea capillata (Scyphozoa), Clava multicornis, Ectopleura larynx, Laomedea flexuosa, and Pennaria tiarella (Hydrozoa) from the Marine Biological Laboratory at Woods Hole (www.mbl.edu) and Nemopsis bachei from the Gulf Specimen Marine Lab (www.gulfspecimen.org). Tissues from Catostylus mosaicus, Linuche unguiculata, and Rhizostoma pulmo (Scyphozoa) were kindly provided by Dr Michael Dawson, individuals of Obelia longissima (Hydrozoa) by Dr Annette F. Govindarajan, a specimen of Pelagia noctiluca (Scyphozoa) collected at the Station Marine d'Endoume (Marseille, France) by Dr Alexander Ereskovsky, and a DNA aliquot for Lucernaria janetae from the East Pacific Rise by Dr Janet Voight. The identities of hydrozoan species were confirmed morphologically by Dr Peter Schuchert and assessed for all species by comparing the partial cox1 and rnl sequences to the National Center for Biotechnology Information database. We extracted total DNA using phenol–chloroform extraction followed by proteinase K digestion.
We amplified and sequenced parts of cob, cox1, cox2, nad5, rnl, and rns using conserved primers for animals (Burger et al. 2007). We used the sequences obtained from these amplifications to design species-specific primers for long PCR amplifications. We amplified nearly complete mitochondrial genomes in a single piece between either cob–cox1 or cob–cox2, or in two parts between rnl–nad5 and rns–cox1. We extended our sequences using a modified step-out protocol (Burger et al. 2007) and reamplified the contigs using the standard PCR. We then sheared the long PCR products and processed them for multiplex sequencing (Meyer et al. 2008) using either the 454 high-throughput or Illumina Sequencing platforms, carried out at the Center for Genomics and Bioinformatics of Indiana University and the Office of Biotechnology DNA Facility of Iowa State University, respectively. For C. convolvulus, H. “sanjuanensis,” and L. unguiculata, we constructed random clone libraries from the purified PCR product using the TOPO Shotgun Subcloning Kit from Invitrogen (for details, see Burger et al. 2007) and sequenced at the Office of Biotechnology DNA Facility of the Iowa State University.
We identified mitochondrial sequences from the partial genomic reads of A. moseri generated by the Iridian Genomes project. We used specific primers and step-out protocol on a tissue sample from another individual of A. moseri to extend our sequences toward the end of the mitochondrial chromosomes. Because of the high degree of segmentation of cubozoan mtDNA, we PCR amplified nearly complete coding sequences using the standard PCRs (for rns–nad1 and cox2–cox3) and the step-out protocols (for cob, cox1, nad4, nad2–nad5, ORF314–polB, and rnl) and cloned the products into TOPO vectors. Plasmid preparation and sequencing were processed at the Iowa State University Office of Biotechnology DNA facility. We used enzymatic digestion of total DNA with the methylation-sensitive restriction enzyme HpaII to confirm that PCR products amplified in this study are genuine mitochondrial sequences (Kumar and Bendich 2011).
Genome Assembly, Gene Annotation, and Analysis
We assembled all sequences using the STADEN software suite (Staden 1996). We used both the tRNAscan-SE and ARWEN programs (Lowe and Eddy 1997; Laslett and Canbäck 2008) to identify tRNA genes. We reconstructed the secondary structures of and by comparing with their homologues in other cnidarian species. Other genes were identified by similar searches in the local databases using the FASTA program (Pearson 1994). We predicted the boundaries of rRNA genes by aligning them to rRNA sequences from the published cnidarians and checking the alignments manually. We also aligned individual protein genes with ClustalW 1.82 (Thompson et al. 1994) using default parameters. We manually verified all alignments based on either amino acid alignments (for protein coding genes) or inferred rRNA or tRNA secondary structures (for RNA-coding genes) to A. aurita and H. oligactis (Shao et al. 2006; Kayal and Lavrov 2008). For the analysis of sequence similarities, we calculated sequence identities using local scripts based on the BioPerl modules (Stajich et al. 2002). We estimated codon usage with the CUSP program in the EMBOSS package (Rice et al. 2000).
Results
The Linear Mitochondrial Genomes of Medusozoa
We amplified and sequenced mitochondrial (mt) genomes of eight species representing the two clades of Scyphozoa (Coronatae: L. unguiculata; Discomedusae: C. andromeda, C. frondosa, C. mosaicus, Chrysaora sp, C. capillata, P. noctiluca, and R. pulmo (partial)), eight species representing the two clades of Hydrozoa (Hydroidolina: C. multicornis, E. larynx, L. flexuosa, M. platyphylla, N. bachei, O. longissima, and P. tiarella; Trachylina: C. aphrodite), and three species representing both orders of Staurozoa (Cleistocarpida: C. convolvulus; Eleutherocarpida: H. “sanjuanensis” and L. janetae). In addition, we obtained substantial mitochondrial sequences from five species belonging to the two major cubozoan clades (Carybdeida: A. moseri, C. barnesi, and C. xaymacana; Chirodropida: C. fleckeri and C. quadrumanus).
The mtDNA of all scyphozoan, staurozoan, and hydrozoan species sampled for this study mapped into single linear molecules (see below). Cubozoan mtDNA was composed of eight mitochondrial chromosomes, each encoding one to five mitochondrial genes with the same transcriptional orientation. This is in agreement with earlier DNA hybridization experiments, which suggested that the cubozoan mtDNA was composed of several, up to 4 kb, linear chromosomes (Ender and Schierwater 2003). All cubozoan mitochondrial genes were similar in size to their homologues in other medusozoans and contain neither internal stop codons (TAA or TAG) nor frameshifts. In addition, incubation of the A. moseri total DNA with the methylation-sensitive enzyme HpaII (method described in Kumar and Bendich 2011) inhibited PCR amplifications of the contigs harboring the restriction sites (supplementary fig. S1, Supplementary Material online). Together, this evidence strongly suggested that our cubozoan contigs were genuine mitochondrial sequences, as opposed to nuclear pseudogenes (NUMTs).
All medusozaon mtDNA contained a set of genes for 13 protein subunits conserved in animals and involved in the electron transport chain of oxidative phosphorylation (atp6-8, cob, cox1-3, nad1-6), two ribosomal RNAs (rRNA) (rnl and rns), and two transfer RNAs (tRNA) (trnM(cau) and trnW(uca)). trnW(uca) was missing from the partial mitochondrial genome of the coronate L. unguiculata and no tRNA genes were found in the partial sequences from cubozoans (see below). We also found some level of class-specific conservation at the ends of the linear mitochondrial chromosomes: duplicated cox1 in most hydrozoans (Hydroidolina), polB and an extra ORF in cubozoans, scyphozoans, staurozoans, and the trachyline hydrozoan C. aphrodite (see below).
Medusozoan mitochondrial genomes displayed a compact organization with few, if any intergenic regions (IGRs), and several pairs of overlapping genes. We found overlapping nad3-nad6 genes in the mtDNA of all hydrozoans, scyphozoans, and staurozoan species (11 ± 3, 35 ± 17, and 9 ± 2 nucleotides respectively). In addition, nad4L-nad3 overlapped by 10 nucleotides in all scyphozoan species, nad1-nad4 overlapped by 10 ± 3 nucleotides in all hydrozoans, atp6-atp8 overlapped by seven nucleotides, and nad2-nad5 by 13 nucleotides in all staurozoans. Similarly, nad2-nad5 overlapped by 26 and 44 nucleotides in the staurozoans H. “sanjuanensis” and L. janetae, respectively. Finally, in the aplanulate E. larynx we found cob-cox1 overlapping by 44 nucleotides. The same two genes overlapped by eight nucleotides in Hydra magnipaillata and H. vulgaris (Voigt et al. 2008), which are also part of the Aplanulata clade (Collins et al. 2005).
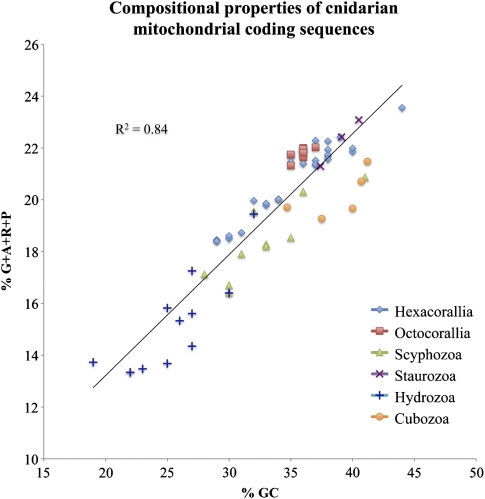
We compared the variation in the mtDNA nucleotide composition among different cnidarian classes (table 1). The linear mtDNA in medusozoans displayed GC-skew values close to zero: cubozoans, hydrozoans, and scyphozoans all shared a small positive GC-skew (on average 0.04 standard deviation [SD] = 0.03) between the two strands of the coding sequences, while staurozoans had a slightly negative GC-skew (−0.05 SD = 0.02). In comparison, the circular mtDNA in anthozoans displayed a positive GC-skew (0.20 SD = 0.07 in hexacorals and 0.09 SD = 0.01 in octocorals). AT-skews in all studied medusozoan species were negative for the coding strand (between −0.11 and −0.21) and similar to those in anthozoans. The A + T content of coding regions varied substantially between different medusozoan classes. Hydrozoan mtDNA had the highest A + T content (74% SD = 4), and cubozoan mtDNA had the lowest (61% SD = 3). Scyphozoans and staurozoans had intermediate %A + T values (67% SD = 4 and 67% SD = 2 respectively) similar to those in anthozoans. We found a strong correlation (R2 = 0.84) between nucleotide composition and the proportion of GC-rich amino acids (the “GARP” amino acids: glycine, alanine, arginine, and proline) (fig. 1). This result was consistent with previous studies, which found that nucleotide composition can serve as a proxy for amino acid compositions of protein sequences and can be an important source of systematic errors in phylogenetic inference (Delsuc et al. 2005 and references therein).
Table 1.
Gene Properties in the mtDNA of Medusozoa
| Cubozoa |
Hydrozoa |
Scyphozoa |
Staurozoa |
|||||||||||||
| Sizea | %ATb | Startc | Endd | Size | %AT | Start | End | Size | %AT | Start | End | Size | %AT | Start | End | |
| atp6 | 708 ± 7 | 63 ± 2 | AG | * | 704 ± 1 | 75 ± 4 | A | A* | 704 ± 3 | 69 ± 4 | A | A* | 708 ± 0 | 63 ± 1 | A | A |
| atp8 | 210 ± 2 | 64 ± 4 | AG | AG* | 206 ± 3 | 83 ± 5 | A | A* | 208 ± 7 | 73 ± 4 | AG | AG* | 204 ± 0 | 62 ± 4 | A | A |
| cob | 1149 | 62 ± 2 | A | G | 1148 ± 12 | 73 ± 3 | AG | A | 1146 ± 8 | 66 ± 2 | A | AG | 1068 | 60 ± 2 | A | ? |
| cox1 | 1569 | 58 ± 3 | A | A | 1569 | 67 ± 3 | AG | AG | 1580 ± 7 | 64 ± 3 | A | AG | 1578 ± 0 | 61 ± 1 | A | A |
| cox2 | 737 ± 2 | 61 ± 2 | A | AG* | 744 ± 10 | 73 ± 4 | A | AG | 746 ± 8 | 67 ± 4 | A | AG* | 747 ± 0 | 62 ± 1 | A | AG |
| cox3 | 786 ± 0 | 59 ± 3 | A | AG | 786 ± 0 | 72 ± 4 | A | AG | 786 ± 0 | 64 ± 3 | A | AG | 786 ± 0 | 61 ± 1 | A | AG |
| nad1 | 987 ± 8 | 62 ± 3 | AG | AG | 989 ± 4 | 73 ± 4 | A | AG | 972 ± 5 | 66 ± 4 | AG | A* | 987 ± 0 | 59 ± 0 | A | A |
| nad2 | 1341 | 63 ± 7 | A | G* | 1328 ± 32 | 79 ± 5 | A | AG* | 1323 ± 13 | 70 ± 5 | A | AG | 1346 ± 2 | 59 ± 4 | A | A |
| nad3 | 351 ± 0 | 62 ± 4 | AG | * | 355 ± 4 | 77 ± 4 | A | * | 357 ± 6 | 69 ± 4 | AG | A* | 354 ± 0 | 65 ± 4 | A | A* |
| nad4 | 1446 | 59 | A | G | 1458 ± 2 | 76 ± 4 | A | AG* | 1441 ± 2 | 68 ± 5 | A | AG* | 1461 ± 0 | 61 ± 3 | A | AG* |
| nad4L | 290 ± 2 | 67 ± 3 | A | G* | 299 ± 2 | 79 ± 4 | A | * | 303 ± 1 | 72 ± 5 | AG | A* | 299 ± 2 | 64 ± 1 | A | * |
| nad5 | 1824 | 62 ± 1 | AG | G | 1832 ± 2 | 76 ± 4 | A | AG* | 1830 ± 19 | 68 ± 5 | AG | A* | 1860 ± 13 | 60 ± 2 | A | AG |
| nad6 | 542 ± 4 | 64 ± 3 | A | G* | 556 ± 8 | 79 ± 5 | A | AG* | 564 ± 12 | 70 ± 5 | A | AG* | 553 ± 2 | 62 ± 2 | A | A |
| ORF314 | 315 | 64 | A | A | 291 | 78 | A | G | 313 ± 7 | 73 ± 8 | A | A | 288 | 62 | A | A |
| polB | 873 | 58 | G | A | ? | ? | ? | ? | 969 | 70 ± 8 | A | A | 1119 | 58 | ATG | ? |
| rnl | 769 | 57 | NA | NA | 1746 ± 9 | 76 ± 4 | NA | NA | 1818 ± 34 | 69 ± 5 | NA | NA | 1830 | 57 ± 1 | NA | NA |
| rns | 672 | 62 | NA | NA | 910 ± 21 | 74 ± 2 | NA | NA | 950 ± 10 | 69 ± 3 | NA | NA | 914 ± 1 | 57 ± 1 | NA | NA |
| trnM | — | — | NA | NA | 71 ± 1 | 69 ± 2 | NA | NA | 71 ± 0 | 64 ± 5 | NA | NA | 69 ± 0 | 53 ± 2 | NA | NA |
| trnW | — | — | NA | NA | 70 ± 1 | 65 ± 3 | NA | NA | 70 ± 0 | 64 ± 5 | NA | NA | 71 ± 0 | 52 ± 2 | NA | NA |
NOTE.—The average size in nucleotides (and standard deviation), AT composition (and standard deviation), and putative start and stop codons are reported for each of the Medusozoa subclasses. NA, not applicable; ?, data not available.
Average size in nucleotides, with standard deviation.
AT composition, with standard deviation.
Start A and G stand for ATG and GTG start codons, respectively.
Stop A and G stand for TAA and TAG stop codons, respectively; an asterisk corresponds to an incomplete stop codon (see text).
FIG. 1.—
Compositional properties of cnidarian mitochondrial coding sequences. The total G-C content of mitochondrial protein genes is plotted against the percentage of amino acids encoded by G- and C-rich codons (glycine, alanine, arginine, and proline [G + A + R + P]). The trend line and correlation coefficient are displayed. Hydrozoa are characterized by very A-T rich genomes and relatively fewer [G + A + R + P] codons. Staurozoan have relatively higher GC content similar to anthozoans.
Gene Arrangements in Medusozoan mtDNA
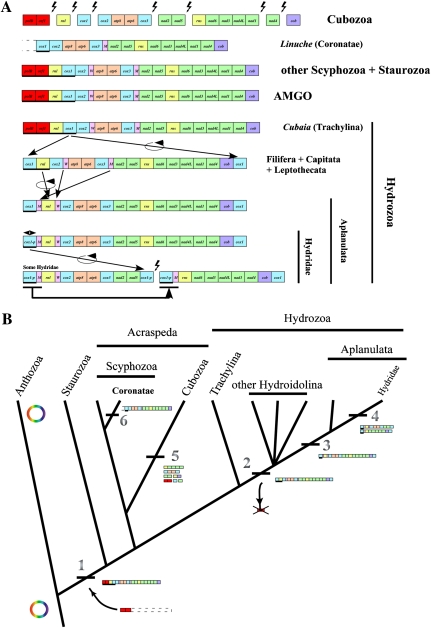
Analysis of the newly determined mitochondrial sequences revealed a well-conserved mitochondrial gene order within Medusozoa (fig. 2A). Eleven representatives of both discomedusan Scyphozoa and Staurozoa sampled for this study had a completely conserved gene order, identical to that found in A. aurita (Shao et al. 2006). In these groups, the cluster formed by cox1-rnl-ORF314-polB had a different transcriptional orientation to the rest of the genes (fig. 2A). The two clusters were separated by a relatively large noncoding region of about 80 nucleotides, potentially involved in the control of mitochondrial transcription (see below). In the coronate L. unguiculata, trnW was not found either in its conserved position between cox2 and atp8 or in the rest of the genome. We were also unable to identify sequences for polB and ORF314 in this species. However, given that the sequence of L. unguiculata is incomplete, it is possible that these genes are present in the mitochondrial genome, downstream of cox1. The gene arrangement was also conserved in all cubozoan species, despite the presence of multiple chromosomes (fig. 2A). Single gene chromosomes harbored cob, cox1, nad4, and rnl genes. Other chromosomes contained gene pairs nad2-nad5 and ORF314-polB, and gene clusters cox2-atp8-atp6-cox3 and rns-nad6-nad3-nad4L-nad1. When placed together, the gene order across the eight chromosomes in cubozoan mtDNA is nearly identical to that in most scyphozoans and staurozoans except for the absence of tRNA genes.
FIG. 2.—
Evolution of mitochondrial genomes in Medusozoa (Cnidaria) based on phylogenetic relationships according to Collins et al. (2006), Collins et al. (2008), and Cartwright et al. (2008). (A) Comparison of mitochondrial gene orders between various medusozoan clades and the putative mitochondrial AMGO. Breaks in contigs mark the ends of linear mitochondrial chromosomes. Genes are transcribed from left to right unless underlined. cox1-p corresponds to the partial copy of cox1 at the end of the mtDNA molecule(s) in the Hydridae family. polB is an inferred member of the B DNA polymerase gene family and ORF314 is an unidentified ORF located upstream of it. Lightning bolts represent breaks in the mitochondrial chromosomes. Black arrows depict directions of inferred genome rearrangements. (B) Evolution of genome organization of the linear mtDNA in Medusozoa. The phylogeny is based on Collins et al. (2006). Major genomic rearrangements are labeled. 1) Linearization of the mtDNA by insertion of a linear plasmid of unknown source; 2) loss of polB and ORF314, and duplication of cox1 at each end of the linear chromosome within Hydrozoa after the divergence of Trachylina; 3) displacement of tRNAs and inversion of rnl in Aplanulata; 4) genome segmentalization of the mtDNA in several Hydridae species with consequent trnM and cox1 duplication; 5) high level segmentalization of the mtDNA in cubozoan; and 6) displacement of trnW in the coronate L. unguiculata.
The mitochondrial genome organization within Hydrozoa was more variable, with at least four different gene orders. The genome organization in the trachyline C. aphrodite was identical to that in discomedusan Scyphozoa and Staurozoa (fig. 2A). All species from the clades Capitata (M. platyphylla and P. tiarella), Filifera III (C. multicornis) and VI (N. bachei), and Leptothecata (L. flexuosa and O. longissima) had a genome organization resembling that of C. aphrodite, with the exception of a duplicated cox1 downstream of cob and the loss of ORF314-polB (fig. 2A; for the description of hydrozoan subclades, see Cartwright et al. 2008). The genome organization in the aplanulate E. larynx was similar to H. oligactis, where rnl was inverted and both tRNA genes (trnM and trnW) moved compared with the other hydrozoans. Interestingly, all nontrachyline hydrozoan species sequenced in this study had two identical copies of cox1, one at each end of the mitochondrial chromosome. In comparison, the three previously published Hydridae mitochondrial genomes (Hydra magnipapillata, H. oligactis, and H. vulgaris) were reported to have only one complete copy of cox1 downstream of cob, with the other copy(ies) being partially degenerated and lacking the 5′ end of the gene (Kayal and Lavrov 2008; Voigt et al. 2008). Furthermore, in H. magnipapillata and H. vulgaris, the mtDNA was split into two nearly equal sized chromosomes, a feature also reported for several other species belonging to the family Hydridae (Warrior and Gall 1985; Bridge et al. 1992; Ender and Schierwater 2003).
Overall, the mitochondrial gene order in medusozoans is well conserved, with only four unique gene orders found so far (ignoring genome fragmentation and cox1 degeneration in Hydridae). We inferred the evolution of mitochondrial genome organization in medusozoans using the currently accepted cnidarian phylogeny (fig. 2B; Collins et al. 2006; Cartwright et al. 2008). Gene order similar to the scyphozoan A. aurita is found in all four of the recognized medusozoan classes. Given the reportedly low rate of genome rearrangement in animals, it is most parsimonious to reconstruct the mitochondrial ancestral medusozoan gene order (AMGO) as identical to the gene order found in A. aurita (fig. 2B).
Protein Gene Similarity in Medusozoan mtDNA
Sizes of orthologous protein genes in medusozoan mtDNA showed little variation (0–4%), as compared to anthozoans (0–11%). By contrast, the overall amino acid pairwise identity of protein genes for Medusozoa was highly variable, ranging between 24–96% (average 49% SD = 12) (table 1). Such variability indicates that mtDNA proteins can be good markers for reconstructing the evolutionary history of medusozoans at lower taxonomic levels. Interestingly, the amino acid pairwise identity of protein genes between coronates and other scyphozoans (50% SD = 2) were similar to the values for all other cnidarian classes (50% and 51% with hexa- and octocorals, 38% with cubozoans, 47% with hydrozoans, and 48% with staurozoans), with a mean of 49% SD = 4 to all cnidarians.
The minimally derived genetic code (TGA = tryptophan) was inferred for mitochondrial protein synthesis in all the medusozoan genomes analyzed in this study. Codon usage bias estimated as the effective number of codons Nc (Wright 1990) was highly correlated with the GC content of the mtDNAs (R2 = 0.97), where the AT-rich hydrozoan mtDNA displayed the highest codon usage bias (35 SD = 4), and the lowest values were found in cubozoans and staurozoans (47 SD = 4 and 47 SD = 1 respectively). Within a given codon family, codons ending with A or T were favored over those ending with C or G. CGN codons were nearly absent from the mtDNA of cubozoans, hydrozoans, and scyphozoans, where they represented < 15% of arginine residues with no CGG codon used in hydrozoans. In contrast, CGN codons encoded about 50% of arginine residues in staurozoans.
ATG was inferred as the initiation codon for most mitochondrial coding sequences, yet, we inferred GTG as a start codon for two genes in Hydrozoa (cob in L. flexuosa and O. longissima, and cox1 in E. larynx) and five genes in Schyphozoa (atp8 in L. unguiculata, nad1 in P. noctiluca, nad3 in all but two species, nad4L in C. mosaicus, and nad5 in Chrysaora sp) (table 1). GTG was also the inferred initiation codon in at least 5 out of the 13 protein genes in Cubozoa, and mostly present in the order Chirodropida. Both stop codons TAA and TAG were found in all medusozoan classes (80% and 20% of the genes respectively, where the former value represents both TAA and incomplete TA stop codons). Incomplete stop codons TA were inferred for several protein genes in medusozoan mtDNA, predominantly in atp6, atp8, nad3, and nad4L (table 1). In the aplanulatan E. larynx the nearest stop codon for nad5 is located 30 nucleotides past the 5′ end of rns. Interestingly, no stop codon was inferred for nad5 in the scyphozoan P. noctiluca, as the nearest stop codon is found 77 nucleotides within rns.
RNA Genes in Medusozoan mtDNA
All complete medusozoan mt-genomes analyzed in this study contained four RNA genes typical for Cnidaria: two genes coding for large and small subunit rRNA (rnl and rns), and two for methionine and tryptophan tRNAs (trnM(cau) and trnW(uca)). The genes for the small and large ribosomal RNA subunits were on average 17% smaller in size and displayed less size variation in medusozoans compared to their homologues from anthozoans (table 1). The nucleotide composition of RNA genes was slightly more AT-rich than that of the protein coding regions.
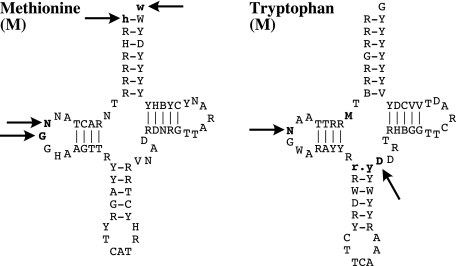
Both medusozoan mt-tRNA genes were more variable in primary sequence and inferred secondary structure than their homologues in anthozoans and demosponges (Wang and Lavrov 2008). The initiator was on average 70 nucleotides long, ranging from 68 in staurozoans to 71 in hydrozoans and scyphozoans. The shorter in Staurozoa lacked two nucleotides at positions 16 and 17 of the D-loop (fig. 3). The anticodon arm in all hydrozoan and staurozoan had an extra base pair typical to type 7 tRNA (Watanabe et al. 1994; Steinberg and Cedergren 1995; Steinberg et al. 1997), resulting in a shorter variable loop (three nucleotides) in the hydrozoans, but a standard four-nucleotides long variable loop in staurozoans. The of all medusozoans but Hydra species had two nucleotides (8 and 9) in the connector region between the acceptor and the D-arm, which makes the loss of nucleotide 9 in mitochondrial a synapomorphy for the Hydridae family. Finally, all hydrozoans but C. aphrodite and all staurozoans lacked nucleotide 16 in the D-loop of The partial cubozoans mitochondrial sequences determined for this study harbored no tRNA genes.
FIG. 3.—
Consensus secondary structure of tRNAs encoded by the mtDNA of Hydrozoa, Scyphozoa, and Staurozoa. Bold letters with black arrows correspond to position that are missing in some taxa: in , two nucleotides at positions 16 and 17 of the D-loop in Staurozoa, and positions 1 and 71 in the acceptor stem of the hydrozoan N. bachei; in , position 9 of the connector region between the acceptor and the D-arm in the Hydridae family, position 16 of the D-loop in staurozoan and all hydrozoan but the trachyline Cubaia aphrodite, and position 42 of the variable loop in all hydrozoan. Bold letters without arrow correspond to an extra base pair in the anticodon arm of in Hydrozoa and Staurozoa, typical to type 7 tRNAs.
Intergenic and Putative Control Regions in Medusozoan mtDNA
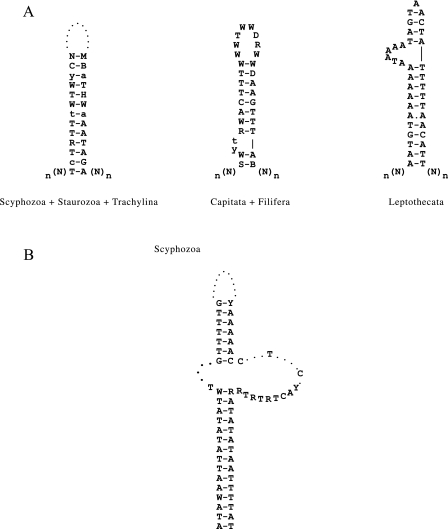
We found a single, relatively large (60–94 nucleotides) and AT-rich (> 70%) IGR between cox1 and cox2 in all scyphozoan and staurozoan species, and in the trachyline hydrozoan C. aphrodite mtDNA (fig. 2A). This IGR delimits two sets of genes with opposite transcriptional polarity. A large portion of this region can be folded into a conserved stem-loop motif with up to 13-base pairs in the stem, most of them (23 bases) completely conserved in all scyphozoans sampled in this study and some (12 bases) also conserved in staurozoans and in the hydrozoan C. aphrodite (fig. 4A). In most other hydrozoan groups (Filifera, Capitata, and Leptothecata), the largest IGR was located between cox2 and rnl, two genes with opposite transcriptional polarities (fig. 2A). This IGR also harbored a 20 nucleotide-long sequence that, while displaying low sequence conservation between hydrozoan species, could be folded into a strong stem-loop element (fig. 4A).
FIG. 4.—
Putative control region and terminal stem-loop found in medusozoan mtDNA. (A) Consensus sequence of the putative control regions (CR) found in the mtDNA of Hydrozoa, Scyphozoa, and Staurozoa. This CR corresponds to the largest IGR that delimits two sets of genes with opposite transcriptional polarity. (B) Putative stem-loop structure found at the end of scyphozoan mtDNA.
In aplanulatan hydrozoans, we found a relatively large (42–52 nt) noncoding region between cox3 and nad2 that displayed up to 70% similarity with trnW sequences. Finally we did not find major IGRs in cubozoan mtDNA.
The “Ends” of Medusozoan mtDNA: Extra ORFs versus Duplicated Genes
All species of Staurozoa, discomedusan Scyphozoa (the sequence of the corresponding region in the coronate scyphozoan L. unguiculata remains undetermined), and the trachyline hydrozoan C. aphrodite contained two ORFs at one end of the molecule. In Cubozoa, analogous ORFs were found on a separate mitochondrial chromosome. The sequences of the larger ORF were similar with polB described in A. aurita (Shao et al. 2006) and formed a monophyletic group in a phylogenetic analysis that included polB from other organisms (supplementary fig. S2, Supplementary Material online). The sequence of the smaller ORF (ORF314) was poorly conserved within Medusozoa, with only two identical amino acid residues between the three classes. This ORF encoded about 100 amino acids and was always located upstream of polB. Although preliminary analysis using 3D modeling of the tertiary structure of the putative product of ORF314 suggests similarities with elongation factors and a potential DNA-binding activity (data not shown), further studies are needed to investigate the role of this gene.
The mtDNA of all hydrozoans but the trachyline C. aphrodite lacked the two extra ORFs (polB and ORF314) (fig. 2A). Furthermore, we were not able to identify copies of these genes in the nuclear genome of H. magnipapillata (Chapman et al. 2010). Instead, we found a complete copy of cox1 at each end of the mitochondrial chromosome (fig. 2A). The two copies of cox1 had opposite transcriptional polarities and formed part of the inverted terminal repeats (ITRs, fig. 2A). In addition, we found a relatively conserved 116 bp sequence downstream of cox1 in A. aurita and Cassiopea spp. mtDNAs that could be folded into a complex stem-loop structure (fig. 4B). The presence of ITRs is a hallmark for the linear mitochondrial genomes and has been reported in several distantly related organisms (Valach et al. 2011 and references therein).
Discussion
Linear mitochondrial DNA has been well documented in several nonmetazoan groups such as plants and fungi. In yeast, where linear mtDNA has been extensively studied, the distribution of linear and circular mitochondrial genomes has no apparent phylogenetic signal, with closely related species having either linear or circular mtDNA (Nosek et al. 1998; Nosek and Tomaska 2008). In animals, strictly linear mtDNA has been described only in Medusozoa (Warrior and Gall 1985; Bridge et al. 1992), for which only six genomes have been published to date (Shao et al. 2006; Kayal and Lavrov 2008; Voigt et al. 2008; Park, Hwang, et al. 2011). The only other report of linear mtDNA in animals comes from the crustacean isopod, Armadillidium vulgare, where the mtDNA consists of a circular ∼28 kb dimer and a linear ∼14 kb monomer (Raimond et al. 1999; Marcadé et al. 2007). Our study represents the first thorough survey of the evolution of linear mtDNA in Medusozoa. In order to better understand the evolutionary history of mtDNA linearity in this group, we surveyed species belonging to all medusozoan classes, as well as their primary subclades.
We analyzed medusozoan mitochondrial genomes in a phylogenetic framework and propose the following hypotheses for the evolution of linear mtDNA in this group: 1) a single origin for the linear mtDNA in the lineage leading to Medusozoa, most likely by the integration of a linear plasmid; 2) loss of polB and ORF314, and the duplication of cox1 at each end of the linear mitochondrial chromosome after the divergence of Trachylina from the rest of the hydrozoans (Hydroidolina); 3) gene rearrangement at the stem of Aplanulata; 4) partial loss of cox1 sequence in Hydridae and split of the mitochondrial chromosome in several species within this clade; 5) high level of mtDNA fragmentation in the branch leading to Cubozoa; 6) displacement of trnW in the coronate L. unguiculata (fig. 2B). It is worth noting that the fragmentation of the mitochondrial molecules appears to be an irreversible process as no rearranged mitochondrial genomes have been observed in the taxa where mtDNA is fragmented, as one would expect if different pieces would fuse randomly.
Fukuhara et al. (1993) suggested a positive correlation between the mitochondrial gene order conservation and the linear as opposed to the circular structure of the yeast mtDNA. Our study revealed a similar trend. If we ignore the genome fragmentation and the cox1 duplication, only four unique gene orders can be distinguished in the medusozoan mtDNA: the AMGO is found in all four medusozoan classes, with modified gene orders found in the coronate L. unguiculata, Hydroidolina, and Aplanulata (fig. 2A). By contrast, at least nine unique gene orders (more if the presence/the absence of ORFs is considered) have been reported in the circular mtDNA of anthozoans (Uda et al. 2011). Furthermore, most of the differences in the medusozoan mitochondrial gene orders can be explained by a few simple gene rearrangement events, whereas multiple and/or complex rearrangement events are needed to explain differences in the anthozoan gene orders. These observations suggest that the linearization has favored the stabilization of the mitochondrial genome organization in Medusozoa. On the other hand, this result may to some extent reflect the difference in the species richness in Anthozoa versus Medusozoa (about twice as many species in the former; Daly et al. 2007) and/or the number of mitochondrial genomes that have been studied in each group (43 vs. 30).
One other difference between the anthozoan and medusozoan mtDNA is the amount and distribution of noncoding intergenic DNA. Anthozoan mtDNA usually has several relatively large IGRs that sometimes contain repetitive sequences and/or ORFs (Park, Song, et al. 2011). By contrast, medusozoan mitochondrial genomes are compactly arranged with few if any intergenic nucleotides and, sometimes, overlapping genes. Most mitochondrial genomes investigated in this study contained only a single moderately large (<100 bp) IGR separating the two regions of mtDNA with opposite transcriptional polarities. The conservation of sequence and inferred secondary structure of this region suggest that it may be involved in the control of mtDNA transcription and/or replication in Scyphozoa, Staurozoa, and trachyline Hydrozoa. Similarly, the largest IGR in other hydrozoans (situated between trnM and cox1 in Aplanulata and between cox2 and rnl in other hydroidolinans) may be involved in the control of transcription as suggested earlier (Voigt et al. 2008). Compactly arranged mtDNA with a single large IGR involved in the initiation of replication (Desjardins and Morais 1990) and transcription (L'Abbé et al. 1991) is also a feature of bilaterian animals. Interestingly, the maturation of a polycistronic pre-mRNA in bilaterian mitochondria involves the excision of tRNA sequences located between most coding sequences in the genome, resulting in monocistronic mRNAs (Ojala et al. 1981). Based on the near absence of IGRs, one can assume that the transcription of medusozoan mtDNA is also polycistronic. However, the loss of all but two tRNA genes necessitates a different mechanism for mRNA maturation in medusozoan mitochondria. Finally, the absence of large IGRs and the conservation of unidirectional transcriptional orientation of all genes in cubozoan mtDNA suggest that the initiation of replication and transcription occurs at the end(s) of each of the mitochondrial chromosomes.
We found two distinctive genetic elements at the end of the medusozoan mitochondrial chromosomes: duplicated cox1 in the hydroidolinan hydrozoans and two extra ORFs (polB and ORF314) in all the other medusozoan classes (Cubozoa, discomedusan Scyphozoa, Staurozoa, and trachyline Hydrozoa). In an earlier study, Shao et al. (2006) showed that polB from the scyphozoan A. aurita mtDNA shares several conserved motifs characteristic of the polymerase domain in family B-DNA polymerases. The authors also attributed the origin of the two extra protein genes to an ancient invasion event by a linear plasmid that resulted in the linearization of the mtDNA in Medusozoa. Similar polB-like sequences are found in the linear mtDNA of several nonanimal groups such as fungi and algae, where they are hypothetically associated with the integration of linear plasmids in the mitochondrial genome (Mouhamadou et al. 2004). The grouping of medusozoan polB sequences into a monophyletic clade suggests a single invasion event early in the evolutionary history of the group that coincided with the origin of its linear mtDNA at the stem of the medusozoan tree. Yet, the low level of sequence conservation in polB-like sequences between medusozoan and nonanimal species hinders our attempts to predict the source of the invading element. Nevertheless, the conservation of both sequence length and position of the two ORFs suggests some level of selection pressure for their maintenance in the mtDNA of most medusozoans.
Short ITRs have been previously reported in the mtDNA of A. aurita (Shao et al. 2006) and the hydrozoans H. magnipapillata, H. oligactis, and H. vulgaris (Kayal and Lavrov 2008; Voigt et al. 2008). ITRs are one out of the few telomeric structures found in the linear mtDNAs (Nosek et al. 1998; Nosek and Tomáska 2003) and involved in the maintenance and replication of the linear chromosomes. In yeast, short ITRs are associated with type III linear mtDNA, where a TP covalently binds at the single-stranded 5′ end of the linear molecules (Nosek and Tomaska 2008). The linear molecules generally encode TPs, either as part of a DNA polymerase gene or by a yet unidentified ORF (Fricova et al. 2010 and references therein). In the medusozoan mtDNA, the putative product of ORF314, if shown to have DNA-binding properties, may act as a TP. Consequently, assuming polB and ORF314 are functional genes, we predict that the mtDNA in cubozoans, scyphozoans, staurozoans, and trachyline hydrozoans uses mechanisms of maintenance and replication similar to the type III linear mtDNA as found in yeasts, linear plasmids, and adenoviral DNA. This assertion is further supported by the similarity of polB sequences between the medusozoan and linear plasmids. As described above, both polB and ORF314 are absent from nontrachyline hydrozoan mtDNAs, and no homologues could be found in the completely sequenced nuclear genome of H. magnipapillata (Hydridae, Hydrozoa). Thus, unlike what has been suggested by an earlier study (Voigt et al. 2008), hydrozoans appear to employ an alternative mechanism for the maintenance and replication of their mtDNA. However, the recruitment of a nuclear encoded TP for the maintenance of linear mtDNA in these hydrozoans cannot be ruled out. In addition, the duplication of cox1 at each end of the mitochondrial chromosome(s) is limited to the lineage leading to Hydroidolina, suggesting that a novel mechanism evolved after the divergence of Trachylina and the rest of hydrozoans. Future studies that explore the end conformations of the medusozoan mtDNA will shed light on the maintenance and processing of these linear molecules.
Despite our efforts, we were not able to verify the expression of polB and ORF314 at the RNA level, most likely as a consequence of poor RNA conservation in the preserved tissue samples. We were also unable to investigate the processes involved in the transcription and the replication of the mtDNA in medusozoans. The analysis of a data set containing ESTs from the scyphozoan C. capillata was also unsuccessful, highlighting the difficulties associated in working with the RNA in this group. Interestingly, in expressed sequence tag (EST) assemblies obtained from the staurozoan H. “sanjuanensis,” we found a single mitochondrial hit that spans from the 3′-end of cox1 to the 5′-end of rnl, suggesting that these genes are transcribed in a polycistronic manner. Yet, additional studies are needed to shed light on the role of the two extra ORFs (polB and ORF314) and their putative products in the maintenance of linear mitochondrial chromosomes in medusozoans, and on the mechanisms of mitochondrial gene expression in the group.
Conclusion
Medusozoan mtDNA is typically a small compact genome composed of one or several linear chromosomes with a highly conserved gene order, and ITRs at the end of the molecule(s). The linearity of the medusozoan mtDNA is most likely the product of a unique invasion event by a linear plasmid-type element harboring a polB-like and a putative TP gene, with secondary loss of the two extra ORFs within hydrozoans. Medusozoan mitochondrial genomes lack large IGRs, contain several overlapping genes, and harbor shorter protein and ribosomal genes than the representatives from other nonbilaterian clades (Signorovitch et al. 2007; Wang and Lavrov 2008). Besides their strictly linear mitochondrial genomes, a unique feature in animals, the meduzosoan mtDNA display characteristics that can be described as intermediary between bilaterian and nonbilaterian animals. On the one hand, medusozoans use a minimally derived genetic code for mitochondrial protein synthesis and show a relatively low evolution rate of their mitochondrial genes, as found in most nonbilaterian animals. On the other hand, the compactness of their genomes and a relatively stable gene order, both, mirror the evolution of the bilaterian mtDNA. Put into a broader perspective, our results show that the evolution of the animal mitochondrial genomes does not follow a single linear pathway as proposed in an earlier study (Lavrov 2007). This is well illustrated in the cnidarian clade where both bilaterian-like and nonbilaterian-like mitochondrial features are found. Future studies that focus on undersampled animal taxa may further expose the rather unpredictable evolutionary pattern of genome evolution in animal mitochondria, revealing a more complex picture of mtDNA evolution in Metazoa. In addition, we predict that a larger survey of linear mtDNA in medusozoans may uncover original/new solutions to the “the challenge of the ends” (Nosek et al. 1998).
Supplementary Material
Supplementary figs. S1 and S2 are available at Genome Biology and Evolution online (http://www.gbe.oxfordjournals.org/).
Acknowledgments
We would like to thank Dr Michael Dawson and Dr Alexander Ereskovsky for their contributions to the sampling effort of Scyphozoa, Dr Janet Voight for providing us with DNA from the staurozoan L. janetae, and Dr Annette F. Govindarajan for providing us with the hydrozoxan O. longissima. We also thank Dr Casey Dunn and the Cnidarian Tree of Life project (DEB-0531779 to Paulyn Cartwright and A.G.C.) for granting access to EST data from the scyphozoan C. capillata and H. “sanjuanensis.” We are very grateful to Dr Peter Schuchert for the identification of our hydrozoan samples. B.B. wishes to acknowledge funding through the USNational Science Foundation Doctoral Dissertation Grant DEB 0910237. E.K. wishes to acknowledge the funding through the Smithsonian Predoctoral Fellow grant. This work was supported by a grant from the National Science Foundation to D.V.L. (DEB-0829783) and by the funding from the Iowa State University.
References
- Beagley CT, Okimoto R, Wolstenholme DR. The mitochondrial genome of the sea anemone Metridium senile (Cnidaria): introns, a paucity of tRNA genes, and a near-standard genetic code. Genetics. 1998;148:1091–1108. doi: 10.1093/genetics/148.3.1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bendich AJ. Reaching for the ring: the study of mitochondrial genome structure. Curr Genet. 1993;24:279–290. doi: 10.1007/BF00336777. [DOI] [PubMed] [Google Scholar]
- Boore JL. Animal mitochondrial genomes. Nucleic Acids Res. 1999;27:1767–1780. doi: 10.1093/nar/27.8.1767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bridge D, Cunningham CW, Schierwater B, DeSalle R, Buss LW. Class-level relationships in the phylum Cnidaria: evidence from mitochondrial genome structure. Proc Natl Acad Sci U S A. 1992;89:8750–8753. doi: 10.1073/pnas.89.18.8750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burger G, Gray MW, Lang BF. Mitochondrial genomes: anything goes. Trends Genet. 2003;19:709–716. doi: 10.1016/j.tig.2003.10.012. [DOI] [PubMed] [Google Scholar]
- Burger G, Lavrov DV, Forget L, Lang BF. Sequencing complete mitochondrial and plastid genomes. Nat Protoc. 2007;2:603–614. doi: 10.1038/nprot.2007.59. [DOI] [PubMed] [Google Scholar]
- Cartwright P, et al. Phylogenetics of Hydroidolina (Hydrozoa: cnidaria) J Mar Biol Assoc UK. 2008;88:1663–1672. [Google Scholar]
- Chapman JA, et al. The dynamic genome of Hydra. Nature. 2010;464:592–596. doi: 10.1038/nature08830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen C, Chiou C-Y, Dai C-F, Chen CA. Unique mitogenomic features in the scleractinian family pocilloporidae (scleractinia: astrocoeniina) Mar Biotechnol (NY). 2008;10:538–553. doi: 10.1007/s10126-008-9093-x. [DOI] [PubMed] [Google Scholar]
- Collins AG, Winkelmann S, Hadrys H, Schierwater B. Phylogeny of Capitata and Corynidae (Cnidaria, Hydrozoa) in light of mitochondrial 16S rDNA data. Zool Scr. 2005;34:91–99. [Google Scholar]
- Collins AG, et al. Medusozoan phylogeny and character evolution clarified by new large and small subunit rDNA data and an assessment of the utility of phylogenetic mixture models. Syst Biol. 2006;55:97–115. doi: 10.1080/10635150500433615. [DOI] [PubMed] [Google Scholar]
- Collins AG, et al. 2008. Phylogenetics of Trachylina (Cnidaria: Hydrozoa) with new insights on the evolution of some problematical taxa. J Mar Biol Assoc UK. 88:1673. [Google Scholar]
- Daly M, et al. The phylum Cnidaria: a review of phylogenetic patterns and diversity 300 years after Linnaeus*. Zootaxa. 2007;182:127–128. [Google Scholar]
- Delsuc F, Brinkmann H, Philippe H. Phylogenomics and the reconstruction of the tree of life. Nat Rev Genet. 2005;6:361–375. doi: 10.1038/nrg1603. [DOI] [PubMed] [Google Scholar]
- Desjardins P, Morais R. Sequence and gene organization of the chicken mitochondrial genome. A novel gene order in higher vertebrates. J Mol Biol. 1990;212:599–634. doi: 10.1016/0022-2836(90)90225-B. [DOI] [PubMed] [Google Scholar]
- Ender A, Schierwater B. Placozoa are not derived Cnidarians: evidence from molecular morphology. Mol Biol Evol. 2003;20:130–134. doi: 10.1093/molbev/msg018. [DOI] [PubMed] [Google Scholar]
- Faure E, Casanova J-P. Comparison of chaetognath mitochondrial genomes and phylogenetical implications. Mitochondrion. 2006;6:258–262. doi: 10.1016/j.mito.2006.07.004. [DOI] [PubMed] [Google Scholar]
- Fricova D, et al. The mitochondrial genome of the pathogenic yeast Candida subhashii: GC-rich linear DNA with a protein covalently attached to the 5’ termini. Microbiology. 2010;156:2153–2163. doi: 10.1099/mic.0.038646-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukuhara H, et al. Linear mitochondrial DNAs of yeasts: frequency of occurrence and general features. Mol Cell Biol. 1993;13:2309–2314. doi: 10.1128/mcb.13.4.2309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gissi C, Iannelli F, Pesole G. Evolution of the mitochondrial genome of Metazoa as exemplified by comparison of congeneric species. Heredity. 2008;101:301–320. doi: 10.1038/hdy.2008.62. [DOI] [PubMed] [Google Scholar]
- Helfenbein KG, Fourcade HM, Vanjani RG, Boore JL. The mitochondrial genome of Paraspadella gotoi is highly reduced and reveals that chaetognaths are a sister group to protostomes. Proc Natl Acad Sci U S A. 2004;101:10639–10643. doi: 10.1073/pnas.0400941101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hikosaka K, et al. Divergence of the mitochondrial genome structure in the apicomplexan parasites, Babesia and Theileria. Mol Biol Evol. 2010;27:1107–1116. doi: 10.1093/molbev/msp320. [DOI] [PubMed] [Google Scholar]
- Kayal E, Lavrov DV. The mitochondrial genome of Hydra oligactis (Cnidaria, Hydrozoa) sheds new light on animal mtDNA evolution and cnidarian phylogeny. Gene. 2008;410:177–186. doi: 10.1016/j.gene.2007.12.002. [DOI] [PubMed] [Google Scholar]
- Kumar RA, Bendich AJ. Distinguishing authentic mitochondrial and plastid DNAs from similar DNA sequences in the nucleus using the polymerase chain reaction. Curr Genet. 2011;57(4):287–295. doi: 10.1007/s00294-011-0342-6. [DOI] [PubMed] [Google Scholar]
- L'Abbé D, Duhaime JF, Lang BF, Morais R. The transcription of DNA in chicken mitochondria initiates from one major bidirectional promoter. J Biol Chem. 1991;266:10844–10850. [PubMed] [Google Scholar]
- Laslett D, Canbäck B. ARWEN: a program to detect tRNA genes in metazoan mitochondrial nucleotide sequences. Bioinformatics. 2008;24:172–175. doi: 10.1093/bioinformatics/btm573. [DOI] [PubMed] [Google Scholar]
- Lavrov DV. Key transitions in animal evolution: a mitochondrial DNA perspective. Integr Comp Biol. 2007;47:734–743. doi: 10.1093/icb/icm045. [DOI] [PubMed] [Google Scholar]
- Lowe TM, Eddy SR. tRNAscan-SE: a program for improved detection of transfer RNA genes in genomic sequence. Nucleic Acids Res. 1997;25:955–964. doi: 10.1093/nar/25.5.955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcadé I, et al. Structure and evolution of the atypical mitochondrial genome of Armadillidium vulgare (Isopoda, Crustacea) J Mol Evol. 2007;65:651–659. doi: 10.1007/s00239-007-9037-5. [DOI] [PubMed] [Google Scholar]
- Medina M, Collins AG, Takaoka TL, Kuehl JV, Boore JL. Naked corals: skeleton loss in Scleractinia. Proc Natl Acad Sci U S A. 2006;103:9096–9100. doi: 10.1073/pnas.0602444103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer M, Stenzel U, Hofreiter M. Parallel tagged sequencing on the 454 platform. Nat Protoc. 2008;3:267–278. doi: 10.1038/nprot.2007.520. [DOI] [PubMed] [Google Scholar]
- Miyamoto H, Machida RJ, Nishida S. Complete mitochondrial genome sequences of the three pelagic chaetognaths Sagitta nagae, Sagitta decipiens and Sagitta enflata. Comp Biochem Physiol Part D Genomics Proteomics. 2010;5:65–72. doi: 10.1016/j.cbd.2009.11.002. [DOI] [PubMed] [Google Scholar]
- Moret BME, Warnow T. Advances in phylogeny reconstruction from gene order and content data. Methods Enzymol. 2005;395:673–700. doi: 10.1016/S0076-6879(05)95035-0. [DOI] [PubMed] [Google Scholar]
- Mouhamadou B, Barroso G, Labarère J. Molecular evolution of a mitochondrial polB gene, encoding a family B DNA polymerase, towards the elimination from Agrocybe mitochondrial genomes. Mol Genet Genomics. 2004;272:257–263. doi: 10.1007/s00438-004-1050-4. [DOI] [PubMed] [Google Scholar]
- Nosek J, Tomaska L. Mitochondrial telomeres: alternative solutions to the end-replication problem. In: Krupp G, Parwaresch R, editors. Telomeres, telomerases and cancer. New York: Kluwer/Plenum; 2002. pp. 396–441. [Google Scholar]
- Nosek J, Tomáska L. Mitochondrial genome diversity: evolution of the molecular architecture and replication strategy. Curr Genet. 2003;44:73–84. doi: 10.1007/s00294-003-0426-z. [DOI] [PubMed] [Google Scholar]
- Nosek J, Tomaska L. Mitochondrial telomeres: an evolutionary paradigm for the emergence of telomeric structures and their replication strategies. In: Nosek J, Tomaska L, editors. Origin and evolution of telomeres. Austin (TX): Landes Bioscience; 2008. pp. 162–171. [Google Scholar]
- Nosek J, Tomáska L, Fukuhara H, Suyama Y, Kovác L. Linear mitochondrial genomes: 30 years down the line. Trends Genet. 1998;14:184–188. doi: 10.1016/s0168-9525(98)01443-7. [DOI] [PubMed] [Google Scholar]
- Ojala D, Montoya J, Attardi G. tRNA punctuation model of RNA processing in human mitochondria. Nature. 1981;290:470–474. doi: 10.1038/290470a0. [DOI] [PubMed] [Google Scholar]
- Papillon D, Perez Y, Caubit X, Le Parco Y. Identification of chaetognaths as protostomes is supported by the analysis of their mitochondrial genome. Mol Biol Evol. 2004;21:2122–2129. doi: 10.1093/molbev/msh229. [DOI] [PubMed] [Google Scholar]
- Park E, Song JI, Won YJ. The complete mitochondrial genome of Calicogorgia granulosa (Anthozoa: octocorallia): potential gene novelty in unidentified ORFs formed by repeat expansion and segmental duplication. Gene. 2011;486(1–2):81–87. doi: 10.1016/j.gene.2011.07.003. [DOI] [PubMed] [Google Scholar]
- Park E, Hwang DS, Lee JS, Song JI, Seo TK, Won YJ. Estimation of divergence times in cnidarian evolution based on mitochondrial protein-coding genes and the fossil record. Mol Phylogenet Evol. doi: 10.1016/j.ympev.2011.10.008. Advance Access published October 20, 2011, doi:10.1016/j.ympev.2011.10.008. [DOI] [PubMed] [Google Scholar]
- Pearson WR. Using the FASTA program to search protein and DNA sequence databases. Methods Mol Biol. 1994;24:307–331. doi: 10.1385/0-89603-246-9:307. [DOI] [PubMed] [Google Scholar]
- Pont-Kingdon G, et al. Mitochondrial DNA of the coral Sarcophyton glaucum contains a gene for a homologue of bacterial MutS: a possible case of gene transfer from the nucleus to the mitochondrion. J Mol Evol. 1998;46:419–431. doi: 10.1007/pl00006321. [DOI] [PubMed] [Google Scholar]
- Raimond R, et al. Organization of the large mitochondrial genome in the isopod Armadillidium vulgare. Genetics. 1999;151:203–210. doi: 10.1093/genetics/151.1.203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rice P, Longden I, Bleasby A. EMBOSS: the European Molecular Biology Open Software Suite. Trends Genet. 2000;16:276–277. doi: 10.1016/s0168-9525(00)02024-2. [DOI] [PubMed] [Google Scholar]
- Shao Z, Graf S, Chaga OY, Lavrov DV. Mitochondrial genome of the moon jelly Aurelia aurita (Cnidaria, Scyphozoa): a linear DNA molecule encoding a putative DNA-dependent DNA polymerase. Gene. 2006;381:92–101. doi: 10.1016/j.gene.2006.06.021. [DOI] [PubMed] [Google Scholar]
- Signorovitch AY, Buss LW, Dellaporta SL. Comparative genomics of large mitochondria in placozoans. PLoS Genet. 2007;3:e13. doi: 10.1371/journal.pgen.0030013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staden R. The Staden sequence analysis package. Mol Biotechnol. 1996;5:233–241. doi: 10.1007/BF02900361. [DOI] [PubMed] [Google Scholar]
- Stajich JE, et al. The Bioperl toolkit: perl modules for the life sciences. Genome Res. 2002;12:1611–1618. doi: 10.1101/gr.361602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinberg S, Cedergren R. A correlation between N2-dimethylguanosine presence and alternate tRNA conformers. RNA. 1995;1:886–891. [PMC free article] [PubMed] [Google Scholar]
- Steinberg S, Leclerc F, Cedergren R. Structural rules and conformational compensations in the tRNA L-form. J Mol Biol. 1997;266:269–282. doi: 10.1006/jmbi.1996.0803. [DOI] [PubMed] [Google Scholar]
- Thompson JD, Higgins DG, Gibson TJ. CLUSTAL W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res. 1994;22:4673–4680. doi: 10.1093/nar/22.22.4673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uda K, et al. Complete mitochondrial genomes of two Japanese precious corals, Paracorallium japonicum and Corallium konojoi (Cnidaria, Octocorallia, Coralliidae): notable differences in gene arrangement. Gene. 2011;476:27–37. doi: 10.1016/j.gene.2011.01.019. [DOI] [PubMed] [Google Scholar]
- Valach M, et al. Evolution of linear chromosomes and multipartite genomes in yeast mitochondria. Nucleic Acids Res. doi: 10.1093/nar/gkq1345. Advance Access published January 25, 2011, doi:10.1093/nar/gkq1345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voigt O, Erpenbeck D, Wörheide G. A fragmented metazoan organellar genome: the two mitochondrial chromosomes of Hydra magnipapillata. BMC Genomics. 2008;9:350. doi: 10.1186/1471-2164-9-350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe Y, et al. Higher-order structure of bovine mitochondrial tRNA(SerUGA): chemical modification and computer modeling. Nucleic Acids Res. 1994;22:5378–5384. doi: 10.1093/nar/22.24.5378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Lavrov DV. Seventeen new complete mtDNA sequences reveal extensive mitochondrial genome evolution within the Demospongiae. PLoS One. 2008;3:e2723. doi: 10.1371/journal.pone.0002723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warrior R, Gall J. The mitochondrial DNA of Hydra attenuata and Hydra littoralis consists of two linear molecules. Arch Sci (Geneva). 1985;38:439–445. [Google Scholar]
- Wolstenholme DR. Animal mitochondrial DNA: structure and evolution. Int Rev Cytol. 1992;141:173–216. doi: 10.1016/s0074-7696(08)62066-5. [DOI] [PubMed] [Google Scholar]
- Wright F. The “effective number of codons” used in a gene. Gene. 1990;87:23–29. doi: 10.1016/0378-1119(90)90491-9. [DOI] [PubMed] [Google Scholar]