Abstract
Objective
Acute low back pain (ALBP) may limit mobility and impose functional limitations in active duty military personnel. Although some manual therapies have been reported effective for ALBP in military personnel, there have been no published randomized controlled trials (RCTs) of osteopathic manipulative treatment (OMT) in the military. Furthermore, current military ALBP guidelines do not specifically include OMT.
Methods
This RCT examined the efficacy of OMT in relieving ALBP and improving functioning in military personnel at Fort Lewis, Washington. Sixty-three male and female soldiers ages 18 to 35 were randomly assigned to a group receiving OMT plus usual care or a group receiving usual care only (UCO).
Results
The primary outcome measures were pain on the quadruple visual analog scale, and functioning on the Roland Morris Disability Questionnaire. Outcomes were measured immediately preceding each of four treatment sessions and at four weeks post-trial. Intention to treat analysis found significantly greater post-trial improvement in ‘Pain Now’ for OMT compared to UCO (P = 0·026). Furthermore, the OMT group reported less ‘Pain Now’ and ‘Pain Typical’ at all visits (P = 0·025 and P = 0·020 respectively). Osteopathic manipulative treatment subjects also tended to achieve a clinically meaningful improvement from baseline on ‘Pain at Best’ sooner than the UCO subjects. With similar baseline expectations, OMT subjects reported significantly greater satisfaction with treatment and overall self-reported improvement (P<0·01).
Conclusion
This study supports the effectiveness of OMT in reducing ALBP pain in active duty military personnel.
Keywords: Low back pain, Manual medicine, Manipulation
Introduction
Pain and disability associated with acute low back pain (ALBP) continues to be an important area of research in the military environment.1 Although it is generally accepted that ALBP is likely to resolve over a brief period of time under normal conditions in civilian populations, active duty military personnel work under unique conditions that continuously stress the musculoskeletal system.2 Thus, interest continues in designing optimum interventions and prevention programs addressing ALBP in active duty military personnel. In 2007, George et al.3 published a prevention protocol for military personnel to avoid low back injuries. However, to date, no outcome research for that protocol has been published. The Department of Defense (DoD) 2009 guidelines recommend increasing core strength in military trainees to prevent problems with low back pain (LBP).4 Those guidelines are based on earlier studies and case reports with limited findings associating core stability with risks for low back injuries.5–7 The continued importance of discovering optimum methods for preventing or rapidly reducing pain and disability associated with ALBP is underscored in Cohen et al.1 in which LBP was found to be a major factor in soldiers not returning to combat duty following sick leave status. Prevention research is of critical importance, but we also need to explore the effectiveness of interventions that may rapidly reduce ALBP and restore soldiers to peak performance.
Current evidence
In a 2009 systematic review of published research in targeted manual therapies and/or exercise for treating LBP in civilians, Kent et al.8 reviewed only four RCTs and found insufficient evidence to support any specific treatment. Following that review, the Cochrane Collaboration reported in April 2010 that insufficient evidence is available to support or refute chiropractic interventions for improving LBP or disability compared to other interventions.9 That same report indicated that no controlled clinical trials had been published comparing combined chiropractic treatments to no-treatment. Subsequently, in November 2010, the American Osteopathic Association published guidelines10 for the treatment of LBP, citing multiple clinical trials on the efficacy of osteopathic manipulative treatment (OMT) for LBP.11–13 In those clinical trials, OMT has been found to be effective in civilian patients in decreasing pain and medication use and improving functioning.
Osteopathic manipulation (referred to in this study as OMT) is ‘a full-body system of hands-on techniques to alleviate pain, restore function, and promote health and well-being’.14 As a system of techniques, OMT differs from spinal manipulation alone, which is one of a set of techniques. Definitions of OMT and spinal manipulation have been used in previous relevant publications with consistency.9,10,15–18
If OMT can rapidly reduce the severity and length of an episode of ALBP in civilian patients, it may be an important combat environment treatment modality that currently is not routinely utilized. Current military medical care guidelines however, do not specifically include OMT, but do include chiropractic, physical therapy, patient education, and options for pain relief medication.19
Rationale for this clinical trial
Given the lack of published research on the effectiveness of OMT for resolving pain and disabilities associated with ALBP in military personnel, we conducted a randomized controlled trial (RCT) at Fort Lewis, Washington, that was registered at ClinicalTrials.Gov, and completed in 2008.20 Previous research has reported short-term benefits of other manual therapies,21 but this study used a specific four-week OMT protocol, with a baseline assessment, three treatment outcomes assessments at one-week intervals, and one outcomes assessment four-weeks after the last treatment (end-point). Active duty military personnel meeting inclusion and exclusion criteria were randomly assigned to one of two treatment groups, OMT plus usual care or UCO (usual care only).
The primary aim for this study was to determine whether OMT reduced pain more effectively than UCO. We were also interested in whether the OMT group improved more quickly than soldiers under UCO.
Research design and methodological issues
Research designs and methods for studies of manual therapy modalities have matured in the past decade, using refined placebos and measurements, and integrating new perspectives on plausible ‘placebo effects’. There are three refinements relevant to this study. First, researchers have attempted to isolate factors that may clinically predict recovery from ALBP. Although patients with lower than average initial pain intensity and shorter duration of symptoms may recover more quickly than other patients, self-reported level of functioning and pain adaptation seems to predict return to work more reliably than the measure of pain itself.22,23 Our study utilized two reliable self-reported measures of functioning and pain adaptation, the Roland Morris Disability Questionnaire (RMDQ)24 and the Quadruple Visual Analog Scale (QVAS).25
Second, research has suggested that clinically meaningful improvement requires a minimum of 30% change from baseline on pain using the QVAS, and on functioning using the RMDQ.26 Third, Myers et al.27 recently reported that higher expectations for recovery were associated with greater functional improvement. This RCT included a measure of treatment expectation for both study groups.
Research hypotheses
The primary hypothesis for this RCT was that soldiers receiving OMT would experience a greater reduction in pain compared to soldiers under UCO. A secondary hypothesis was that OMT would improve self-reported functioning to a greater extent than UCO. Because of the value placed on rapid restoration of health in military personnel, we were also interested to know whether OMT would improve pain sooner than UCO. We also considered whether treatment expectations would influence treatment outcomes, satisfaction with treatment, and perceived overall improvement. This research was presented as a poster at the 2008 American Osteopathic Association Research Conference. To the best of our knowledge, this is the first published RCT examining the effectiveness of OMT in treating ALBP an active duty military population.
Methods
Organization of the study
This study occurred at the Madigan Army Medical Center (MAMC) in Fort Lewis, Washington. The Osteopathic Research Center at the University of North Texas Health Science Center was the clinical research organization (CRO) for this RCT. The CRO team consisted of two blinded osteopathic physicians, one un-blinded social scientist, and an un-blinded study biostatistician. The CRO worked closely with the MAMC study team led by the site principal investigator (site PI), an active duty osteopathic family physician, who served as the blinded study evaluating physician (SEP). The SEP screened and examined all subjects in a blinded fashion for medical exclusion criteria prior to enrollment and before each study visit, managed all medications related to back pain, and retained these data in separate confidential records on each study subject.
The non-blinded study treatment physicians (STPs) included three osteopathic and one allopathic physicians with previous training in manual medicine who were commissioned officers in the medical corps. The site clinical research coordinator (CRC) reported to the site PI. The CRO designed the protocol and the data collection forms, trained the site STPs and CRC, and managed the data entry and analysis. Sealed randomization envelopes were prepared by the CRO and mailed to the study CRC for insertion into blank study charts for opening by the STP at the first study visit. Assessment charts were kept physically separate from the study treatment charts that were accessible only to the STPs, thereby maintaining blinding of the SEP and the CRC. A Data and Safety Monitoring Board (DSMB) was created to monitor patient safety, establish retention, dropping and stopping criteria, and ensure data collection integrity.
DoD LBP guidelines
The DoD usual care protocol for military personnel with ALBP at the time of the study consisted of the following.
Advice: assuring the soldier that most episodes of ALBP will resolve uneventfully within 6 weeks; encouraging the soldier to maintain as close to normal activity as is tolerable and to avoid bed rest of longer than 24 hours; and advising the use of non-steroidal anti-inflammatory drugs unless contraindicated.
Prescribing muscle relaxants for up to one week or low dose opiates (codeine; propoxyphene) unless contraindicated.
Passive modalities such as ice or heat for symptomatic relief. If the soldier’s pain was not improved in two weeks, guidelines required that the soldier be re-evaluated for ‘red flags,’ a different non-steroidal anti-inflammatory drug considered, and be given a referral to physical therapy while continuing to be followed in the primary care clinic.
Participants
Figure 1 provides details on subjects’ recruitment, enrollment, and retention, and the final number of subjects in the analyses. A power analysis performed prior to the study had indicated that 36 subjects would be needed in each group to detect a medium (0·36) effect size with alpha at 0·05 and 85% power. A total of 63 patients were randomized to a treatment group.
Figure 1.
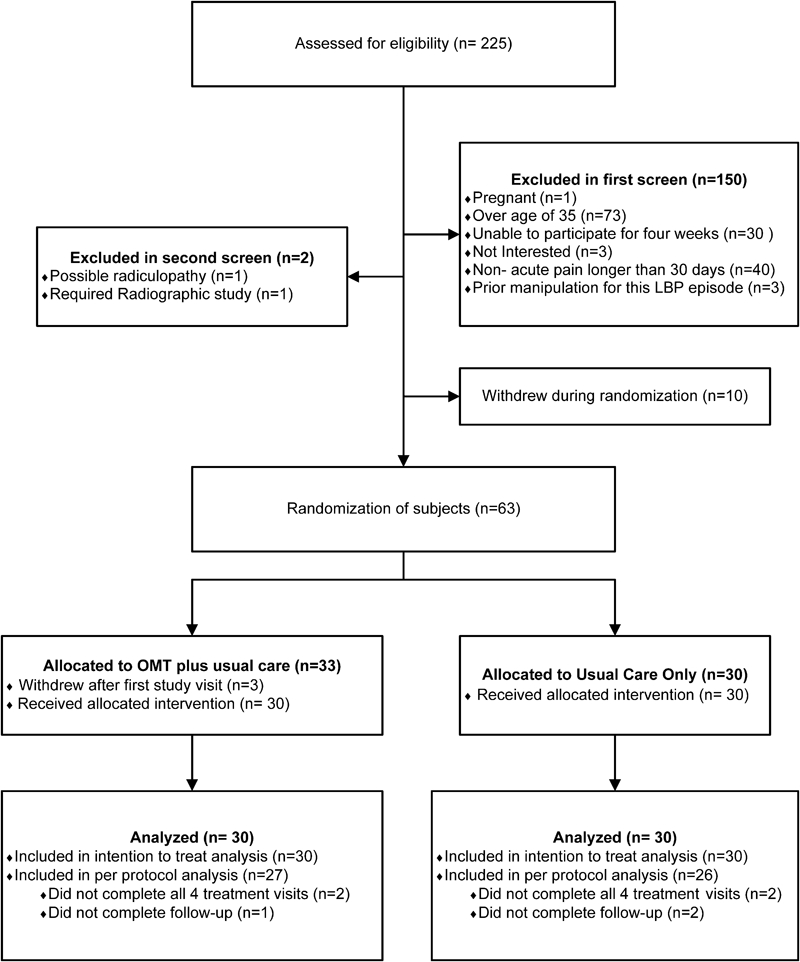
Participant flow.
Soldiers presenting with a new complaint of ALBP, defined as a minimum of 30 days hiatus of pain from previous LBP episodes, were recruited daily at the MAMC clinics. The CRC verified that a soldier met the first level screening criteria. To pass the first screening level, a soldier had to be male or female, of any racial or ethnic origin, and between 18 and 35 years old. If a woman’s onset of her last menstrual cycle was >28 days prior to enrollment, she was given a urine pregnancy test, and excluded from the study if pregnant.
The second level screening was performed by the blinded SEP. A soldier could not enroll in the study if the SEP found evidence of a serious neurological, rheumatologic, or orthopedic condition present on examination, including spondylolysis, spondylolisthesis, fracture, nerve impingement, tumors, or infections. Also, soldiers were not eligible if there was clinical evidence of a leg length discrepancy greater than 13 mm or if their leg pain was worse than their back pain indicating possible radiculopathy. Soldiers could not have had manual therapy for this episode of ALBP. Last, they could not enroll in the study if there was any known inability to give informed consent or the soldier knew at that time that he or she would be unable to stay in the study for the four week protocol and participate in the end-point outcomes measures for the trial.
After enrollment, a subject would be dropped from the study if the SEP determined the need for additional clinical tests such as radiographs or orthopedic consultation, or found a red-flag condition. Red-flag conditions were those the SEP believed required immediate medical diagnostics and/or evaluation for treatment including physical signs or symptoms of abnormal reflexes, neurogenic bowel or bladder, erectile dysfunction, lower extremity weakness or paralysis, or dermatomal sensory loss suggestive of a spinal cord or nerve root injury. One subject was dropped from the study for red-flag symptoms during that person’s second week of enrollment. The DSMB found no other significant patient safety concerns during the study.
Interventions
Figure 2 displays the OMT protocol used in this study. The OMT protocol used in this study has been previously published as it was also used in another research study.28 Treatments occurred once per week for four weeks, not closer than 7 or more than 10 days apart. Treatments were administered by the non-blinded STPs trained in the protocol. Subjects were instructed to refrain from receiving other manual therapies during the four weeks of the trial. The STPs completed four hands-on training sessions in the study OMT protocol taught by two CRO osteopathic physicians, board certified in neuromusculoskeletal medicine and osteopathic manipulative medicine. Two training sessions occurred prior to the start of, and two occurred during the trial. A DVD refresher video was also provided to ensure maintenance of protocol skills and minimize variation among STPs in the application of the treatments.
Figure 2.
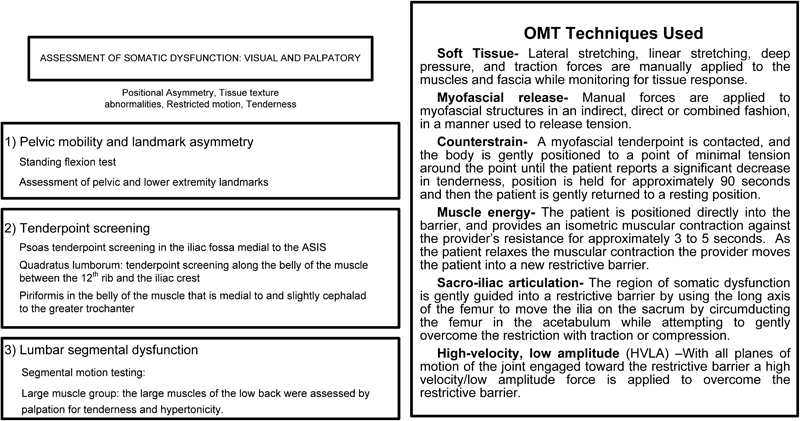
Osteopathic manipulative treatment protocol.
Study outcome measures
The primary outcome of interest in this RCT was pain, with a secondary outcome being back related functioning. The QVAS for aspects of pain intensity was administered using a 10 point scale for each of these aspects: ‘Pain Now’, ‘Pain Typical’, ‘Pain at Worst’, and ‘Pain at Best’. The scale ranged from 1 to 10 with 1 corresponding to ‘No pain’, and 10 corresponding to ‘Worst possible pain’. The RMDQ measured back-specific functioning, with 24 statements that subjects endorse if it describes them at the time of the assessment. We also took a measure of quality of life using the Short Form Health Survey (SF-36) at baseline only to determine if the two treatment groups differed in their self-perceived overall health status. Other measures included a Patient Expectation Questionnaire (PEQ), developed specifically for this trial, one question about perceived overall improvement during the trial on a scale of 1 to 7, and one question regarding satisfaction with treatment received using a scale of 1 to 10.
Medication usage can be addressed using a variety of methods. Medication logs are sometimes used in this type of study. However, asking soldiers to maintain a medication log became impractical and unreliable. Therefore, for this study the blinded SEP’s prescribing practices were obtained from the records. Prescribing practices for both Schedule 1 medications (naproxen, ibuprofen, and acetaminophen) and Schedule 2 medications (cyclobenzaprine and acetaminophen with codeine) were identified in the data collection form.
Study procedures
All subjects followed the same study procedures. There were five study visits, visit 1 through visit 4 for the treatment protocol, and visit 5 for a follow-up end-point interview. Outcome measures for treatment effects were collected one week after the first, second, and third treatment sessions, and four weeks after the last treatment session. Participants received and signed informed consent documents prior to enrollment.
After enrolling in the study, and before randomization and treatment, each subject completed the SF-36, the QVAS, the RMDQ, the PEQ and the one question regarding satisfaction with treatment. Immediately following the completion of baseline measures, the subject proceeded to see the Study Treating Physician (STP) for randomization and the first treatment session. The STP was located in an area of the clinic physically separated from the CRC and SEP offices to protect blinding.
At visit 1, the STP opened the randomization envelope and assigned the study subject to either the OMT group or the usual care only (UCO) group. Subjects assigned to the UCO group left the STP office with instructions to follow the DoD guidelines. Subjects randomized to the treatment group received the first OMT treatment and the DoD guideline instructions.
At visits 2, 3, and 4, the subjects met first with the blinded SEP for red-flag screening and medication evaluation, next with the blinded CRC to complete the QVAS and the RMDQ, and finally with the STP who followed the protocol for each group. Thus, study subjects completed assessments of pain and functioning one-week after each of the first three manipulative medicine treatments. The physical separation between the SEP and CRC offices and the STP treatment room was sufficiently extensive to maintain blinding.
Visit 5 occurred four weeks after the last treatment session at visit 4, at which time the CRC collected a last round of outcome measures, and asked the single question regarding satisfaction with treatment.
The list below specifies the sequence of study visits.
V1 (Randomization): Outcomes at Baseline, followed by Treatment Session 1
V2 (One week Post-Treatment 1): Outcomes Post-treatment 1 followed by Treatment Session 2
V3 (One week Post-Treatment 2): Outcomes Post-treatment 2 followed by Treatment Session 3
V4 (One week Post-Treatment 3): Outcomes Post-treatment 3 followed by Treatment Session 4
V5 (Four weeks Post-Treatment 4): End-point Interview Outcomes Post-Treatment Session 4
Thus, five rounds of outcome measurements for pain and functioning were collected by the CRC.
Data management and statistical methods
Data were recorded in study booklets by the blinded CRC. Study booklets were copied and de-identified, using only a study ID number linked to a list maintained by the CRC, and mailed to the CRO where the data were entered in a blinded fashion. Only the study biostatistician and the DSMB members were informed of the subjects’ respective study groups. As required by the DSMB, the study biostatistician at the CRO performed a preliminary data analysis mid-point during the study for the DSMB, producing open and closed reports. Closed reports were provided to the CRO, the site PI and the CRC. Final analysis was completed in May 2007, and the DSMB released the final reports in June 2008 to the CRO and the sponsor.
Statistical analysis
The DSMB determined that only those subjects with two completed treatment visits would be considered as viable study participants for the analysis. Study subjects with only one visit would have had only pre-treatment baseline data, and no post-treatment data. Therefore, any subjects with only one study visit were not included in the study, and thus not included in any analysis. Data were analyzed using the statistical software package SPSS (version 17·0; SPSS Inc., Chicago, IL, USA). We utilized both intention to treat (ITT) and per-protocol analyses to explore whether the results were different when including only those subjects who completed all outcome measures.
Outcome variables of pain and functioning were treated as continuous variables for analysis, based on the distribution of the underlying construct as well as inspection of baseline distributions for the sample. Chi-square and t-test analyses were used to compare the two treatment groups at baseline.
A repeated measures t-test was used to examine changes between baseline and trial endpoint. We utilized a repeated measures Analysis of Variance to test both the primary hypothesis that the OMT group would report greater improvement in pain and functioning than the UCO group, and to examine the question of whether OMT improved pain and functioning sooner than UCO.
In addition, we were interested in when a clinically meaningful improvement of at least 30% from baseline occurred in pain and functioning. Thus we utilized a Cox Regression Analysis to examine the time to clinically meaningful improvement for the OMT group compared to the UCO group.
Last, we calculated correlation coefficients to examine the possible relationships between treatment expectations and pain and functioning, and performed t-tests to compare the two groups on patient expectations, overall satisfaction and self-reported improvement scores.
Results
As shown in Fig. 1, 60 subjects were included in the analysis. For the ITT analysis, there were 30 subjects in the OMT and 30 in the under UCO group. For the per-protocol analysis, we retained 27 subjects’ data in the OMT group, and 26 in the UCO group, all of whom had completed all five study visits.
Baseline characteristics
Table 1 displays the baseline characteristics for each treatment group. There were no differences between the two groups for age, gender, race/ethnicity, or marital status. There were no significant differences in physical characteristics such as BMI or days of pain, or for their overall self-reported health status as measured by the SF-36. Furthermore, there were no significant differences between the OMT and UCO groups at baseline for pain or functioning. Table 2 displays the QVAS and RMDQ scores for both groups at entry to the study. Specifically, ‘Pain Now’ averages at baseline were 5·23 and 5·53 for the OMT and UCO groups respectively.
Table 1. Descriptive data associated with subjects in each group.
| OMT (n = 30) | UCO (n = 30) | P value* | |
| Age, mean (SD) | 26·3 (5·1) | 27·1 (4·8) | 0·501 |
| Days of pain, mean (SD) | 11·0 (9·7) | 13·3 (22·9) | 0·621 |
| BMI, mean (SD) | 26·4 (3·9) | 27·4 (4·6) | 0·359 |
| Gender, n (%) | |||
| Female | 14 (46·7%) | 13 (43·3%) | 0·795 |
| Male | 16 (53·3%) | 17 (56·7%) | |
| Marital status, n (%) | |||
| Single | 11 (36·7%) | 12 (40·0%) | 0·852 |
| Married | 16 (53·3%) | 14 (46·7%) | |
| Divorced or separated | 3 (10·0%) | 4 (13·3%) | |
| Race/ethnicity, n (%) | |||
| White | 20 (66·7%) | 15 (50·0%) | 0·432 |
| Black | 4 (13·3%) | 8 (26·7%) | |
| Hispanic | 3 (10·0%) | 5 (16·7%) | |
| Other | 3 (10·0%) | 2 (6·7%) | |
| SF36 general health, mean (SD) | 18·8 (4·2) | 18·9 (2·4) | 0·861 |
| SF36 mental health, mean (SD) | 21·1 (3·4) | 20·8 (2·9) | 0·687 |
| SF36 physical functioning, mean (SD) | 22·2 (4·2) | 20·4 (5·1) | 0·147 |
Note: OMT = osteopathic manipulative treatment group; UCO = usual care only group.
*P values represent the probability of difference tested using independent samples t-tests for continuous variables and chi-square tests for categorical variables.
Table 2. Baseline measurements of back pain and functioning.
| OMT (n = 30) | UCO (n = 30) | Difference (95% CI)* at baseline | |
| Mean (SD) | Mean (SD) | ||
| Pain Now | 5·23 (2·11) | 5·53 (2·21) | −0·30 (−1·42 to 0·82) |
| Pain Typical | 5·73 (1·78) | 6·40 (1·99) | −0·67 (−1·64 to 0·31) |
| Pain at Best | 2·60 (1·16) | 3·0 (1·88) | −0·40 (−1·21 to 0·41) |
| Pain at Worst | 8·3 (1·18) | 8·43 (1·48) | −0·13 (−0·82 to 0·56) |
| RMDQ | 12·37 (5·30) | 12·50 (6·02) | −0·13 (−3·06 to 2·80) |
Note: OMT = osteopathic manipulative treatment group; UCO = usual care only group; RMDQ = Roland Morris Disability Questionnaire.
*Represents the difference tested using independent samples t-tests.
Pain and functioning outcomes
Using ITT analysis, we compared the changes in the scores for pain using the QVAS and for functioning using the RMDQ, between baseline and trial end-point (four weeks after the last treatment). Table 3 provides the results of t-tests comparing the changes between groups in pain and functioning between baseline and end-point. There was significantly greater improvement for the OMT group compared to the UCO group on the QVAS measurement for ‘Pain Now’ between baseline and end-point (P = 0·026).
Table 3. Changes in scores baseline to end-point.
| OMT (n = 30) | UCO (n = 30) | Difference (95% CI)* at end-point | |||
| Baseline | End-point | Baseline | End-point | ||
| Mean (SD) | Mean (SD) | Mean (SD) | Mean (SD) | ||
| Pain Now | 5·23 (2·11) | 1·96 (1·47) | 5·53 (2·21) | 3·73 (2·39) | −1·47 (−2·75 to −0·18) |
| Pain Typical | 5·73 (1·78) | 2·15 (1·48) | 6·40 (1·99) | 3·65 (2·16) | −0·84 (−2·09 to 0·41) |
| Pain at Best | 2·60 (1·16) | 1·48 (0·96) | 3·0 (1·88) | 2·15 (1·65) | −0·27 (−1·13 to 0·58) |
| Pain at Worst | 8·3 (1·18) | 4·04 (2·62) | 8·43 (1·48) | 5·23 (2·55) | −1·06 (−2·47 to 0·35) |
| RMDQ | 12·37 (5·30) | 4·44 (5·92) | 12·50 (6·02) | 7·31 (6·3) | −2·73 (−6·32 to 0·86) |
Note: OMT = osteopathic manipulative treatment group; UCO = usual care only group; RMDQ = Roland Morris Disability Questionnaire.
*Represents the difference tested using repeated measures t-tests.
To determine whether OMT improved pain and functioning sooner than UCO, we examined the effects of time and treatment group on pain and functioning with a repeated measures ANOVA. This statistical method provides information about the possible influence of the rate of pain and functioning change in addition to the magnitude of any changes at each study visit and end-point. Figure 3 displays the mean VAS and RMDQ scores for each treatment group at each study visit.
Figure 3.
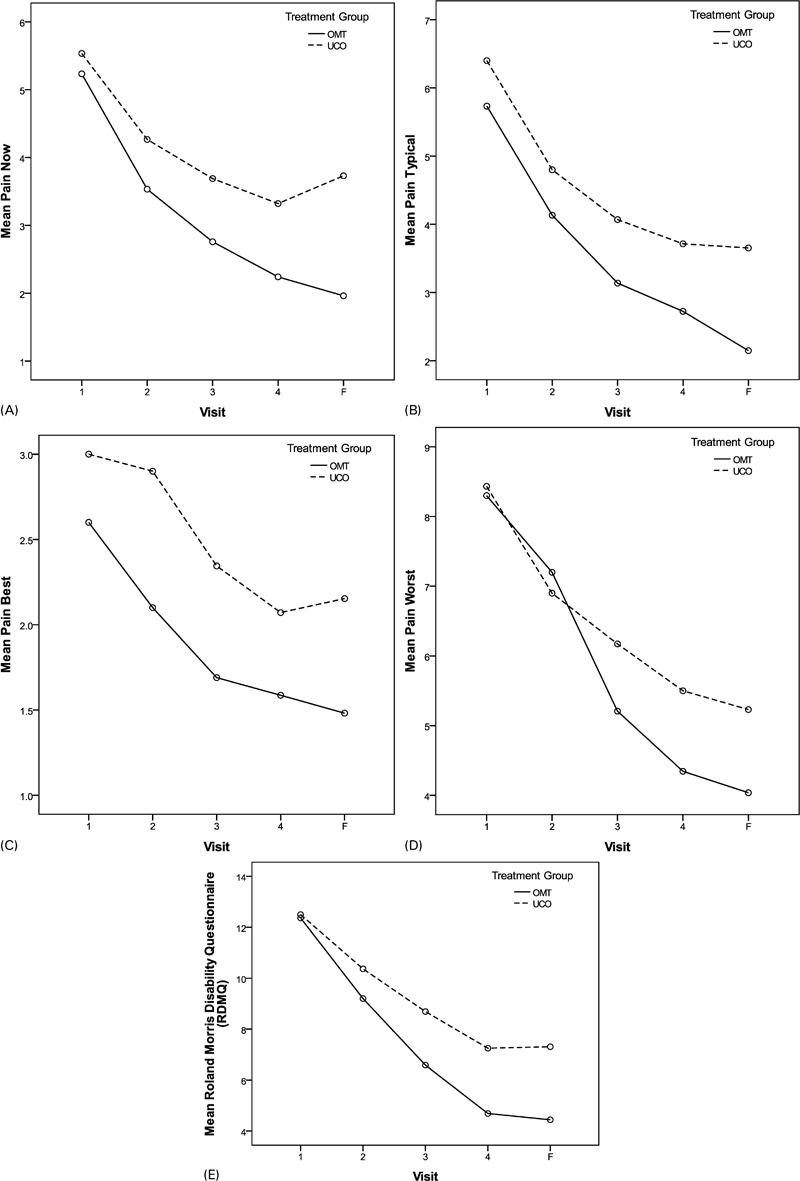
Mean Pain Now, Typical, Best and Worst, and RMDQ by group and visit. OMT = osteopathic manipulative treatment; UCO = usual care only. (A) Effect of time (P<0·001) and treatment group (P = 0·025) were significant, but interaction (P = 0·175) was not. (B) Effect of time (P<0·001) and treatment group (P = 0·020) were significant, but interaction (P = 0·473) was not. (C) Effect of time (P<0·001) was significant; however, group effect (P = 0·065) was not. (D) Effect of time (P<0·001) was significant. Group (P = 0·198) and the time x group interaction (P = 0·080) were not significant. (E) Effect of time (P<0·001) was significant; however, the interaction (P = 0·691) and treatment group (P = 0·197) were not.
There was a significant effect of time on improvement in pain and functioning, with both groups improving on average over time. However, treatment group assignment also influenced the reported levels of ‘Pain Now’ and ‘Typical Pain’ (P = 0·025 and P = 0·020 respectively) with the OMT group reporting greater improvement. Being in the OMT group also appears to have some influence on improvement in the QVAS measure of ‘Pain at Best’ (P = 0·065).
To examine if there were differences in outcomes for patients who completed all four study visits and the follow-up end-point interview, a per-protocol analysis was performed using data from the 53 subjects with all visits. The per-protocol findings were all consistent with the ITT analysis, with the only significant difference being between the treatment groups for change from baseline to end-point for ‘Pain Now’ (P = 0·014).
Clinically meaningful change
In keeping with the previously cited recommendations26 regarding minimally important improvements as measured by the QVAS and the RMDQ, we calculated a minimally important change for each subject, defined as the visit at which the subject experienced a 30% improvement over baseline. Cox Regression survival analysis (see Fig. 4) revealed a significant difference for time to improvement for ‘Pain at Best’, with OMT subjects more likely to achieve a 30% improvement on this pain measure at an earlier visit than the UCO group (P=0.004). It is important to note that 76·7% of the OMT group reported clinically meaningful reductions in ‘Pain at Best’, compared to 43·3% of the UCO group (P = 0·008). For all other QVAS pain measures, improvements between groups were not significantly different. The overall proportion of all subjects regardless of group who reported 30% or greater improvements ranged from 66·7% for ‘Pain at Worst’ to 81·7% for ‘Pain Now’.
Figure 4.
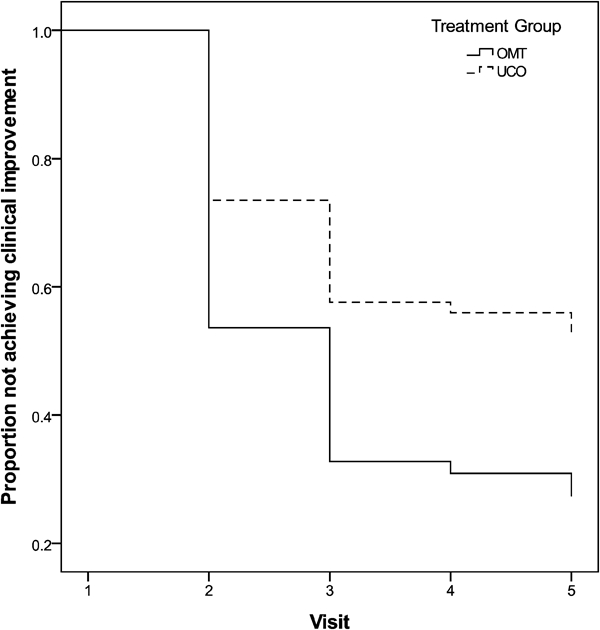
Time to clinical improvement in Pain Best for OMT and UCO groups.
Medications
We considered whether the blinded SEP’s prescribing practices differed for the two treatment groups. We found no differences in the SEP’s prescribing practices for Schedule 1 medications (naproxen, ibuprofen, and acetaminophen) or Schedule 2 medications (cyclobenzaprine and acetaminophen with codeine) between the two groups (P = 0·688, P = 0·791 respectively). Schedule 1 medications were prescribed for 86·7% in the OMT group and 90% in the UCO group. Schedule 2 medications were prescribed for 36·7% of the OMT group and 40% of the UCO group.
Subjects’ reported treatment expectation, overall improvement and treatment satisfaction
For this study we also used a measure of ‘treatment expectation’ with a PEQ to evaluate the extent to which subjects’ expectation or belief in one or another treatment might influence the results. The PEQ was completed at baseline following enrollment, but before randomization. All subjects rated the following four statements on a scale of 1 to 5, with 5 being ‘strongly agree’.
I believe manipulation with standard care will help my LBP.
I believe that standard care alone will help my LBP.
I believe that manipulation plus standard care will improve my level of functioning.
I believe that standard care alone will improve my level of functioning.
We also asked all study subjects to rate their overall satisfaction with their treatment and their perceived overall improvement during the study. Table 4 shows that both groups expressed similar expectations for their respective treatments. Nonetheless, we chose to examine whether the expressed treatment expectation was related to each group’s reported changes in pain and functioning. Pearson correlation coefficient analysis found no significant relationships between overall improvement, patient satisfaction and treatment expectations.
Table 4. Subjects’ expectations and confidence in treatment.
| OMT (n = 30) | UCO (n = 30) | Difference (95% CI)* | |
| Mean (SD) | Mean (SD) | ||
| I believe manipulation with standard care will help my low back pain | 3·77 (0·73) | 4·03 (0·62) | −0·27 (−0·62 to 0·08) |
| I believe that standard care alone will help my low back pain | 2·80 (0·85) | 3·20 (0·93) | −0·40 (−0·86 to 0·06) |
| I believe that manipulation plus standard care will improve my level of functioning | 3·73 (0·83) | 4·03 (0·56) | −0·30 (−0·67 to 0·07) |
| I believe that standard care alone will improve my level of functioning | 2·93 (0·87) | 3·33 (0·96) | −0·40 (−0·87 to 0·07) |
Note: OMT = osteopathic manipulative treatment group; UCO = usual care only group.
*Represents the difference tested using independent samples t-tests.
Finally, we compared the two groups on their overall satisfaction with their respective treatments and self-reported ‘overall improvement’ scores. The results (Table 5) indicate that the OMT group reported significantly greater satisfaction with treatment and significantly greater overall improvement (P<0·01).
Table 5. Subjects’ satisfaction and perceived improvement.
| OMT (n = 30) | UCO (n = 30) | Difference (95% CI)* | |
| Mean (SD) | Mean (SD) | ||
| Patient satisfaction | 8·74 (1·71) | 5·81 (2·74) | 2·93 (1·75 to 4·12) |
| Self-reported overall improvement | 5·96 (1·33) | 4·65 (1·39) | 1·31 (0·61 to 2·01) |
Note: OMT = osteopathic manipulative treatment group; UCO = usual care only group.
*Represents the difference tested using independent samples t-tests.
Discussion
Current DoD guidelines for treating ALBP in active duty military personnel do not include OMT. This study provides evidence however, of the effectiveness of OMT for improving pain and functioning due to ALBP. Since the completion of this study, we have found no published reports of research in OMT for ALBP in military personnel.
In addition to the significant differences in the magnitude and temporal improvement in pain favoring the OMT group, there are other plausible trends that merit comment. For example, at the study end-point, the UCO group reported worsening pain for the QVAS items ‘Pain Now’ and ‘Pain at Best’, whereas the OMT group showed improvement. A longer follow-up period would be needed to determine if there may be a trend for the OMT effect to last longer than improvements experienced with UCO.
It is of interest to note that at visit 2 the OMT group reported worsening pain on average. In a natural treatment environment, osteopathic physicians caution patients that they may feel slightly worse after the initial OMT before the improvement takes effect. As the intent of OMT is to reduce musculoskeletal dysfunctions, improved physiological function, and support homeostasis, OMT may have a broad impact on the whole person system. Thus the study subjects in the OMT group may have experienced a period of readjustment while assimilating the structural changes before experiencing improvements.
At the end of the study, the soldiers in the OMT group reported being ‘much better’ on average in their overall improvement. The UCO group on average however, reported being between ‘moderately worse’ and ‘better.’ In fact 76% of soldiers in the OMT group reported that their pain was much better or completely gone compared to 20% of the UCO group reporting this degree of improvement in their pain.
This study proposed that an OMT protocol would be effective in improving pain and functioning due to ALBP for active duty military personnel. Since this study was conducted, new recommendations have been published that have guided the analysis of this study. The results of this RCT support OMT as a modality to use in conjunction with standard care to reduce a variety of pain experiences including Pain Now, Pain at Best, and Pain Typical. However, contrary to the hypothesis, there were no between group differences found for change in functioning with the RDMQ. While both groups showed improvement in terms of functioning over time, there were no differences in magnitude or rate of this change between the OMT and UCO group.
Osteopathic manipulative treatment did not appear to have improved low back functioning in this population, despite the evidence of its effectiveness in improving functioning in civilian patients.11–13 Based on this current study it is difficult to determine why functioning did not improve in the OMT group similarly to pain. While this may have been due to small sample size and a limited follow-up period, it may also be due to the unsuitability of the RMDQ for measuring low back disability in military personnel. Military personnel face different types of physical demands than civilian patients, and have fewer options for limiting activity or resting. Typical RDMQ statements, for example, ‘I sit down for most of the day because of my back’ may not be suitable for active duty military. Thus, although the RMDQ has been shown to be a reliable and valid method for examining back specific functioning in civilians, it is possible that it is not appropriate for use in the active duty military population.
Study Strengths and Limitations
This RCT had several limitations, including the relatively small sample size, challenges with retention of soldiers in the study for the full trial, due to high military operational tempo, and the relevance of the RMDQ to military lifestyle. The study was scheduled to run for 12 months, and encountered delays in start-up at the site. In addition, the site PI had to complete the study prior to a change of duty assignment, thus shortening the window of recruitment time. These types of challenges are not unusual to encounter at an active military base. The logistics and restrictions associated with studies in military installations can also affect the enrollment rate and retention. The original targeted enrollment of 72 was ultimately restricted to 63 randomized and 60 qualified for inclusion in the final data set.
The study completion rate was approximately 85%, with 53 individuals (27 in OMT and 26 in UCO) completing study through end-point. In this military environment, soldiers who were unable to complete all study visits were unavailable to the research team after several attempts to contact them. However, it is important to note, that overall loss to follow-up rates between the groups were similar.
This is the first known randomized controlled clinical trial testing the effectiveness of OMT in reducing pain and improving functioning in active duty soldiers against the usual care guidelines for LBP treatment that must be followed by military medical personnel. This study was conducted under real-world conditions on a military training installation, under normal demands and requirements accompanying such an environment. There is no opportunity to control the level of physical activity required of a soldier, or to require rest. Thus many factors might account for the changes in back pain and functioning among the subjects in this study.
Conclusions
Compared to the UCO group, the OMT group reported greater improvement between baseline and end point of this study for ‘Pain Now’. Furthermore, the OMT group reported a reduction in ‘Pain at Best’ sooner than the UCO group. If OMT can reduce the severity and duration of ALBP, as demonstrated by these findings, this may be an important treatment modality for the combat environment that is not currently routinely utilized.
Acknowledgments
This work was supported by a grant from the Samueli Institute for Information Biology (SIIB) through an award from the Uniformed Services University of the Health Sciences (USUHS) under Award No. MDA905-03-C-0003. The views, opinions and/or findings contained in this report are those of the authors and should not be construed as an official DoD position, policy or decision unless so designated by other documentation.
We would also like to acknowledge the consultation of Daisha Cipher, PhD and Sejong Bae, PhD during this research study.
At the time of the study Dr Stoll was the PI, Associate Professor and Chair, Department of Osteopathic Manipulative Medicine, TCOM, Fort Worth, Texas.
References
- 1.Cohen SP, Nguyen C, Kapoor SG, Anderson-Barnes VC, Foster L, Shields C, et al. Back pain during war: an analysis of factors affecting outcome. Arch Intern Med 2009;20:1916–23 [DOI] [PubMed] [Google Scholar]
- 2.Knapik JJ, Jones SB, Darakjy S, Hauret KG, Bullock SH, Sharp MA, et al. Injury rates and injury risk factors among U.S. Army wheel vehicle mechanics. Mil Med 2007;9:988–96 [DOI] [PubMed] [Google Scholar]
- 3.George SZ, Childs JD, Teyhen DS, Wu SS, Wright AC, Dugan JL, et al. Rationale, design, and protocol for the prevention of low back pain in the military (POLM) trial (NCT00373009). BMC Musculoskelet Disord 2007;8:92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jonas WB, O’Connor F, Deuster P, Macedonia C. Total force fitness for the 21st Century: a new paradigm. Mil Med; 2010;175;1–78 [Google Scholar]
- 5.Cohen SP, Griffith S, Larkin TM, Villena F, Larkin R. Presentation, diagnoses, mechanisms of injury, and treatment of soldiers injured in Operation Iraqi Freedom: an epidemiological study conducted at two military pain management centers. Anesth Analg 2005;4:1098–103 [DOI] [PubMed] [Google Scholar]
- 6.Green BN, Sims J, Allen R. Use of conventional and alternative treatment strategies for a case of low back pain in a F/A-18 aviator. Chiropr Osteopat 2006;14:11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wilson E, Payton O, Donegan-Shoaf L, Dec K. Muscle energy technique in patients with acute low back pain: a pilot clinical trial. J Orthop Sports Phys Ther 2003;9:502–12 [DOI] [PubMed] [Google Scholar]
- 8.Kent P, Mjosund HL, Petersen DH. Does targeting manual therapy and/or exercise improve patient outcomes in nonspecific low back pain? A systematic review. BMC Med 2010;8:22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Walker BF, French SD, Grant W, Green S. A Cochrane review of combined chiropractic interventions for low-back pain. Spine 2011;3:230–42 [DOI] [PubMed] [Google Scholar]
- 10.Clinical Guideline Subcommittee on Low Back Pain American Osteopathic Association guidelines for osteopathic manipulative treatment (OMT) for patients with low back pain. JAOA 2010;11:653–66 [PubMed] [Google Scholar]
- 11.Licciardone JC, Stoll ST, Fulda KG, Russo DP, Siu J, Winn W, et al. Osteopathic manipulative treatment for chronic low back pain: a randomized controlled trial. Spine 2003;13:1355–62 [DOI] [PubMed] [Google Scholar]
- 12.Licciardone JC. The unique role of osteopathic physicians in treating patients with low back pain. JAOA 2004;104:S13–8 [PubMed] [Google Scholar]
- 13.Licciardone JC, Brimhall AK, King LN. Osteopathic manipulative treatment for low back pain: a systematic review and meta-analysis of randomized controlled trials. BMC Musculoskelet Disord 2005;6:43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Barnes PM, Bloom B, Nahin RL. Complementary and alternative medicine use among adults and children: United States, 2007. Hyattsville, MD: National Center for Health Statistics (US); 2008. Report No. 12 [PubMed] [Google Scholar]
- 15.Andersson GBJ, Lucente T, Davis AM, Kappler RE, Lipton JA, Leurgans S. A comparison of osteopathic spinal manipulation with standard care for patients with low back pain. New Engl J Med 1999;341:1426. [DOI] [PubMed] [Google Scholar]
- 16.Brontfort G, Haas M, Evans RL, Bouter LM. Efficacy of spinal manipulation and mobilization for low back pain and neck pain: a systematic review and best evidence synthesis. Spine J 2004;4:335. [DOI] [PubMed] [Google Scholar]
- 17.Evans DW. Why do spinal manipulation techniques take the form they do? Towards a general model of spinal manipulation. Manual Ther 2010;15:212. [DOI] [PubMed] [Google Scholar]
- 18.Leininger B, Brontfort G, Evans R, Reiter T. Spinal manipulation or mobilization for radiculopathy: a systematic review. Phys Med Rehabil Clin N Am 2011;22:105. [DOI] [PubMed] [Google Scholar]
- 19.Farley DO, Vernez G, Nicholas W, Quiter ES, Dydek GJ, Pieklik S, et al. Evaluation of the low back pain practice guideline implementation in the army medical department. Santa Monica, CA: RAND Corporation; 2004 [Google Scholar]
- 20.Manual manipulative therapy for back pain in active duty military personnel. 2006. Available from: http://clinicaltrials.gov/ct2/show/NCT00394264?term = NCT00394264&rank = 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sutlive TG, Mabry LM, Easterling EJ, Durbin JD, Hanson SL, Wainner RS, et al. Comparison of short-term response to two spinal manipulation techniques for patients with low back pain in a military beneficiary population. Mil Med 2009;174:750–6 [DOI] [PubMed] [Google Scholar]
- 22.Hancock MJ, Maher CG, Latimer J, Herbert RD, McAuley JH. Can rate of recovery be predicted in patients with acute low back pain? Development of a clinical prediction rule. Eur J Pain 2009;1:51–5 [DOI] [PubMed] [Google Scholar]
- 23.Baldwin ML, Butler RJ, Johnson WG, Cote P. Self-reported severity measures as predictors of return-to-work outcomes in occupational back pain. J Occup Rehabil 200;4:683–700 [DOI] [PubMed] [Google Scholar]
- 24.Roland M, Morris R. A study of the natural history of back pain. Part I: development of a reliable and sensitive measure of disability in low-back pain. Spine 1983;2:141–4 [DOI] [PubMed] [Google Scholar]
- 25.Johnson EW. Visual analog scale (VAS). Am J Phys Med Rehabil 2001;10:717. [DOI] [PubMed] [Google Scholar]
- 26.Ostelo RW, Deyo RA, Stratford P, Waddell G, Croft P, Von Korff M, et al. Interpreting change scores for pain and functional status in low back pain: towards international consensus regarding minimal important change. Spine 2008;1:90–4 [DOI] [PubMed] [Google Scholar]
- 27.Myers SS, Phillips RS, Davis RB, Cherkin DC, Legedza A, Kaptchuk TJ. Patient expectations as predictors of outcome in patients with acute low back pain. J Gen Intern Med 2008;2:148–53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Licciardone JC, King HH, Hensel KL, Williams DG. Osteopathic health outcomes in chronic low back pain: the osteopathic trial. Osteopath Med Prim Care 2008;2:5. [DOI] [PMC free article] [PubMed] [Google Scholar]


