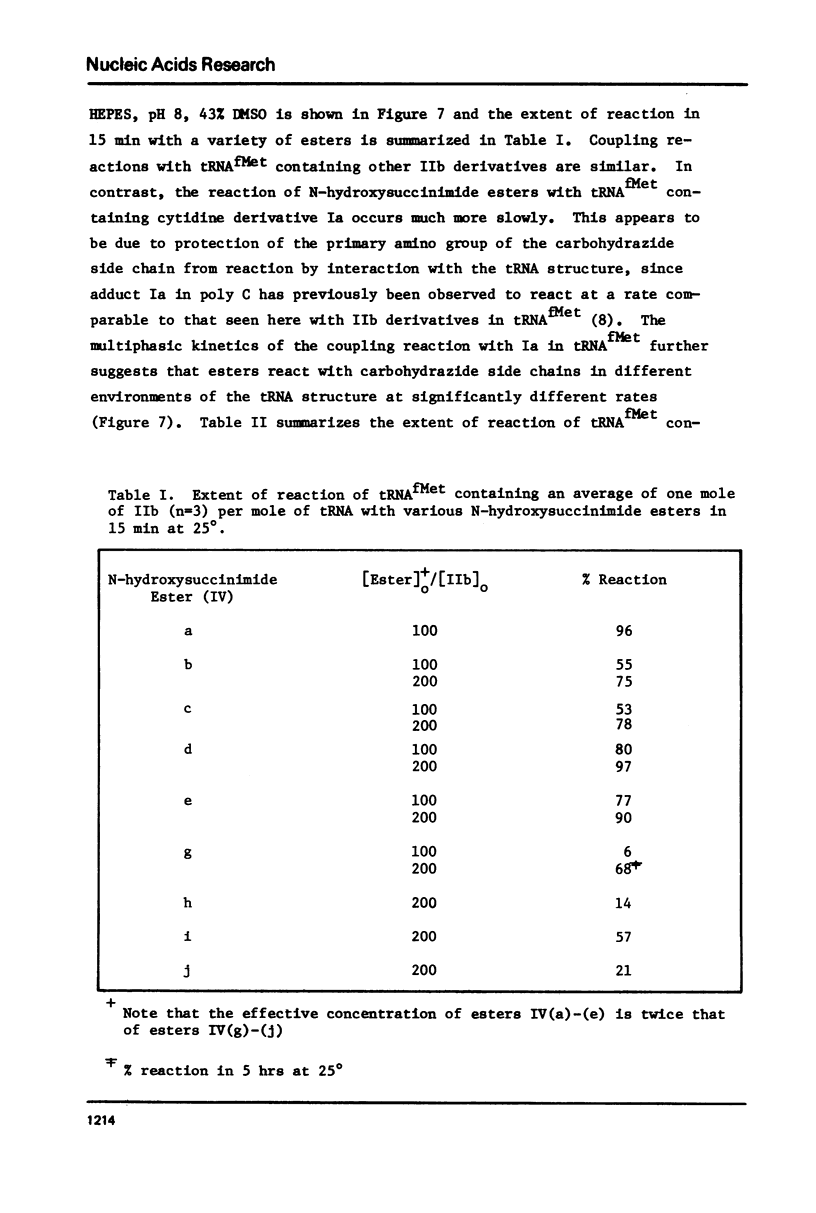
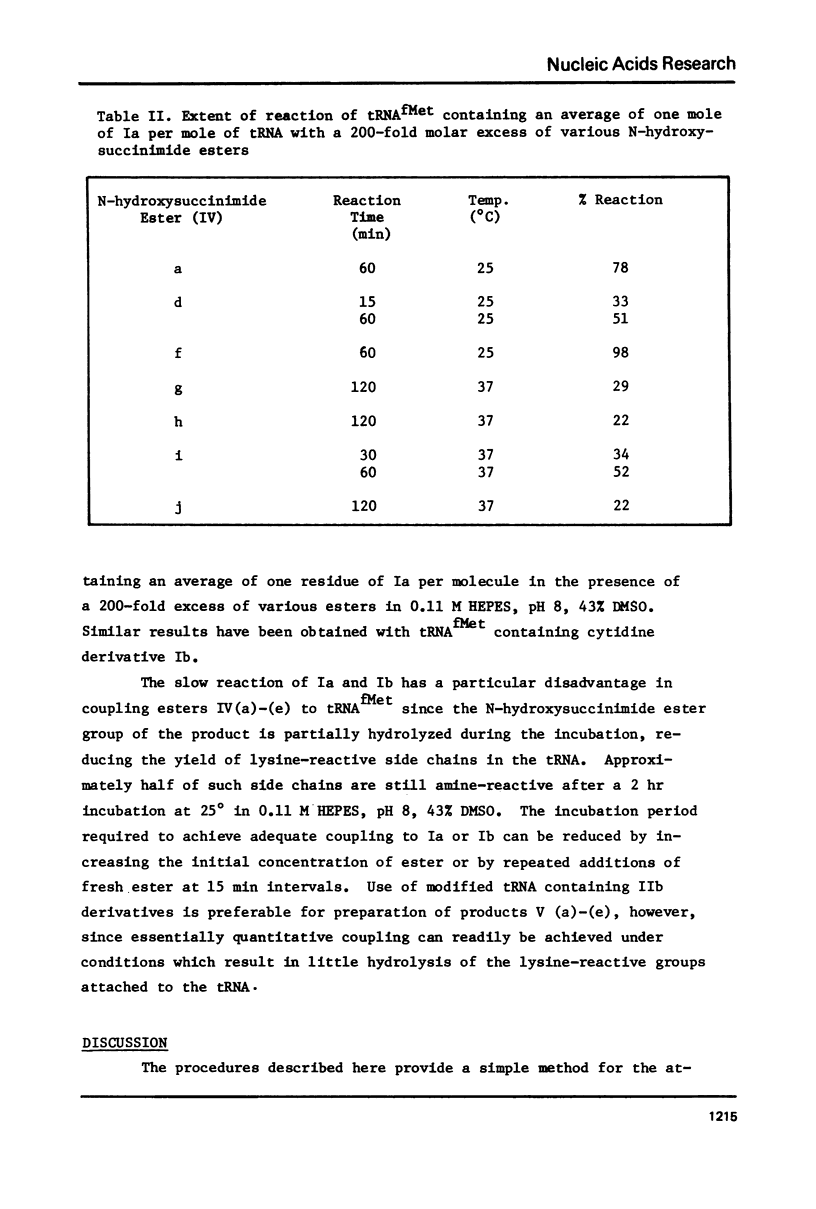
Abstract
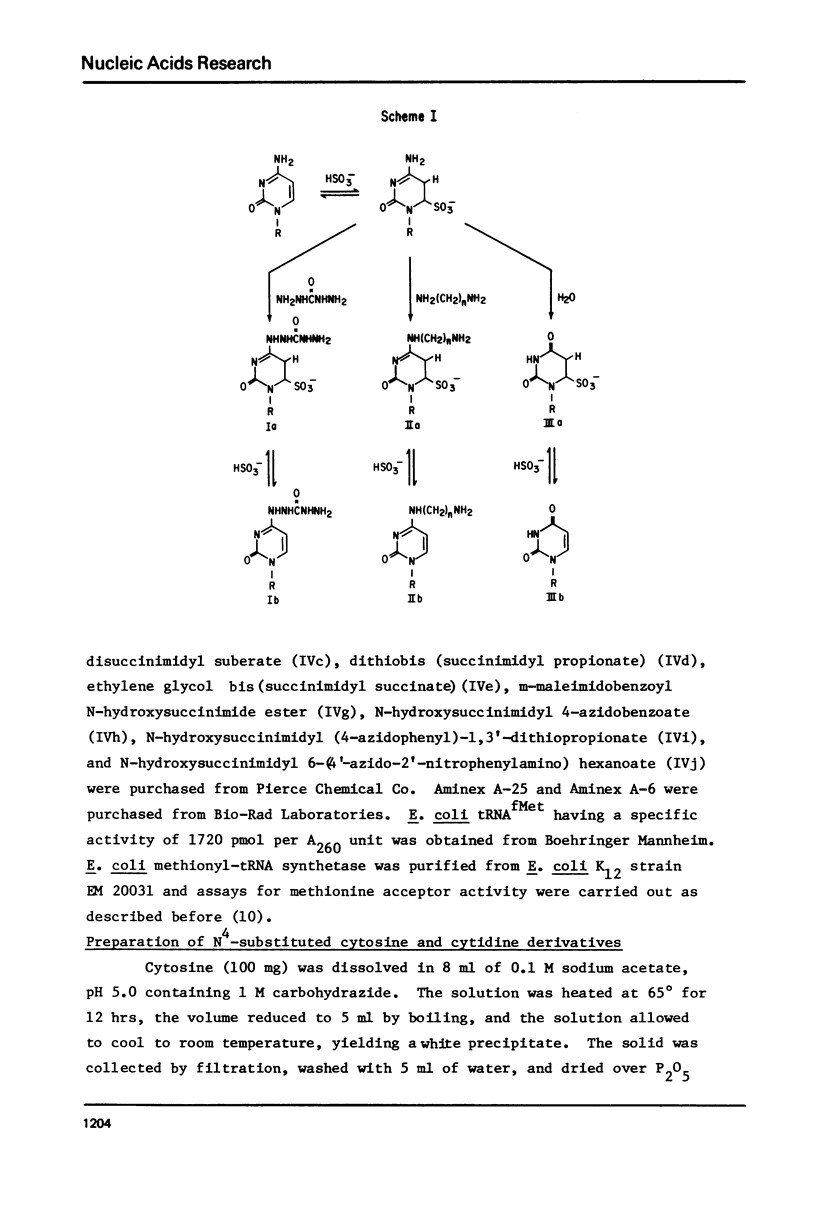
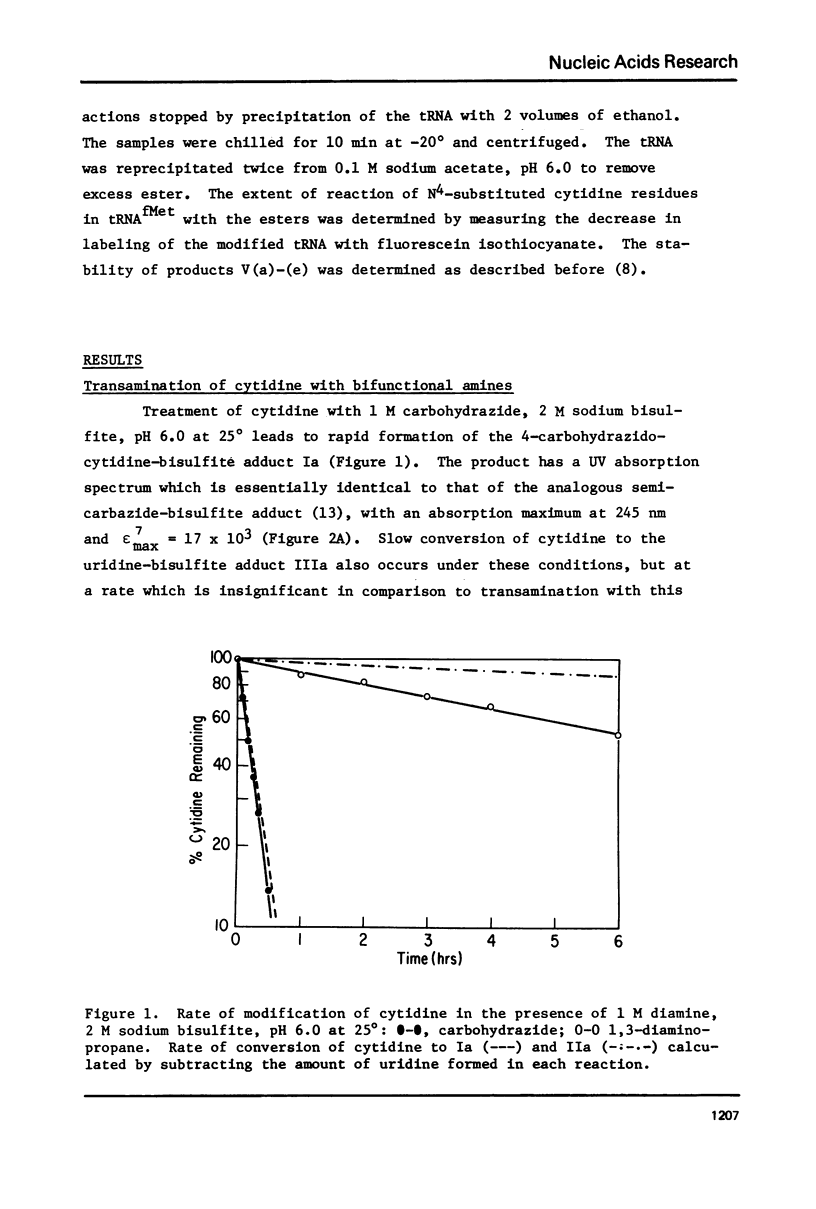
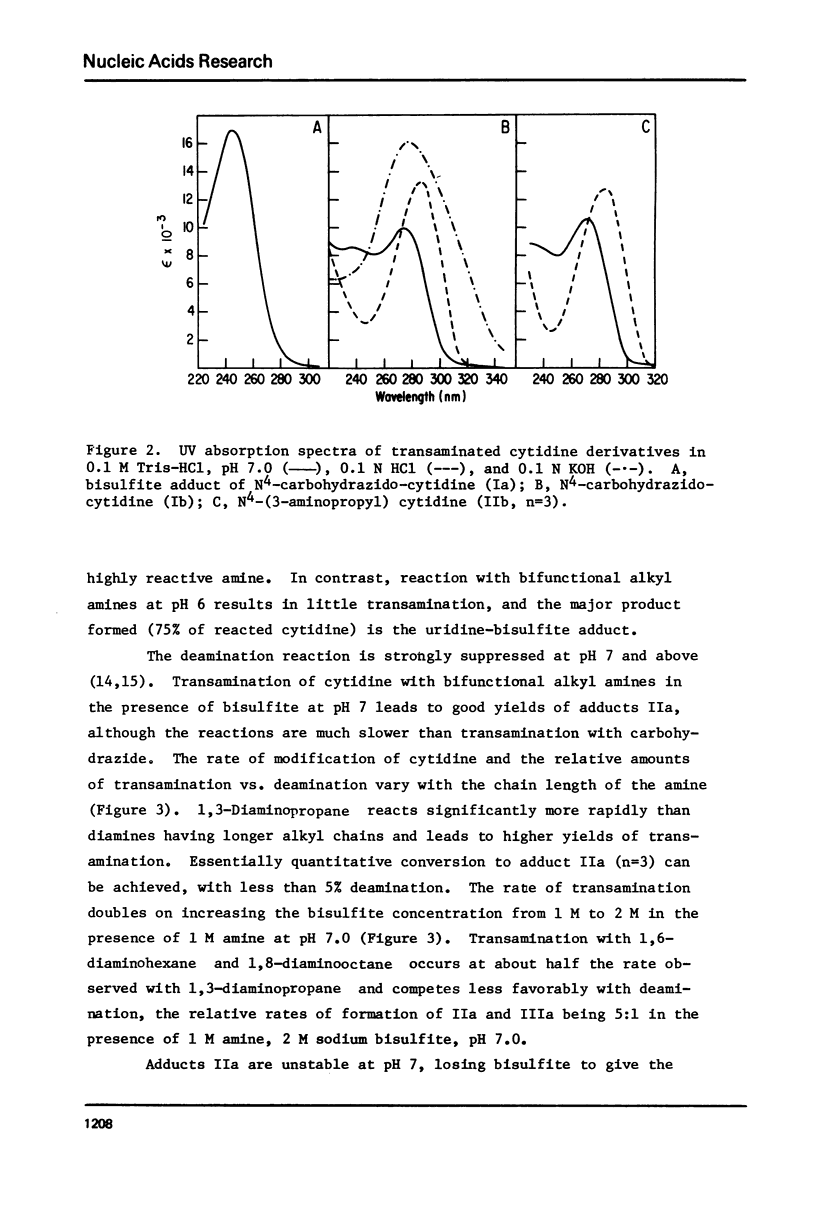
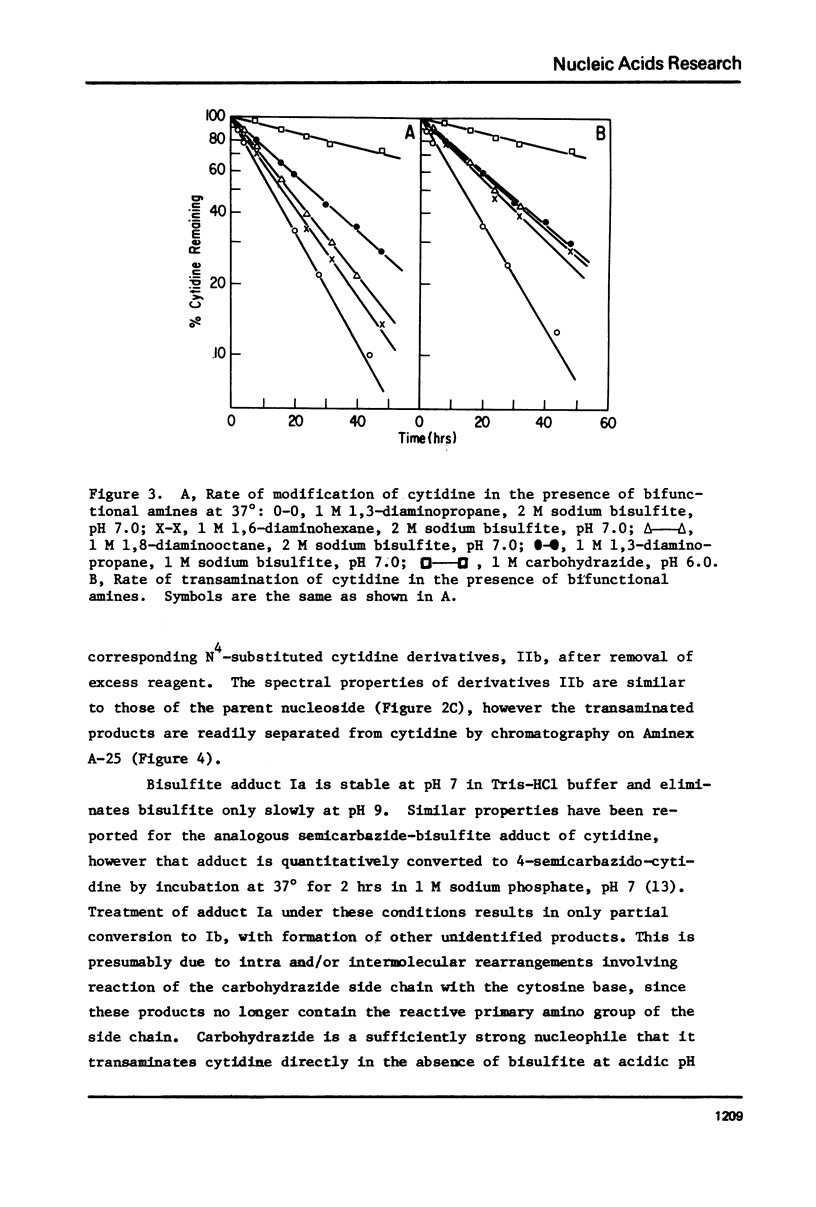
Transamination with bifunctional amines in the presence of bisulfite has been used to attach side chains of variable length to the N4-position of single stranded cytidine residues in E. coli tRNAfMet. Such side chains, terminating in reactive primary amino groups, have been coupled to a variety of N-hydroxysuccinimide esters. The resulting modified tRNAs carry protein affinity labeling groups capable of covalent reaction with a variety of amino acids.
Full text
PDF


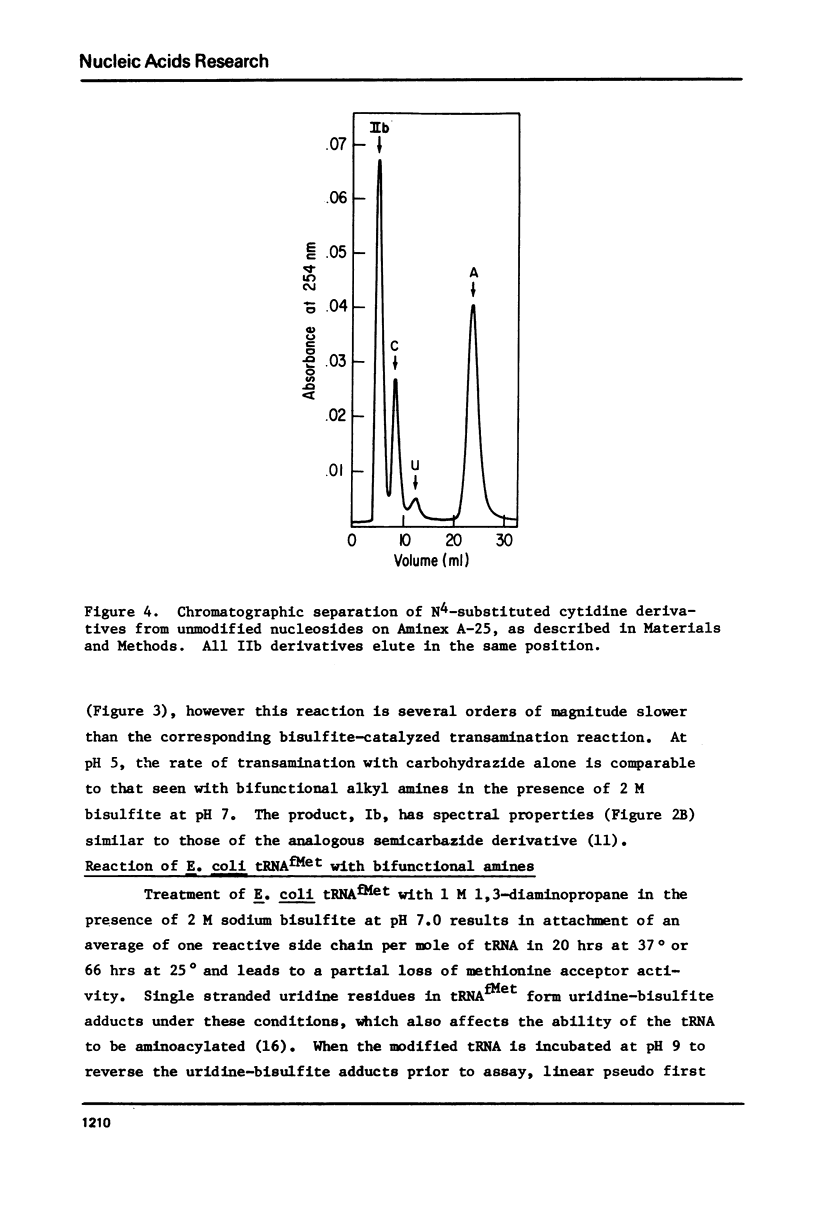
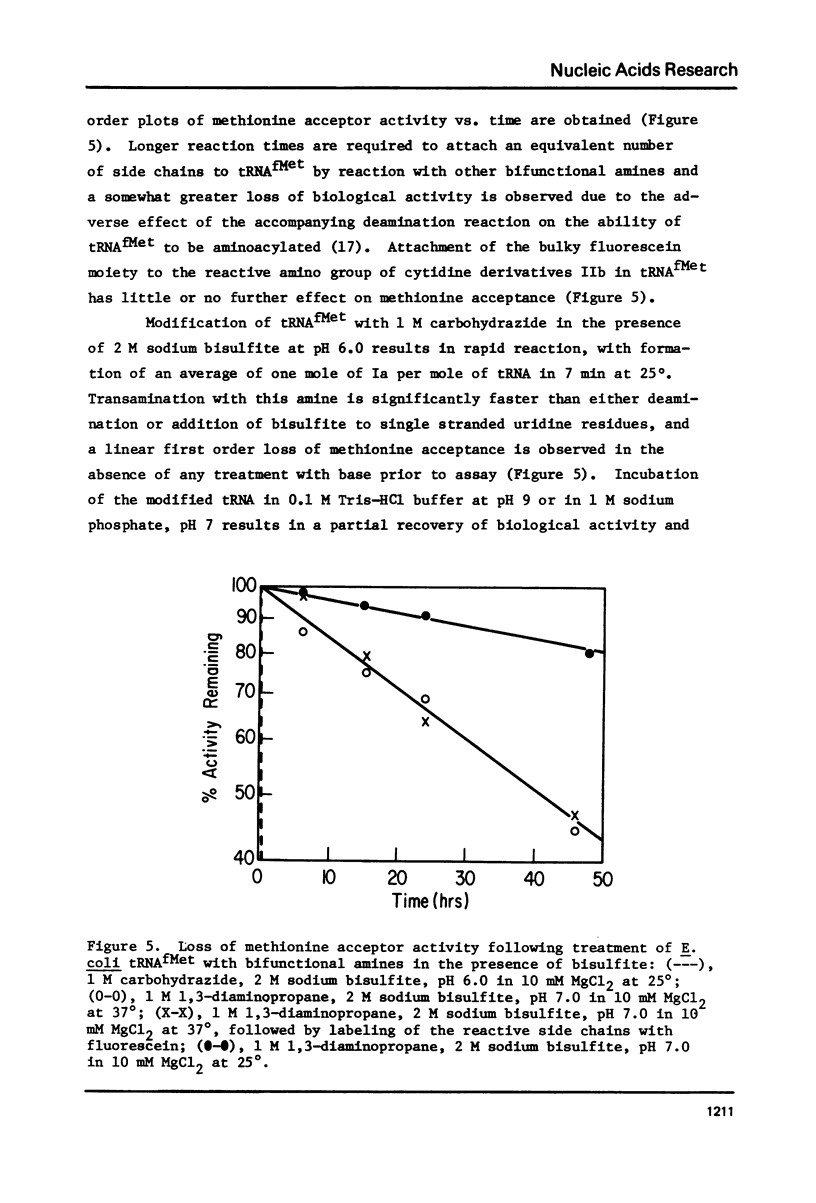
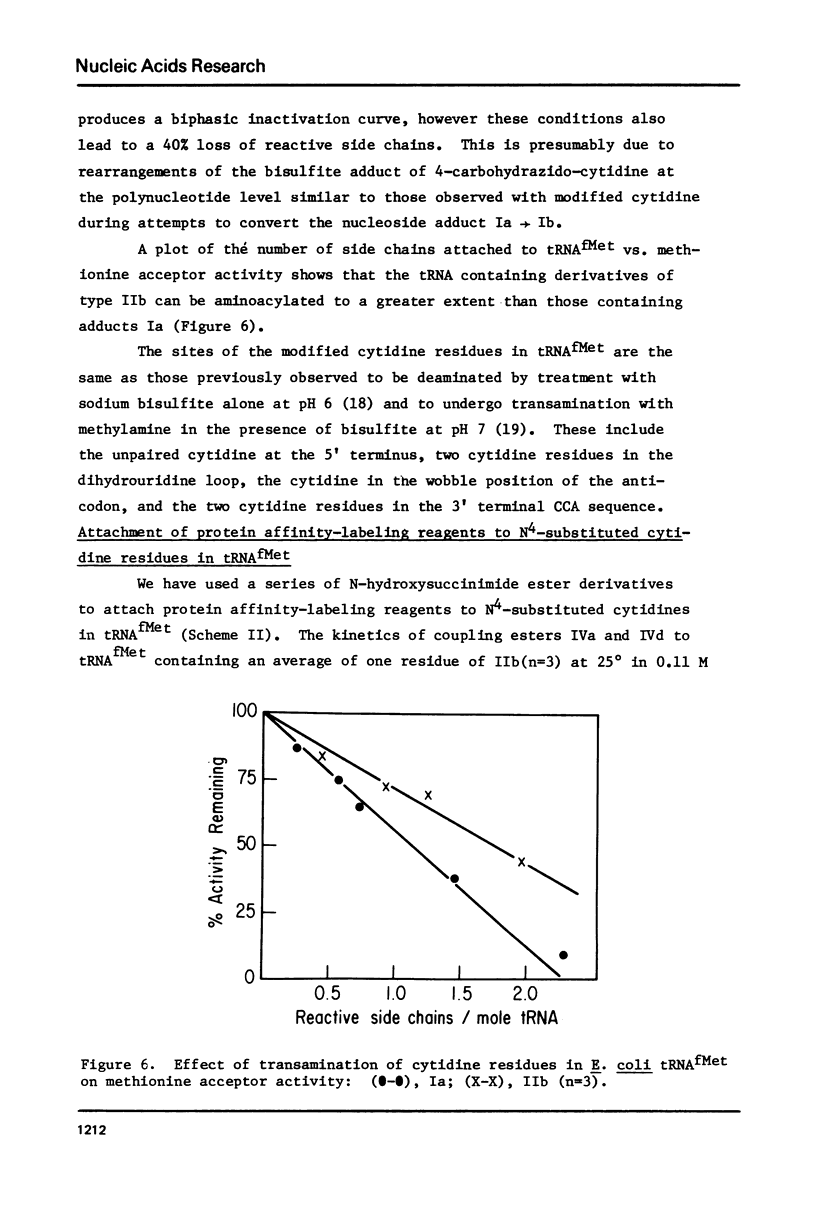
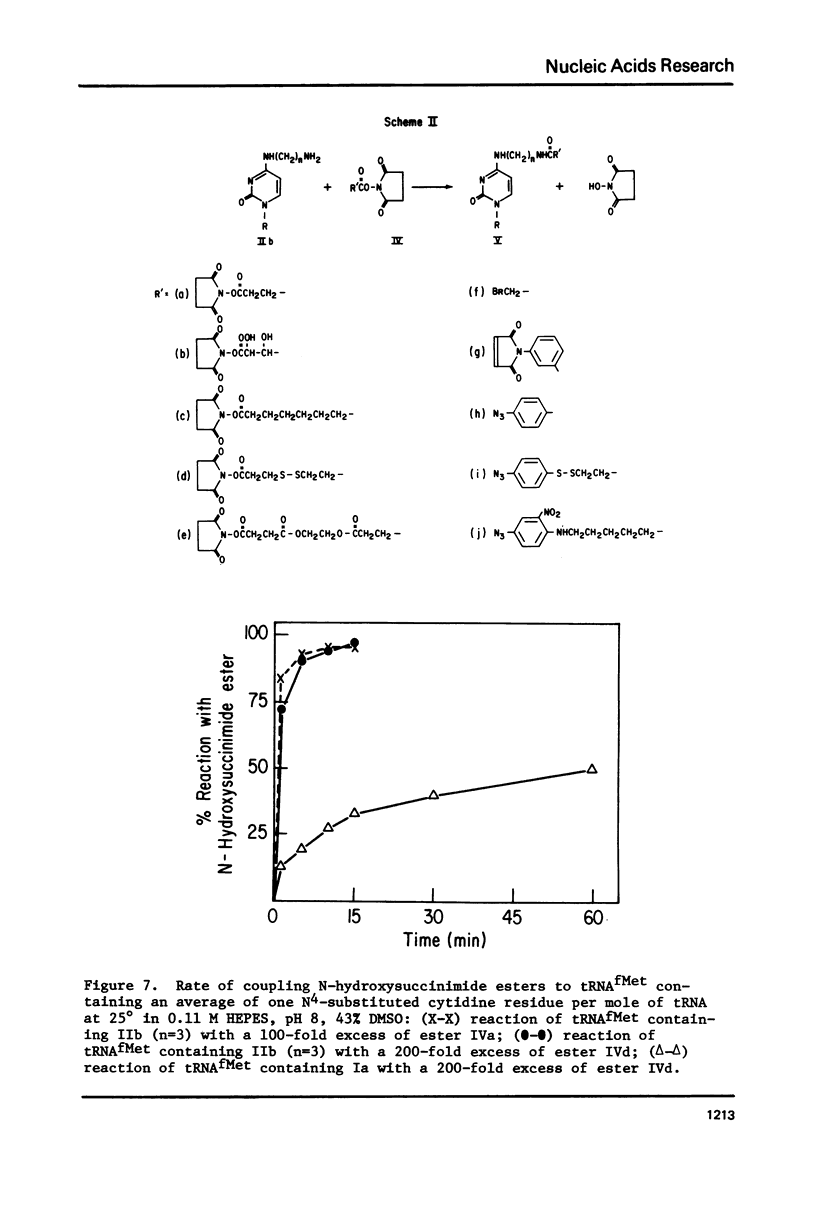


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abdella P. M., Smith P. K., Royer G. P. A new cleavable reagent for cross-linking and reversible immobilization of proteins. Biochem Biophys Res Commun. 1979 Apr 13;87(3):734–742. doi: 10.1016/0006-291x(79)92020-5. [DOI] [PubMed] [Google Scholar]
- Boni I. V., Budowsky E. I. Transformation of non-covalent interactions in nucleoproteins into covalent bonds induced by nucleophilic reagents. I. The preparation and properties of the products of bisulfite ion-catalyzed reaction of amino acids and peptides with cytosine derivatives. J Biochem. 1973 Apr;73(4):821–830. doi: 10.1093/oxfordjournals.jbchem.a130145. [DOI] [PubMed] [Google Scholar]
- Budowsky E. I., Sverdlov E. D., Monastyrskaya G. S. New method of selective and rapid modification of the cytidine residues. FEBS Lett. 1972 Sep 1;25(1):201–204. doi: 10.1016/0014-5793(72)80485-x. [DOI] [PubMed] [Google Scholar]
- Draper D. E., Gold L. A method for linking fluorescent labels to polynucleotides: application to studies of ribosome-ribonucleic acid interactions. Biochemistry. 1980 Apr 29;19(9):1774–1781. doi: 10.1021/bi00550a008. [DOI] [PubMed] [Google Scholar]
- Friedman S., Li H. J., Nakanishi K., Van Lear G. 3-(3-amino-3-carboxy-n-propyl)uridine. The structure of the nucleoside in Escherichia coli transfer ribonucleic acid that reacts with phenoxyacetoxysuccinimide. Biochemistry. 1974 Jul 2;13(14):2932–2937. doi: 10.1021/bi00711a024. [DOI] [PubMed] [Google Scholar]
- Goddard J. P., Schulman L. H. Conversion of exposed cytidine residues to uridine residues in Escherichia coli formylmethionine transfer ribonucleic acid. J Biol Chem. 1972 Jun 25;247(12):3864–3867. [PubMed] [Google Scholar]
- Hayatsu H. Reaction of cytidine with semicarbazide in the presence of bisulfite. A rapid modification specific for single-stranded polynucleotide. Biochemistry. 1976 Jun 15;15(12):2677–2682. doi: 10.1021/bi00657a030. [DOI] [PubMed] [Google Scholar]
- Hayatsu H., Takeishi K. I., Ukita T. The modification of nucleosides and nucleotides. 3. A selective modification of cytidine with semicarbazide. Biochim Biophys Acta. 1966 Sep;123(3):445–457. doi: 10.1016/0005-2787(66)90213-9. [DOI] [PubMed] [Google Scholar]
- Hayatsu H., Wataya Y., Kai K., Iida S. Reaction of sodium bisulfite with uracil, cytosine, and their derivatives. Biochemistry. 1970 Jul 7;9(14):2858–2865. doi: 10.1021/bi00816a016. [DOI] [PubMed] [Google Scholar]
- Hayatsu H., Wataya Y., Kazushige K. The addition of sodium bisulfite to uracil and to cytosine. J Am Chem Soc. 1970 Feb 11;92(3):724–726. doi: 10.1021/ja00706a062. [DOI] [PubMed] [Google Scholar]
- Lomant A. J., Fairbanks G. Chemical probes of extended biological structures: synthesis and properties of the cleavable protein cross-linking reagent [35S]dithiobis(succinimidyl propionate). J Mol Biol. 1976 Jun 14;104(1):243–261. doi: 10.1016/0022-2836(76)90011-5. [DOI] [PubMed] [Google Scholar]
- Schulman L. H., Goddard J. P. Loss of methionine acceptor activity resulting from a base change in the anticodon of Escherichia coli formylmethionine transfer ribonucleic acid. J Biol Chem. 1973 Feb 25;248(4):1341–1345. [PubMed] [Google Scholar]
- Schulman L. H., Pelka H. Alteration of the kinetic parameters for aminoacylation of Escherichia coli formylmethionine transfer RNA by modification of an anticodon base. J Biol Chem. 1977 Feb 10;252(3):814–819. [PubMed] [Google Scholar]
- Schulman L. H., Pelka H. Alteration of the kinetic parameters for aminoacylation of Escherichia coli formylmethionine transfer RNA by modification of an anticodon base. J Biol Chem. 1977 Feb 10;252(3):814–819. [PubMed] [Google Scholar]
- Schulman L. H., Shapiro R., Law D. C., Louis J. B. A simplified method for study of RNA conformation--reaction of formylmethionine transfer RNA with [14C]methylamine-bisulfite. Nucleic Acids Res. 1974 Oct;1(10):1305–1316. doi: 10.1093/nar/1.10.1305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shapiro R., DiFate V., Welcher M. Deamination of cytosine derivatives by bisulfite. Mechanism of the reaction. J Am Chem Soc. 1974 Feb 6;96(3):906–912. doi: 10.1021/ja00810a043. [DOI] [PubMed] [Google Scholar]
- Shapiro R., Weisgras J. M. Bisulfite-catalyzed transamination of cytosine and cytidine. Biochem Biophys Res Commun. 1970 Aug 24;40(4):839–843. doi: 10.1016/0006-291x(70)90979-4. [DOI] [PubMed] [Google Scholar]
- Smith R. J., Capaldi R. A., Muchmore D., Dahlquist F. Cross-linking of ubiquinone cytochrome c reductase (complex III) with periodate-cleavable bifunctional reagents. Biochemistry. 1978 Sep 5;17(18):3719–3723. doi: 10.1021/bi00611a007. [DOI] [PubMed] [Google Scholar]
- Sono M., Wataya Y., Hayatsu H. Role of bisulfite in the deamination and the hydrogen isotope exchange of cytidylic acid. J Am Chem Soc. 1973 Jul 11;95(14):4745–4749. doi: 10.1021/ja00795a044. [DOI] [PubMed] [Google Scholar]
- Uziel M., Koh C. K., Cohn W. E. Rapid ion-exchange chromatographic microanalysis of ultraviolet-absorbing materials and its application to nucleosides. Anal Biochem. 1968 Oct 24;25(1):77–98. doi: 10.1016/0003-2697(68)90083-3. [DOI] [PubMed] [Google Scholar]



