Summary
To further understand the role of ovarian hormones in the function of the serotonin neural system, we investigated the effects of estradiol (E), progesterone (P) and raloxifene on 5HT 1A and 2C receptor protein expression in the dorsal raphe region using western blot analysis. Adult rhesus macaques (Macaca mulatta) were ovariectomized and implanted with Silastic capsules containing E or P. In the first paradigm, animals that had been ovariectomized for 6-16 months were treated for one month with E (E1) or E+P (EP1) and compared to animals that were untreated and ovariectomized for 5 months (n=4/group). In the second paradigm, comparisons were made between animals that were ovariectomized and untreated for 5 months, or ovariectomized and immediately implanted with Silastic capsules containing E or E+P for 5 months (E5, EP5), or administered raloxifene in the diet for 5 months (Ral5) (n=4/group). The dorsal raphe region was harvested, homogenized and a crude membrane fraction was obtained for examination of receptor proteins. In the first paradigm, 5HT1A receptor protein expression was significantly lower in E1 and EP1 treatment groups compared to the Ovx-control group (ANOVA p=0.01; posthoc p<0.03), but 5HT2C receptor expression was unaffected by 1 month of E or EP treatment. In the second paradigm, there was no difference in 5HT1A receptor expression between the Ovx-control group and the E5 group, but 5HT1A receptor expression was significantly suppressed in the EP5 group (ANOVA p = 0.04; posthoc p<0.05). In addition, 5HT2C expression increased in the E5 treatment group relative to the Ovx-control group. Addition of P to the E5 regimen prevented the E5-induced increase in 5HT2C receptor expression and significantly reduced 5HT2C receptor expression to a level below that observed in the Ovx-control group (ANOVA p=0.001; posthoc p < 0.05). Thus, 5HT1A receptor may lose sensitivity to the suppressive effect of E after 5 months whereas the 5HT2C receptor increases. However, addition of P in the EP5 regimen maintains the regulatory effects observed with 1 month of treatment. 5HT1A receptor protein levels were higher with raloxifene treatment than in Ovx-control animals (p<0.01) suggesting that raloxifene may antagonize residual E in ovariectomized animals.
Keywords: estradiol, progesterone, serotonin receptors, macaques, raphe nucleus
Introduction
The serotonin system modulates complex physiological, behavioral and autonomic functions as well as a number of neurological and psychiatric conditions such as cognition, memory, arousal, pain, mood, stress, anxiety, depression, thermoregulation, circadian rhythms, obesity/satiety, sexual behavior, sleep and wakefulness 1-3 The serotonin producing neurons in the brain are located in the raphe nuclei, a heterogeneous collection of neurons which flank the midline along the rostro-caudal extension of the brainstem. The dorsal raphe nucleus projects to almost every area of the forebrain and plays an important role in the diverse neural processes regulated by serotonin 4. The multiple actions of serotonin are achieved through seven major receptor families each of which contain several subtypes and isoforms which utilize a variety of intracellular transduction mechanisms 5,6.
Serotonin neurons are targets for the ovarian hormones estradiol (E) and progesterone (P). E receptor beta (ERβ) and P receptor (PR) both co-localize in the serotonin neurons of the non-human primate dorsal raphe nucleus and PR expression is induced by E treatment 7-10. ERβ and PR are nuclear transcription factors that regulate gene expression and in turn, protein expression 11.
Studies from this lab indicate that E and P regulate the expression of pivotal serotonin-related genes and proteins in a pattern suggestive of increased serotonin neurotransmission 12-14. We have utilized a monkey model of surgical menopause with 1 month of natural E and P replacement. While this model has shown that E and P regulate diverse functions of the serotonin system, it does not approach the longer times of hormone therapy that menopausal women are generally prescribed. Women who have undergone complete hysterectomy may take various estrogenic formulations for many years, whereas women with an intact uterus will generally take estrogenic plus progestin formulations. Thus, we welcomed a donation of brains from ovariectomized monkeys that were treated with natural E alone, E+P or raloxifene for 5 months in order to compare endpoints between our standard 1-month treatment and a longer treatment period.
Of further interest are 2 pivotal feedback mechanisms that operate upon serotonin neurons in the dorsal raphe nucleus. One, the 5HT1A autoreceptor on serotonin neurons reduces serotonin neural firing in response to dendritic release of serotonin 15. Two, the 5HT2C receptor excites gamma aminobutyric acid (GABA) interneurons in response to local serotonin, which in turn, inhibit serotonin neural activity 16.
We previously reported that one month of subcutaneous E treatment, with or without P supplementation, decreased 5HT1A receptor mRNA and 8-hydroxy-2-(di-n-propylamino) tetralin (8-OH-DPAT) binding in the dorsal raphe nucleus 17,18. In addition, this treatment decreased 8-OH-DPAT stimulation of GTPgammaS binding 18. In this study, we questioned whether the steroid-induced decrease in gene expression of the 5HT1A autoreceptor leads to a decrease in protein expression, which could account for the decrease in 8-OH-DPAT binding, and whether 5 months of treatment duplicates 1 month of treatment.
In addition, we questioned whether ovarian hormone therapy regulates the expression of 5HT2C receptor protein, which would impact GABA interneuron excitation. Further, we questioned whether there would be a difference between 1 month of hormone therapy and 5 months of hormone therapy. Lastly, we hypothesized that the selective estrogen receptor modulator (SERM) raloxifene would affect the protein expression of the 5HT1A and 2C receptors in the macaque dorsal raphe nucleus, but because the 5HT1A promoter lacks estrogen response elements (EREs), it was unpredictable whether raloxifene would act as an agonist or antagonist. Therefore, western blot analysis of 5HT1A and 5HT2C receptors was used to quantitate protein levels in the dorsal raphe region of spayed monkeys treated with E or E+P for 1 or 5 months or raloxifene for 5 months.
Results
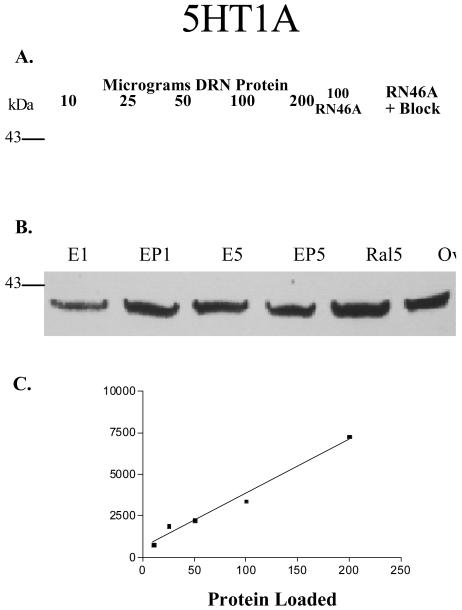
Figure 1, panel A, illustrates the developmental blot and protein linearity for the 5HT1A receptor. The signal bands ran at approximately 45 kDa and increased linearly with increasing concentrations of protein loaded on the gel. Figure 1, Panel C, contains the regression analysis of the tissue linearity and shows that the protein loaded was highly correlated to the band density (correlation coefficient [r] = 0.99; r2= 0.98). From these results, 25 μg of protein was used for 5HT1A analysis in the experimental groups. Positive controls included 50 μg of pineal homogenate (not shown), 100 and 200 μg of RN46A cells and 3 μg of blocking peptide (not shown). This blot was stripped and re-probed with 5HT1A antibody that had been pre-incubated with 20 μg of blocking peptide. No signal was observed upon exposure. This blot was then re-probed for 5HT1A and signal was again present, indicating the block was effective and that 5HT1A protein was still present (data not shown). Figure 1, panel B, illustrates a representative blot containing one animal from each experimental group. Positive controls of 200 μg of RN46A cells and 3 μg of peptide were also included (not shown).
Figure 1.
Digitized photographs of autoradiographic films from western blots. (A). Blot probed for 5HT1A demonstrating the protein linearity. There was an increase in signal intensity at 46 kDa with increasing amounts of protein loaded. Right panel is an image of the RN46 lane from the same blot, which was stripped and re-probed for 5HT1A using antibody pre-incubated with 20 μg of blocking peptide. No signal was observed when the antibody was pre-absorbed with the 5HT1A peptide. (B) Representative blot of samples from each treatment group probed for 5HT1A. Coomassie blue staining of the nitrocellulose membrane revealed visible protein bands that were identical between the lanes indicating that equal amounts of protein were loaded in each lane. (C) Linear regression analysis of the 5HT1A protein linearity shown in panel A. There is a significant correlation between the optical density and the protein loaded (r2=0.98).
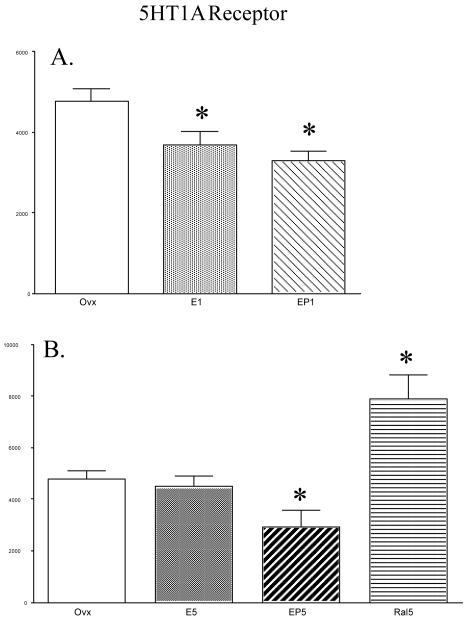
Figure 2 contains histograms illustrating the mean optical density ±SEM (n=4 per group) of 5HT1A receptor signal in each treatment group. Figure 2, panel A, illustrates the effect of 1 month of E or E+P treatment compared to the ovx-control group. Figure 2, panel B, illustrates the effect of 5 months of E, E+P or raloxifene treatment compared to the ovx-control group. The 5-month ovx group with no hormone therapy served as the control for the 1-month treatment groups and the 5-month treatment groups. However, the treatment groups were not directly compared due to the difference in the time of treatment initiation after ovariectomy. After 1 month of E or E+P treatment, there was a significant difference in 5HT1A protein expression between the treatment groups (ANOVA F= 7.0599; p= 0.0143). Post-hoc tests showed the groups treated with E1 and EP1 had significantly lower 5HT1A protein levels than the ovariectomized control group (E1 p=0.03; EP1 p=0.01). There was also a significant difference in 5HT1A protein levels between 5-month treatment groups (ANOVA F= 10.843; p=0.0013). Post-hoc tests showed the E5 group was not different from ovariectomized controls, but the EP5 group had significantly lower 5HT1A protein levels than the ovariectomized control group (p=0.04). There was also a significant increase in 5HT1A protein levels in raloxifene treated monkeys when compared to ovariectomized monkeys (p=0.01).
Figure 2.
Histograms illustrating the mean optical density ± SEM (n=4/group) of 5HT1A signal in each treatment group (abbreviations defined in text). (A) Histogram showing the effect of 1 month of E or E+P treatment compared to the ovx-control group. There was a significant difference in 5HT1A receptor levels between the groups (ANOVA p=0.0143) Post hoc-tests indicated 5HT1A receptor levels in E1 and EP1were lower than the Ovx control group (SNK p <0.3 and p < 0.2, respectively) (B) Histogram showing the effect of 5 months of E, E+P or raloxifene treatment compared to the ovx-control group. There was a significant difference in 5HT1A receptor levels between treatment groups (ANOVA p=0.044). Post hoc-tests indicated 5HT1A receptor protein in EP5 was lower than the Ovx-control group (SNK p< 0.05) while 5HT1A protein in the Ral5 group was higher than the Ovx-control group (SNK p< 0.01).
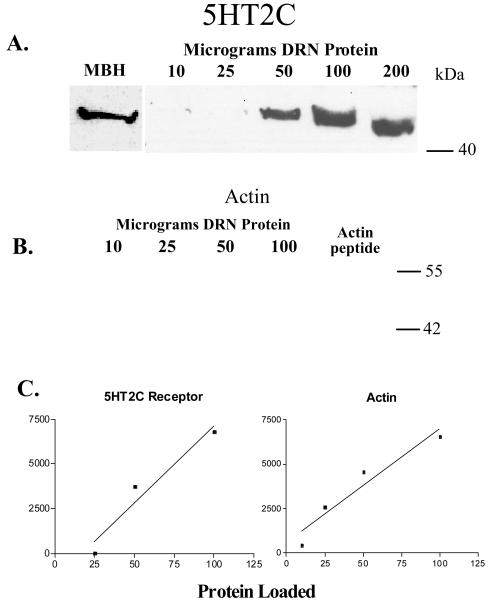
Figure 3, panel A, illustrates the protein linearity for the 5HT2C receptor. The signal bands ran at approximately 50 kDa, and the signal density increased with increasing concentrations of protein loaded on the gel up to 100 μg. Figure 3, panel C contains the regression analysis of the tissue linearity and shows that the protein loaded was highly correlated to the band density (Correlation coefficient [r] = 0.97; r2= 0.94). The positive control for 5HT2C was 50μg protein from the pellet of a homogenized pool of monkey mediobasal hypothalami, which robustly expresses 5HT2C receptors 19. There was no signal from 200 μg of protein from the periaquaductal gray (not shown). From these results, 50 μg of protein was used for 5HT2C analysis in the experimental gels. Figure 3, panel B illustrates the tissue linearity for actin, which was obtained by reprobing the blot in panel A. The signal bands ran at approximately 45 kDa and the signal density increased with increasing concentrations of protein loaded on the gel. Figure 3, panel C contains the regression analysis of the tissue linearity and shows that the protein loaded was highly correlated to the band density (correlation coefficient [r] = 0.96; r2= 0.92). This blot was stripped and re-probed with only the secondary antibody and no signal was observed. It was then probed with the actin primary antibody premixed with 40 μg of blocking peptide and signal was absent. This blot was then re-probed for actin and signal was again present indicating the block was effective and actin remained on the blot (data not shown).
Figure 3.
Digitized photographs of autoradiographic films from western blots of the 5HT2C receptor which exhibited a MW of 50 kDa and for actin which demonstrated a MW of 45kDa(A) Control blot probed for 5HT2C demonstrating protein linearity. There was an increase in optical density with increasing amounts of protein loaded until 200 μg, at which point the densitometry was saturated. The left panel illustrates the signal obtained from 50μg of protein from the pellet of a homogenate of the mediobasal hypothalamus, which robustly expresses the 5HT2C receptor and served as a positive control. (B) The same blot re-probed for actin demonstrating protein linearity. There was an increase in optical density with increasing amounts of protein loaded. (c) Linear regression analysis of the protein linearity shown in panels A and B. There is a significant correlation between the optical density and the protein loaded for 5HT2C up to 100 μg (r2= 0.94) and for actin up to 100 μg (r2= 0.92).
Figure 4 illustrates a representative blot containing one animal from each experimental group. The blot was first probed for 5HT2C, then stripped and probed for actin (bottom panel). Remaining blots were run in this same fashion in order to examine all of the animals. There was no significant difference in actin signal density between any of the groups (ANOVA F=0.60, p=0.70). Therefore, it was not necessary to normalize the 5HT2C signal by the actin signal.
Figure 4.
Representative experimental blot containing one animal from each treatment group probed for 5HT2C (top), stripped and re-probed for actin (bottom). There was no difference in actin indicating that equal amounts of protein were loaded in each lane. However, there is a marked difference in the amount of 5HT2C protein.
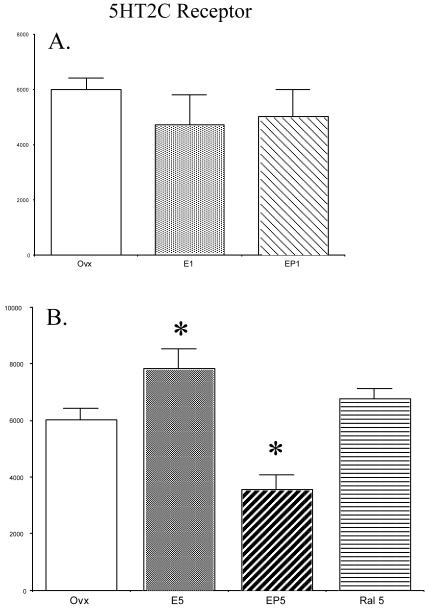
Figure 5 contains histograms illustrating the mean optical density±SEM (n=4 per group) of 5HT2C receptor signal for all of the treatment groups. Figure 5, panel A, illustrates the effect of 1 month of E or E+P treatment compared to the ovx-control group. Figure 5, panel B, illustrates the effect of 5 months of E, E+P or raloxifene treatment compared to the ovx-control group. One month of E or E+P treatment had no effect on 5HT2C protein expression. However, there was a significant difference between the 5-month treatment groups (ANOVA F=10.886; p= 0.0013). Post-hoc tests showed the E5 group had significantly higher 5HT2C protein levels than the control group (p<0.05). Moreover, addition of P to the E treatment for 5 months reversed the stimulatory effect of E and suppressed 5HT2C receptor expression to a level significantly lower than that obtained in the control group (p<0.05). There was no significant difference in 5HT2C protein levels in raloxifene treated monkeys when compared to ovariectomized control monkeys.
Figure 5.
Histograms illustrating the mean optical density ± SEM (n=4/group) of 5HT2C signal in each treatment group (abbreviations defined in text). (A) Histogram showing the effect of 1 month of E or E+P treatment compared to the ovx-control group. There was no difference in 5HT2C receptor levels between the groups. (B) Histogram showing the effect of 5 months of E, E+P or raloxifene treatment compared to the ovx-control group. There was a significant difference in 5HT2C receptor protein between treatment groups (ANOVA p=0.0013). Post hoc-tests indicated 5HT2C receptor protein levels were higher in the E5 treatment group than the ovx-control group (p<0.05) while levels in the EP5 group were lower than control levels (p< 0.01).
Serum concentrations of E and P were previously reported 13. E and P averaged 14 pg/ml and 0.14 ng/ml, respectively, in the Ovx-control and Raloxifene treated groups. E averaged 60 pg/ml and P averaged 3.0 ng/ml in the treatment groups. There was no difference in hormone levels between animals treated for 1 or 5 months.
Discussion
Serotonin neural function governs mood and affective behavior. Studies from this laboratory indicate that ovarian hormones alter serotonin neural function by changing the expression of pivotal genes and proteins 12. To date, women who have undergone complete hysterectomy are prescribed only estrogenic compounds, but women with an intact uterus are prescribed a combination of estrogen and progestin compounds of various chemical formulations. In this study, we show that 1 month E or E+P treatment suppressed the 5HT1A autoreceptor compared to ovariectomized animals whereas, unopposed E for 5 months had no effect on 5HT1A. However, addition of P to the 5-month E regimen suppressed 5HT1A. Thus, one could argue that E lost its efficacy in the regulation of 5HT1A expression after 5 months of unopposed administration, but co-administration of P maintained the efficacy of E.
We also found that there was no effect of 1 month of E or E+P administration on the expression of 5HT2C receptors. However, 5HT2C expression was increased after 5 months of unopposed E administration. This effect was reversed with co-administration of P with E for 5 months. Thus, P also normalized 5HT2C expression in the presence of E.
It may be noted that the 1-month treatments were initiated in animals that had been ovariectomized for an average of 11 months whereas the 5-month treatments were initiated immediately after ovariectomy. Thus, the 1-month treatments were not directly compared to the 5-month treatments. Rather the 1-month and 5-month treatments were independently compared to the Ovx-control group and differences were interpreted. A weakness of the study is that the 1-month E-treated animals had been ovariectomized for 16±3 months whereas the 1-month E+P treated animals had been ovariectomized for 6 months. It could be argued that the difference in time after ovariectomy before treatment initiation could alter the sensitivity to the steroid hormones. At the time these animals were acquired, the length of time between ovariectomy and hormone treatment was not the well-appreciated variable that it has become. Currently, better screening of animals for similar length of ovariectomy is mandated. However, the decrease in 5HT1A receptor protein in response to E and E+P mirrored previous studies identically in which 5HT1A mRNA and 5HT1A binding decreased with E and E+P treatment. Thus, we believe the animals were of similar sensitivity to hormone replacement. Nonetheless, it would be of interest to examine serotonergic endpoints after ovariectomy for different lengths of time.
5HT1A receptor protein levels were lower in the dorsal raphe region with 1 month of E, with or without supplemental P, compared to ovariectomized control monkeys. This is consistent with previous results showing that 1 month of E treatment, with or without P, decreased 5HT1A receptor mRNA and decreased [3H]8-OH-DPAT binding to the autoreceptor and decreased the inhibitory G protein responsible for intracellular signal transduction 17,18. These observations suggest that ovarian hormones may increase serotonin neurotransmission by decreasing both 5HT1A autoreceptors and their intracellular activation of the inhibitory G-protein cascade. In this study, we have extended these results to show that with 5 months of E-only treatment, 5HT1A protein is equal to control levels, but it was suppressed with supplemental P (EP5). This may indicate that 5HT1A protein expression can adapt to chronic E treatment, and that addition of P is necessary to maintain the suppression in long-term treatment paradigms. Reasoning from this data, unopposed long-term estrogen therapy may gradually exhibit decreased efficacy. However, long-term estrogen therapy has been shown to increase well-being in peri- and postmenopausal women 20,21. Hence, the dose of estrogen used may be critical. This study achieved serum estradiol levels of ~60 pg/ml, whereas clinical therapy aims for lower levels. For example, the low dose FemRing™ achieves 35 pg/ml. Moreover, the commonly used ERT formulation of Premarin™ (conjugated equine estrogens) has very little estradiol but instead contains mostly estrone sulfate, which is then converted to estradiol. Serum estradiol after Premarin administration to monkeys equaled 3.9 pmol/L, which is less than 1pg/ml 22.
5HT2C receptor proteins in the dorsal raphe nucleus were not changed with 1 month of treatment, but were increased with 5 months of unopposed E treatment. The lack of an effect after 1 month of treatment is contrary to observations in rodents with either 1 day or 2 weeks of E treatment 23-25. This may indicate that during the normal primate menstrual cycle, there may be little alteration in the expression of the 5HT2C receptors in the dorsal raphe nucleus. However, 5 month unopposed E increased 5HT2C receptor proteins in the dorsal raphe nucleus. An increase in 5HT2C receptors on the GABA interneurons of the dorsal raphe nucleus could lead to increased stimulation of GABA following intra-raphe serotonin release, which in turn could promote inhibition of serotonin neurons. Importantly, 5HT2C receptor protein levels were suppressed to control levels or lower with 5 months of E+P. Thus, the effect of 5 months of unopposed E on the 5HT2C receptors was reversed with the co-administration of P to the E regimen.
Differences in the effect of long- and short-term hormone therapy have been previously observed. We found that there was a difference in the protein expression of the serotonin reuptake transporter (SERT) and MAO-A between 1 and 5 months of E treatment (Smith et al., 2004). SERT behaved in a manner similar to the 5HT2C receptor in that it was unaffected by 1 month of E treatment, but increased with 5 months of treatment and the increase was prevented by P co-administration. However, MAO-A responded in a manner similar to the 5HT1A receptor in that it was decreased by 1 month of estradiol treatment, but it was unchanged from Ovx-control levels with 5 months of E administration. Other studies have observed similar differences. For example, measures of basal forebrain cholinergic function found that short-term treatment with E or P significantly enhanced cholinergic function, but the effects did not persist with prolonged treatment 26.
Hormone therapy has beneficial effects on bone and cardiovascular risk factors 27,28. However, it also increases the risk of breast and uterine cancer so many women decline to use it 29. Hence, SERMS that mimic estrogen actions in the brain, but are not agonistic in peripheral tissues are desirable. Raloxifene is a SERM that has shown anti-anxiety effects in the CNS 13,30,31. In this study, 5 months of oral raloxifene increased 5HT1A expression over the Ovx-control group in the dorsal raphe nucleus. This increase in 5HT1A suggests that raloxifene is antagonizing the minimal levels of residual E in ovariectomized macaques. We previously found that 1 month of raloxifene increased TPH-1 gene expression 32, and 5 months of raloxifene increased TPH protein, TPH phosphorylation and SERT protein in the dorsal raphe of Ovx rhesus macaques in a manner similar to estradiol 13. These initial observations suggested that raloxifene was acting as an estrogen agonist in the context of serotonin neurons, which utilize ERβ. However, in this study raloxifene increased 5HT1A expression which is construed as an antagonistic effect. The promoter region of the 5HT1A gene lacks an estrogen response element (ERE), thus estrogenic regulation of 5HT1A expression is probably not direct. Rather, we have suggested that ligand activated ERβ interferes with the ability of NF B to drive 5HT1A gene expression 12. ERβ, when bound with raloxifene, may not be able to execute this particular function. However, other genes in serotonin neurons, such as TPH, that contain EREs in the promoter region may be able to utilize ERβ when bound to raloxifene, for transcription initiation.
Raloxifene had no effect on 5HT2C expression. The steroid receptor compliment of the GABA neurons in the primate dorsal raphe nucleus that express 5HT2C receptors has not been defined, so the mechanism by which estrogen or raloxifene act on the expression of these receptors is unknown.
In summary, these data suggest that the effect of 5 months of E administration (yielding a serum concentration of 60 pg/ml) differs from 1 month of E administration on the expression of 5HT1A and 2C receptor proteins in the primate dorsal raphe nucleus. Although it is unclear what molecular mechanism may cause the efficacy to change, these data suggest that longer term E administration without supplemental P may lead to an inhibition of serotonin neurotransmission by two mechanisms, ie., through the serotonergic auto-receptor and through stimulation of inhibitory interneurons. Whether these changes impact serotoninergic tone or would produce a clinically meaningful behavioral outcome is unknown. However, these findings could have implications for the effective use of hormone therapy strategies in the aging population.
Materials and Methods
Animal Subjects and Study Design
The Oregon National Primate Research Center (ONPRC) Animal Care and Use Committee approved this study. Unless otherwise stated, reagents were ordered from Sigma Chemical Co., St. Louis, MO.
Twenty-four adult female rhesus monkeys (Macaca mulatta) were ovariectomized and hysterectomized (spayed) by the surgical personnel of the ONPRC according to accepted veterinary protocol. Dental exam was used to determine the age of monkeys that were not born in Oregon. All animals were between 5 and 13 years of age, weighted between 3.8 and 9.2 kg, and were in good health. Monkey chow (Purina) was provided ad libitum and supplemented with fruit and vitamins. Animals were randomly divided into the following treatment groups:
-
1)
Ovariectomized (Ovx): control group was ovariectomized (ovx) for 5 months and then euthanized.
-
2)
Estradiol treatment for 1 month (E1): Ovx for 16±3 months and then implanted with one 4.5 cm Silastic capsule filled with crystalline estradiol for 28 days.
-
3)
Estradiol plus progesterone treatment for 1 month (EP1): Ovx for 6±0.6 months and then implanted with a 4.5 cm E-filled capsule for 28 days and 14 days later implanted with a 6 cm Silastic capsule filled with crystalline progesterone from days 14-28.
-
4)
Estradiol treatment for 5 months (E5): Ovx and immediately implanted with one 4.5 cm E-filled Silastic capsule which was replaced at 2.5 months.
-
3)
Estradiol plus progesterone treatment for 5 months (EP5): Ovx and immediately implanted with a 4.5 cm E-filled Silastic capsule and a 6 cm P-filled Silastic capsule, both of which were replaced at 2.5 months.
-
6)
Raloxifene treatment for 5 months (Ral5): Ovx and raloxifene (Evista, 5 mg/kg daily) was added to the morning feed (0700-0800) for 5 months. Evista cannot be delivered by Silastic capsules and is formulated for oral consumption.
The dose of estradiol and progesterone that is achieved with the Silastic implants is based upon the levels observed during the menstrual cycle. The 4.5 cm E implants achieve serum estradiol levels that duplicate levels measured in the early to mid-follicular phase (60-150 pg/ml). The 6.0 cm P implants duplicate levels measured in the early to mid-luteal phase (3-8 ng/ml). When an ovariectomized animal is treated with an E implant supplemented with a P implant on day 14, the uterine endometrium proliferates and differentiates over a 1-month treatment period in the same manner as observed during a normal menstrual cycle, indicating the immediate efficacy of hormone therapy. The 5-month treatment period was initiated to document the effects of hormone therapy on the pelvic ligaments and the brains were donated to this study. The longer treatment period was of great interest to this program due to the longer duration of hormone therapy in postmenopausal women.
All capsules were implanted subcutaneously in the periscapular region under ketamine anesthesia (10 mg/kg). Crystalline steroid hormones were obtained from Steraloids (Wilton, New Hampshire). Serum E and P concentrations were previously measured with radioimmunoassay in blood samples obtained at necropsy by the ONPRC Endocrine Services Laboratory to verify efficacy of the treatments 13. After treatment all monkeys were euthanized according to procedures recommended by the Panel on Euthanasia of the American Veterinary Association. Monkeys were sedated with ketamine HCl (10 mg/kg, i.m.) and anesthetized with sodium pentobarbital (30 mg/kg, i.v.) and exsanguinated.
The animals treated for 5 months were euthanized over a period of 17 months, and the brains were generously donated to this study by Dr. Amanda Clark, Department of Obstetrics and Gynecology, Oregon Health and Science University. The brains were stored at −80°C until the collection was complete. The animals treated for 1 month were euthanized over an 8-day period and the brains were stored at −80°C for less than one month before homogenization. All homogenizations were done within a one-week period.
Midbrain Dissection and Protein Assay
The brain was removed from the cranium and the midbrain was dissected, wrapped in foil and immediately dropped in liquid nitrogen and stored at −80°C until microdissection of the midbrain for Western analysis as previously described 13. The dissected midbrain block displayed the rounded central canal on its anterior surface and the wing-shaped canal on its caudal surface. This section was microdisected and a small square piece of tissue was harvested which extended from the middle of the central gray (cutting through the middle of the canal) to, but did not include, the decussation of the cerebellar peduncles. The piece was the width of the central gray and contained the major portion of the dorsal raphe (approximately 5mm wide, 6 mm high and 3mm thick). Each piece, called the dorsal raphe nucleus block or DRN, was homogenized in 1 mL of 50 mM Tris (pH 7.5) and 20 mM 2-β-mercaptoethanol and centrifuged at 12000 g for 10 min at 4°C. The supernatant was removed and the pellet was stored at −80°C until resuspension and protein assay for western blot analysis. Pellets from centrifuged homogenized DRN blocks containing membrane-bound proteins were resuspended in 300 μL of Tris (10 mM) and EDTA (1 mM), pH 7.2, containing leupeptin (1 μg/ml), trypsin inhibitor (1 mg/ml), O-phenanthroline (1 mM), iodoacetamide (1 mM), PMSF (250 μM), and pepstatin A (1 μM) and further homogenized with a handheld pestle and mortar (Fisher Scientific, Pittsburgh, PA, USA). Immediately prior to loading the gel, protein assays were performed on the resuspended pellet with the Bio-Rad protein determination reagent according to the method of Bradford 33.
Western Blot Analysis for 5HT1A Receptor
Raphe extracts containing 25 μg of total protein from each animal were dissolved in an equal volume of TPI buffer (10 mM Tris, Complete Mini Protease Inhibitor Cocktail Tablet (Roche Diagnostics, Germany), 1mg/mL trypsin inhibitor, pH 7.2) containing 20% SDS (BioRad). Diluted samples were mixed with an equal volume Laemmli dye containing 8% β-mercaptoethanol and heated in an 80°C water bath for 15 min followed by a 90°C water bath for 10 min before being loaded on a 10% SDS polyacrylamide gel with a 4% stacking gel (Jule Biotechnologies, Milford, CT). Molecular weight markers (Kalidescope, Biorad) were included. Aliquots from a large homogenate of pineals from several monkeys (50 μg), RN46A cells (100 μg, 200 μg), and a blocking peptide (3 μg; Santa Cruz 1459P) were included during development as positive controls. Western blotting was performed as previously described 13. Each blot contained one animal from each treatment group plus markers and a positive control. Thus, 4 blots were required to examine all animals. The blots were incubated in primary antibody overnight. Affinity purified goat anti-human 5HT1A(C-19) polyclonal antibody (sc-1459; Santa-Cruz Biotechnology, Inc., Santa Cruz CA) was used at a dilution of 1/500 in TBS buffer (50 mM Tris and 150 mM NaCl, pH 7.5). The following morning the nitrocellulose membranes were washed with Saline-Tween (3×10 min each; 10 mM Tris, 0.9% NaCl, 0.05% Tween, pH 7.4) before incubation with the secondary antibody for 90 min at RT. Bovine anti-goat antibody conjugated with horseradish peroxidase (sc-2350,Santa Cruz Biotechnology, Inc.) was used as second antibody at a dilution of 1/2000 in 8% nonfat milk. The membranes were again washed with Saline-Tween (3× 10 min each) and the signal was detected by incubating with Supersignal substrate chemilumenescent reagents (Pierce, Rockford, IL) and then exposing the blots to Scientific imaging film (X-OMAT AR film, Eastman Kodak, Rochester, NY) in the darkroom. The nitrocellulose membranes were then incubated in Coomassie Blue to verify equal loading of samples.
Western Blot Analysis for 5HT2C Receptor and Actin
Never-thawed aliquots containing 50 μg of protein from the resuspended raphe pellets were run on new gels as previously described 13 for comparison of 5HT2C receptor and actin protein levels. The pellet from a pool of homogenized monkey mediobasal hypothalami (50 μg) was used as the positive control due to the high concentration of 5HT2C receptor mRNA in this area (Gundlah et al., 1999). The pellet from a homogenized periaquaductal gray area exhibited no 5HT2C signal and served as the negative control. A blocking peptide for actin (5 μg; A2522, Sigma-Aldrich, Inc., Saint Louis, MO) was included in developmental gels as a positive control and an E2F1 peptide (Santa Cruz, sc-193p) was included as a negative control. Blocking peptides for 5HT2C receptor were not available. The blots (n=4) were incubated with affinity purified mouse anti-human 5HT2C receptor monoclonal antibody (556335; BD Pharmingen, San Diego, CA) at a dilution of 0.5 μg/mL in TBS buffer overnight. The blots were washed in Saline-Tween and incubated with goat anti-mouse conjugated with horseradish peroxidase (IgG HRP antibody; A9917; Sigma) at a dilution of 1/5000 in 8% nonfat milk for 120 min. Each blot was incubated with Supersignal and exposed to film as described above.
The blots were then stripped for 15 min in 1x Re-Blot Plus Mild (Chemicon), rinsed in TBS at RT for 10 min, blocked with 8% nonfat milk at RT for 15 min, rinsed TBS again at RT for 10 min, incubated with Supersignal and exposed to film as described above to ensure the signal was absent. The blots were then rinsed in TBS at RT for 10 min and incubated with mouse anti-actin monoclonal antibody (MAB 1501; Chemicon International Inc., Temecula, CA) at a dilution of 1/4000 in TBS buffer overnight. Each blot was washed in Saline-Tween and incubated with goat anti-mouse conjugated with horseradish peroxidase (IgG HRP antibody; A9917; Sigma) at a dilution of 1/10000 in 8% nonfat milk for 120 min. Actin signal was detected by exposing the blot to chemilumenescent substrate and film as above.
Densitometric Analysis of Western Blotting Results
The specific bands on the individual films were captured using an XC-77 CCD video camera (Sony, Towada, Japan) and a framegrabber board on a Mac G4. Densitometric analysis of signal bands was performed using NIH Image Gel Plotting software. The region of interest containing the sample band was marked. The image analysis program scans each lane and converts the size and intensity of each band to a peak. NIH Image then determines the area under the peak in arbitrary units.
Statistical Analysis
The optical densities of the bands on the film in arbitrary units were compared between treatment groups using an ANOVA. Differences between treatment groups were considered significant when p<0.05. Post hoc comparisons were obtained with a Student-Newman-Keuls (SNK) test and differences between treatment groups were considered significant when p<0.05. All statistical analyses were conducted with the Prism Statistic Program (GraphPad, San Diego, CA).
Acknowledgements
The authors are indebted to Dr. Amanda Clark for the generous donation of brains from her experimental animals in which she examined the effect of 5 months of hormone treatment on the structural integrity of the pelvic floor. The authors would like to thank the dedicated staff of the Division of Animal Resources of ONPRC without whose support these experiments would not have been possible.
Supported by NIH grants: MH62677 to CLB, the Eunice Kennedy Shriver NICHD through cooperative agreement U54 HD 18185 as part of the Specialized Cooperative Centers Program in Reproduction and Infertility Research, a training grant in Reproductive Biology T32 HD07133, and RR00163 for the operation of ONPRC
Literature Cited
- 1.Murphy DL. Ann. N. Y. Acad. Sci. 1990;600:282–295. doi: 10.1111/j.1749-6632.1990.tb16890.x. [DOI] [PubMed] [Google Scholar]
- 2.Van de Kar L. Ann. Rev. Pharmacol. Toxicol. 1991;31:289–320. doi: 10.1146/annurev.pa.31.040191.001445. [DOI] [PubMed] [Google Scholar]
- 3.Siever LJ, Kahn RS, Lawlor BA, Trestman RL, Lawrence TL, Coccaro EF. Pharmacol. Rev. 1991;43:509–525. [PubMed] [Google Scholar]
- 4.Azmitia EC, Gannon PJ. Adv. Neurol. 1986;43:407–468. [PubMed] [Google Scholar]
- 5.Fifa E, Fillion G. Pharmacol. Rev. 1992;44:401–458. [PubMed] [Google Scholar]
- 6.Uphouse L. Neurosci. Biobehav. Rev. 1997;21:679–698. doi: 10.1016/s0149-7634(96)00022-x. [DOI] [PubMed] [Google Scholar]
- 7.Bethea CL. Neuroendocrinology. 1993;57:1–6. doi: 10.1159/000126334. [DOI] [PubMed] [Google Scholar]
- 8.Bethea CL. Neuroendocrinology. 1994;60:50–61. doi: 10.1159/000126719. [DOI] [PubMed] [Google Scholar]
- 9.Gundlah C, Kohama SG, Mirkes SJ, Garyfallou VT, Urbanski HF, Bethea CL. Mol. Brain Res. 2000;76:191–204. doi: 10.1016/s0006-8993(99)02475-0. [DOI] [PubMed] [Google Scholar]
- 10.Gundlah C, Lu NZ, Mirkes SJ, Bethea CL. Mol. Brain Res. 2001;91:14–22. doi: 10.1016/s0169-328x(01)00108-5. [DOI] [PubMed] [Google Scholar]
- 11.Tsai MJ, O’Malley BW. Ann. Rev. Biochem. 1994;63:451–486. doi: 10.1146/annurev.bi.63.070194.002315. [DOI] [PubMed] [Google Scholar]
- 12.Bethea CL, Lu NZ, Gundlah C, Streicher JM. Front. Neuroendocrinol. 2002;23:41–100. doi: 10.1006/frne.2001.0225. [DOI] [PubMed] [Google Scholar]
- 13.Smith LJ, Henderson JA, Abell CW, Bethea CL. Neuropsychopharmacology. 2004;29:2035–2045. doi: 10.1038/sj.npp.1300510. [DOI] [PubMed] [Google Scholar]
- 14.Sanchez RL, Reddy AP, Centeno ML, Henderson JA, Bethea CL. Brain Res. Mol. Brain Res. 2005;135:194–203. doi: 10.1016/j.molbrainres.2004.12.011. [DOI] [PubMed] [Google Scholar]
- 15.Barnes NM, Sharp T. Neuropharmacology. 1999;38:1083–152. doi: 10.1016/s0028-3908(99)00010-6. [DOI] [PubMed] [Google Scholar]
- 16.Liu R, Jolas T, Aghajanian G. Brain Res. 2000;873:34–45. doi: 10.1016/s0006-8993(00)02468-9. [DOI] [PubMed] [Google Scholar]
- 17.Pecins-Thompson M, Bethea CL. Neuroscience. 1998;89:267–277. doi: 10.1016/s0306-4522(98)00326-1. [DOI] [PubMed] [Google Scholar]
- 18.Lu NZ, Bethea CL. Neuropsychopharmacology. 2002;27:12–24. doi: 10.1016/S0893-133X(01)00423-7. [DOI] [PubMed] [Google Scholar]
- 19.Gundlah C, Pecins-Thompson M, Schutzer WE, Bethea CL. Brain Res. Mol. Brain Res. 1999;63:325–339. doi: 10.1016/s0169-328x(98)00295-2. [DOI] [PubMed] [Google Scholar]
- 20.Epperson CN, Wisner KL, Yamamoto B. Psychosom. Med. 1999;61:676–97. doi: 10.1097/00006842-199909000-00010. [DOI] [PubMed] [Google Scholar]
- 21.Osterlund MK, Witt MR, Gustafsson JA. Endocrine. 2005;28:235–42. doi: 10.1385/ENDO:28:3:235. [DOI] [PubMed] [Google Scholar]
- 22.Williams JK, Honore EK, Adams MR. Circulation. 1997;96:1970–1975. doi: 10.1161/01.cir.96.6.1970. [DOI] [PubMed] [Google Scholar]
- 23.Sumner BEH, Fink G. Mol. Cell Neurosci. 4:83–92. doi: 10.1006/mcne.1993.1010. [DOI] [PubMed] [Google Scholar]
- 24.Sumner BE, Grant KE, Rosie R, Hegele-Hartung C, Fritzemeier KH, Fink G. Brain Res. Mol. Brain Res. 1999;73:119–28. doi: 10.1016/s0169-328x(99)00243-0. [DOI] [PubMed] [Google Scholar]
- 25.Cyr M, Bosse R, Di Paolo T. Neuroscience. 1998;83:829–836. doi: 10.1016/s0306-4522(97)00445-4. [DOI] [PubMed] [Google Scholar]
- 26.Gibbs RB. Neuroscience. 2000;101:931–938. doi: 10.1016/s0306-4522(00)00433-4. [DOI] [PubMed] [Google Scholar]
- 27.Clarkson TB, Cline JM, Williams JK, Anthony MS. Osteoporos. Int. 1997;7(suppl 1):43–51. doi: 10.1007/BF01674813. [DOI] [PubMed] [Google Scholar]
- 28.Adams MR, Kaplan JR, Manuck SB, Koritnik DR, Parks JS, Wolfe MS, Clarkson TB. Arteriosclerosis. 1990;10:1051–1057. doi: 10.1161/01.atv.10.6.1051. [DOI] [PubMed] [Google Scholar]
- 29.Jacobs HS. Medscape Womens Health. 2000;5:E2. [PubMed] [Google Scholar]
- 30.Strickler R, Stovall DW, Merritt D, Shen W, Wong M, Silfen SL. Obstet. Gynecol. 2000;96:359–365. doi: 10.1016/s0029-7844(00)00937-6. [DOI] [PubMed] [Google Scholar]
- 31.Florio P, Quirici B, Casarosa E, Lombardi I, Luisi M, Genazzani AD, Petraglia F, Genazzani AR. Gynecol. Endocrinol. 2001;15:359–66. [PubMed] [Google Scholar]
- 32.Bethea CL, Mirkes SJ, Su A, Michelson D. Psychoneuroendocrinology. 2002;27:431–445. doi: 10.1016/s0306-4530(01)00054-3. [DOI] [PubMed] [Google Scholar]
- 33.Bradford MM. Analytical Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]