Abstract
VEGF and Angiopoietin (Ang)1 are growth factors that independently improve wound healing outcomes. Using a tet-repressible mouse model coupled with streptozotocin-induced diabetes, we examined wound healing in diabetic and nondiabetic mice engineered to overexpress keratinocyte-specific (K5) VEGF, Ang1 or Ang1-VEGF combined. All nondiabetic mice healed more rapidly than their diabetic counterparts; however overexpression of VEGF, Ang1 or the combination failed to improve wound closure under diabetic conditions. Conversely, under nondiabetic conditions, combining Ang1 and VEGF resulted in rapid wound closure. Molecular analyses of diabetic and nondiabetic K5-Ang1-VEGF skin revealed no differences in VEGF expression but an 80% decrease in Ang1 under diabetic conditions, suggesting an integral role for Ang1. Nondiabetic K5-Ang1 mice healed more quickly and had significant increases in granulation tissue and a 60% decrease in re-epithelialization 7 days after wounding. Furthermore, Ang1 stimulated primary mouse keratinocytes showed significantly less migration into a wound bed in an in vitro wound healing bioassay and had decreased pMAPK, pNFκB, pAkt, and pStat3 signaling. These data suggest that combined Ang1-VEGF overexpression cannot overcome diabetes-induced delays in wound healing but is efficacious under nondiabetic conditions possibly via Ang1-mediated delays in re-epithelialization and enhancement of granulation tissue formation, thereby allowing more rapid secondary intention healing.
Keywords: Streptozotocin, angiogenesis, re-epithelialization, wound healing, transgenic mouse, keratinocyte, skin
Introduction
People with diabetes have a 15 times greater risk of lower limb amputation than nondiabetic individuals, due in part to chronic, nonhealing wounds [1]. The impairments in cutaneous wound healing associated with diabetes result in part but not exclusively, from deficiencies in angiogenesis [2], a reduction in cutaneous sensory innervation [3], dysregulated inflammation [4], and delays in re-epithelialization [5]. Wounds occurring in diabetic patients as well as hyperglycemic mice take significantly longer to close and have reductions in vascular growth factors [6-10]. Although the initial wound closure may not be dependent upon angiogenesis [11-13], full maturation and healing of the wound is dependent on the vascular response, including the release of angiogenic growth factors from skin resident macrophages, keratinocytes, pericytes, and endothelial cells. Blood vessels also participate in the formation of granulation tissue, providing nutrition and oxygen to growing tissues. In human disease and in murine diabetes models, impairments in wound healing are characterized by delays in cellular infiltration and granulation tissue formation, reduced angiogenesis, decreased collagen synthesis, and decreased recruitment of circulating vascular progenitors [14-17]. The reduction in angiogenesis has been attributed to decreased matrix metalloproteinase (MMP) levels and activity [18], decreased numbers and function of endothelial progenitor cells [16, 19, 20], and alterations in expression of vascular specific growth factors and their receptors [21], including VEGF and Tie2 [4, 6, 7, 22-24] and increases in the context specific antagonizing Tie-2 ligand, angiopoietin-2 (Ang2) [6, 25]. Interestingly, Ang2 increases promote vessel regression when VEGF is absent, decreased, or its activity inhibited [26-29]; an environmental scenario like that observed in the diabetic cutaneous wound [6]. This suggests Tie2 activity is required for vessel maintenance [30, 31], and that Ang2 increases occurring concomitant with decreased VEGF facilitate either vascular desta-bilization and vessel sprouting or EC death [27, 32]. The introduction of exogenous Ang1 under these conditions could potentially promote vessel stabilization and angiogenesis.
Current attempts at targeting growth factors have for the most part targeted the loss of VEGF protein associated with impaired wound healing in diabetics. Topical application of VEGF accelerates wound healing in a diabetic setting [11, 17, 23, 24, 33] but also results in increased permeability leading to “leaky” vessels, which may not be beneficial [17, 34-36]. Ang1 when combined with VEGF in the skin, leads to angiogenic remodeling of the cutaneous vasculature, but prevents vessel leakage associated with VEGF exposure alone [35-37]. More recently, others have used Ang1 as a therapeutic for impaired wound healing [38-40], reporting enhanced granulation tissue formation and improved wound closure. However, the effect of targeting Ang1 and VEGF in combination on diabetic and nondiabetic cutaneous wound healing outcomes in vivo has not been examined.
Methods
Animals
Keratinocyte-specific (K5) doxycycline-repressible transgenic mice overexpressing either Angiopoietin 1 (Ang1), vascular endothelial growth factor (VEGF) or Ang1-VEGF together were generated and genotyped as described previously [41]. K5-VEGF and K5-Ang1-VEGF transgenic mice fail to thrive when exposed to high levels of transgenic VEGF expression throughout gestation [41]; therefore for the diabetes wound healing experiments, diabetic and nondiabetic K5-Ang1, K5-VEGF, K5-Ang1-VEGF mice and littermate controls were bred and raised in the presence of doxycycline-supplemented food (200mg/kg; BioServ; Frenchtown, NJ) thereby suppressing transgene expression. Gene expression was initiated by removing the doxycycline food three days prior to wounding. A second cohort of nondiabetic K5-Ang1 mice and their littermate controls were also generated; these mice expressed transgenic Ang1 from conception onward (i.e. no doxycycline exposure at any point).
Diabetes induction
Adult male mice were injected once with either 150 mg/kg of streptozotocin (STZ) to induce diabetes or control citrate buffer (nondiabetic). Blood glucose levels were monitored and diabetes was defined as mice with blood glucose levels greater than 250 mg/dL. Insulin (0-2units of NPH insulin subcutaneously diluted 10 fold) was provided as needed to achieve slow weight gain in the presence of hyperglycemia and glucosuria. Thus, diabetic animals were insulin-deficient but not grossly catabolic. Wound healing experiments were performed in animals after 6-8 weeks of diabetes.
Wound healing
Diabetic and nondiabetic K5-Ang1, K5-VEGF, K5 -Ang1-VEGF and littermate control animals had doxycycline removed (turning transgene expression on in transgenic mice) three days prior to wounding and the dorsal back skin was shaved and depilated in preparation for wounding. Two dorsal wounds were made using a 6 mm punch biopsy tool and wound size at the gross level was measured daily for the duration of the experiments and was presented as a percentage of the original wound size on day 0 to accommodate differences in original wound size; the average of two wound percentages/mouse was used. Skin was collected for RNA and protein isolation and analyses in a subset of mice 7 days following wounding.
Nondiabetic K5-Ang1 mice expressing transgenic Ang1 from conception onward (i.e. no doxycycline) had wounds generated and quantitated as described above. Mice were sacrificed at one of three time points, day 4, 7, or 10 following wounding and tissue was collected for histological analyses.
All animal protocols were approved by the Case Western Reserve University institutional animal care and use committee (IACUC) and conformed to the American Association for Accreditation of Laboratory Animal Care guidelines.
Tissue collection and histological and morphometric analyses
At 4, 7 or 10 days following wounding, nondiabetic K5-Ang1 animals and littermate controls with no doxycycline exposure were euthanized and the wounded skin carefully dissected, the wound bisected in the longest plane and the tissue placed in 10% buffered formalin (Surgipath Medical Industries, Richmond, IL), overnight at 4°C prior to dehydration and paraffin embedding (Sakura Finetech, Torrance, CA). H&E staining was completed on 5 μm thick paraffin sections using standard protocols.
Re-epithelialization of the wound was quantitated in a manner allowing for standardization accounting for small differences in original wound size between mice and regions, as well as other small differences that occur due to experimental variability. Using Image Pro interactive software (MediaCybernetics, Bethesda, Maryland) the wound gap, defined as the distance between the two epithelial tongues (i.e. the keratinocytes migrating into the wound bed); was measured and subtracted from the total length of the original wound (i.e. the distance between the normal skin/wounded skin border on each side of the skin); providing the total re-epithelialization length. This number was then divided by the original length of the wound to supply the percent re-epithelialization. Granulation tissue was measured in the same sections using Image Pro software to trace the boundary of the granulation tissue within each section.
Tissue RNA and protein isolation and analysis
Dorsal skin from nondiabetic and diabetic control and K5-Ang1-VEGF transgenic mice was isolated from mice 7 days following wounding and was homogenized using a Mikro-Dismembrator S (Sartorius Stedim Biotech, Edgewood, NY). RNA and protein were isolated and quantified as described previously [42]. VEGF protein (R&D Systems, Minneapolis, MN), expression was quantitated by ELISA according to the manufacturer's instructions. RNA was reversed transcribed using MMLV reverse transcriptase (Invitrogen, Carlsbad, CA) following the manufacturer's protocol. Quantitative real-time RT-PCR was performed using Taqman technology from Applied Biosystems on a StepOnePlus Realtime system. Probes and primers for Ang1 and 18S were obtained from Applied Biosystems. Expression levels were normalized to 18S.
Primary mouse keratinocyte cultures and in vitro wound assay
Primary mouse keratinocytes were isolated from wildtype CD1 mouse embryos at embryonic day (E) 17.5 according to published protocols [43]. Scratch assays were performed using primary mouse keratinocytes similar to methods previously described [44]. Briefly, 1.5 × 106 cells were plated in a 60 mm plate, and a scratch made across the plate using a pipette tip; plates were then rinsed with PBS followed by media containing 10 ug/ml Mitomycin C (an inhibitor of DNA synthesis) or containing Mitomycin C and 100 ng/ml recombinant mouse Ang1 (R&D, Minneapolis, MN). Images of each scratch (wound) were taken immediately, and the distance between the two keratinocyte beds (wound size) was measured using Image Pro interactive software, providing a baseline measurement of the original in vitro wound size. Images of the same wounds were taken 16 hours later and the wound size re-measured. At least 12 scratches per condition were measured at 0 and 16 hrs per experiment, and the experiment was replicated 6 times.
Signaling analysis
Primary mouse keratinocytes were plated at a cell density of 1 × 106 cells, allowed to attach overnight, and were then stimulated for ten minutes with 100 ng/ml recombinant Ang1 (R&D) or left untreated. Protein lysates were isolated from treated and untreated cells and Western blotting performed using methods published previously [45, 46]. Antibodies used included: anti-phospho-FAK, anti-FAK, anti-phospho Akt, anti-Akt; anti-phospho p44/42 MAPK, anti-MAPK, anti-phospho NFκB, anti-NFκB, anti-phospho-Stat3, anti-Stat3 (all Cell Signaling, Danvers, MA), anti-phospho-Tie2 (Angio-Proteomie, Boston, MA) and anti-Tie2 (Santa Cruz, Santa Cruz, CA). Equal loading of samples was confirmed using a monoclonal antibody against β Actin (Clone AC-15, Sigma, St. Louis, MO). Blots were reproduced using independent samples at least twice.
Statistics
All data are represented as mean ± standard error of the mean (SEM). Between group comparisons were analyzed using either a Student's T test or a Mann Whitney U test and statistical significance was defined as P < 0.05.
Results
K5-Ang-VEGF animals heal more quickly under nondiabetic conditions and this effect is lost following 6-8 weeks of STZ-induced diabetes
Keratinocyte-specific doxycycline repressible transgenic mice overexpressing either Angiopoietin 1 (Ang1), vascular endothelial growth factor (VEGF) or VEGF and Ang1 (Ang1-VEGF) were generated using the K5 promoter. K5-VEGF and K5-Ang1-VEGF mice exposed to transgenic VEGF alone and in combination with Ang1 failed to thrive, and displayed significant morbidity (data not shown) [41] making it difficult to induce diabetes or to study effects on cutaneous wound healing. To circumvent this, we utilized the repressible nature of the transgenic model system and exposed animals to doxycycline food beginning at gestation and up until just prior to wounding. To study the effects of skin-specific overexpression of Ang1, VEGF, and Ang1-VEGF on wound healing under diabetic and nondiabetic conditions, animals were made diabetic using STZ (or injected with control citrate buffer) and following 6-8 weeks of diabetes, transgene expression was turned on (via removal of doxycycline) and three days later mice were wounded on the dorsal surface of their skin.
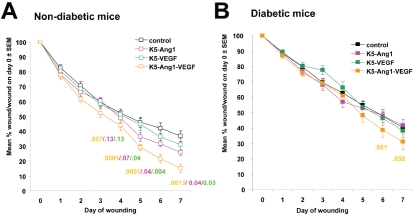
Under nondiabetic conditions K5-Ang1-VEGF mice have significantly smaller wounds than K5-Ang1, K5-VEGF and control littermates beginning ∼4 days following wounding (Figure 1A). As expected, all strains of diabetic mice (control, K5-Ang1, K5-VEGF and K5-Ang1-VEGF) healed more slowly than non diabetic animals (Figure 1A and 1B). However, under diabetic conditions, no statistically significant differences between any of the groups were observed (Figure 1B), and the Ang-VEGF mediated enhancement on wound closure observed under nondiabetic conditions was lost.
Figure 1.
K5-Ang-VEGF animals heal more quickly under nondiabetic conditions and this effect is lost following 6-8 weeks of STZ-induced in diabetes. The size of the wounds on the dorsal surface of each mouse was measured and expressed as a percent of the original wound size. These values were averaged across each group and are presented as mean ± SEM. (A) Under non-diabetic conditions K5-Ang1-VEGF mice have significantly smaller wounds than K5-Ang1, K5-VEGF and control littermates beginning at ∼day 4 following wounding. All nondiabetic mice healed more quickly than their diabetic counterparts. (B) Under diabetic conditions, no statistically significant differences between any of the groups were observed, and the Ang-VEGF mediated effect observed under nondiabetic conditions is lost. Statistical P values for K5-Ang1-VEGF vs controls, K5-Ang1 and K5-VEGF animals are indicated under each timepoint.
STZ-induced diabetes leads to significant decreases in Ang1 expression 7 days after wounding in K5-Ang1-VEGF mice
The observation that nondiabetic rather than diabetic K5-Ang1-VEGF mice healed more quickly versus control animals (Figure 1) suggested that diabetes was changing the cutaneous environment and lead to the examination of growth factor regulation under both conditions. Examination of VEGF and Ang expression in control and K5-Ang1-VEGF mice under diabetic and nondiabetic conditions confirmed significant increases in VEGF (Figure 2A) and Ang1 (Figure 2B) expression in transgenic K5-Ang1-VEGF mice compared to control animals under diabetic and nondiabetic conditions; but that diabetes lead to ∼80% decrease in Ang1 expression in diabetic vs nondiabetic K5-Ang1-VEGF transgenic animals (Figure 2B; P < 0.05).
Figure 2.
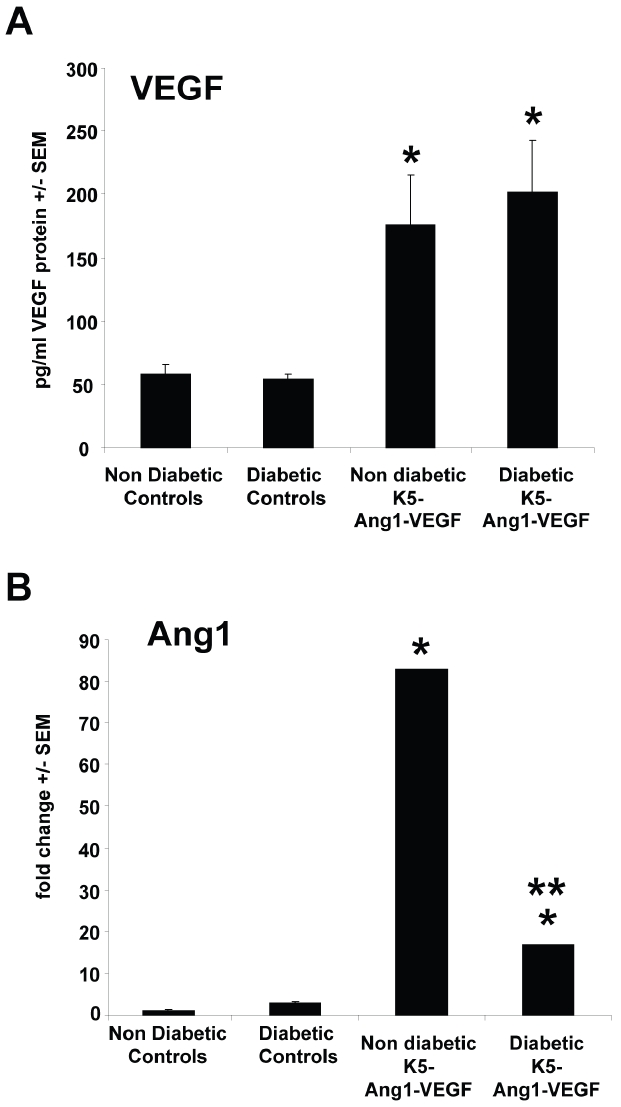
VEGF and Ang1 expression in control and K5-Ang1-VEGF mice. (A) ELISA analyses of VEGF protein expression in nondiabetic and diabetic control littermates reveals significant increases in K5-Ang1-VEGF transgenic mouse skin compared to littermate controls under diabetic and nondiabetic conditions. (B) qRT-PCR of transgenic Ang1 in K5-Ang1-VEGF mouse skin demonstrates significant increases in diabetic and nondiabetic conditions compared to controls; however a ∼4-fold decrease in Ang1 expression was observed in diabetic K5-Ang1-VEGF compared to nondiabetic K5-Ang1-VEGF animals. *P < 0.05 compared to diabetic and nondiabetic control animals; **P < 0.05 compared to nondiabetic K5-Ang1-VEGF mice.
K5-Ang1 animals have improved wound healing compared to littermate controls
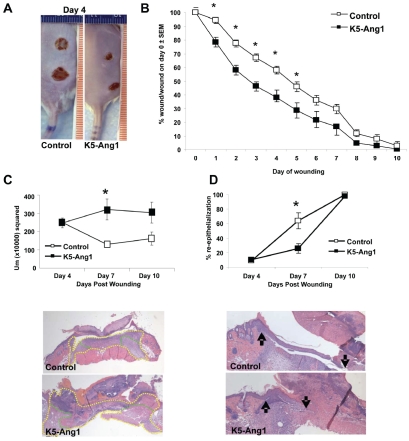
To further examine the importance of Ang1 in wound healing and to elucidate the potential mechanisms by which Ang1 alone could enhance wound closure, an additional group of nondiabetic K5-Ang1 transgenic mice were generated with Ang1 gene expression present throughout development and into adulthood. These mice appear normal at the gross level, show no morbidity and thrive into adulthood [41]. Adult K5-Ang1 transgenic mice and control littermates were wounded and wound closure was monitored daily. Beginning 1 day and extending out to 6 days after wounding, K5-Ang1 mice had smaller wounds (Figure 3A and 3B). At the histological level, no differences between groups were apparent at 4 days following wounding, however at day 7, significant increases in the amounts of granulation tissue in K5-Ang1 mice compared to littermate controls was observed (Figure 3C), coupled with a delay or decrease in the amount of re-epithelialization (Figure 3D). By 10 days, wounds were completely re-epithelialized in all mice and, although no longer statistically significant, granulation tissues in K5-Ang1 mouse skin remained elevated (Figure 3C and 3D).
Figure 3.
K5-Ang1 wounds are significantly smaller than control littermates as early as 1 day following wound induction. (A) Pictures of the dorsal surface of control and K5-Ang1 mice 4 days following wounding. (B) Quantitation of the wound size as a percent of the original wound area illustrates the significant improvement in wound closure in K5-Ang1 mice at days 1-6. (C) Histological analyses of H&E stained tissue sections of wounded K5-Ang1 versus control skin revealed significant increases 7 days following wounding in the wound healing response (fibroplasia and granulation; demarcated in C with yellow) and granulation tissue formation (demarcated in C with green). (D) No differences in re-epithelialization were seen at 4 or 10 days post-wounding between control and K5-Ang1 animals, however a significant delay in re-epithelialization in K5-Ang1 mice was observed at 7 days (arrows in D indicate the original wound margin (left) and the border of the re-epithelialization into the wound area (right)). *P < 0.05 vs littermate controls.
Ang1 stimulated keratinocytes migrate less than control stimulated keratinocytes in an in vitro wound healing bioassay and have decreased kinase signaling
Published work by us and others has demonstrated a lack of the angiopoietin tyrosine kinase receptor, Tie2 in keratinocytes [42, 47]; and see Figure 4B) but has identified β1 integrin as an alternative receptor for Ang1-mediated biological effects, including in skin [46, 47]. To study whether Ang1 could elicit biological effects on keratinocytes directly, we used wildtype primary mouse keratinocytes in an in vitro wound healing assay and examined the effects of Ang1 stimulation on keratinocyte migration into the wound bed. Consistent with our in vivo observations (Figure 3D) of delays in re-epithelialization, Ang1 stimulated keratinocytes showed ∼50% reduction in migration into a scratch wound compared to media alone (Figure 4A).
Figure 4.
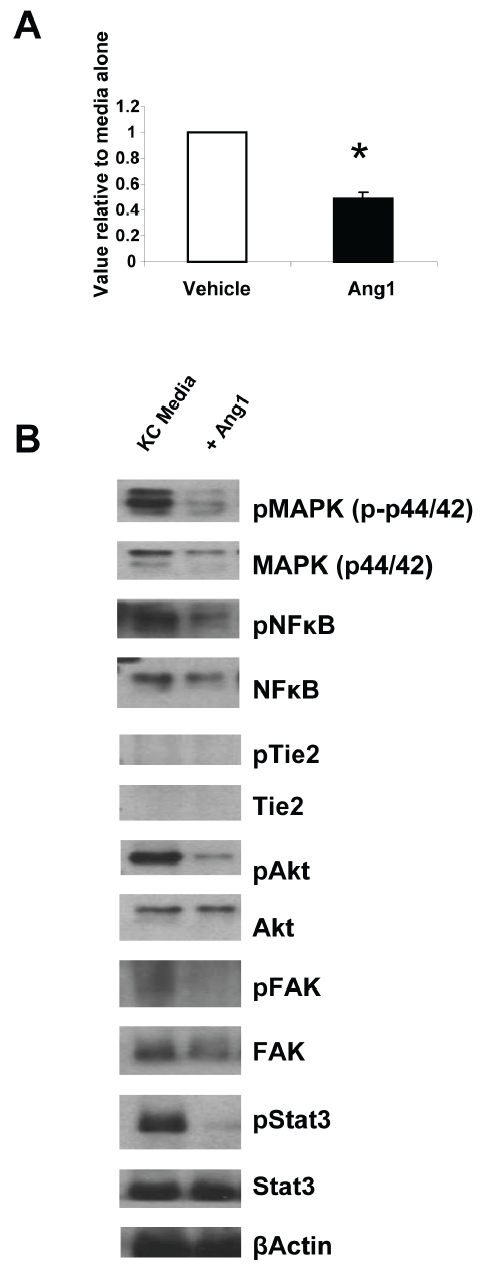
In vitro wound healing assay and kinase signaling following Ang1 stimulation. (A) Pure primary wildtype mouse keratinocytes were stimulated with media containing vehicle or recombinant Ang1; and at time 0 and 16 hours later the size of the wound bed was measured. Data is presented as a value relative to vehicle alone and represents the average of 6 independent experiments. (B) Western blot analyses of common kinase pathways following 10 minutes of Ang1 stimulation (100 ng/ml) demonstrates decreases in MAPK, NFκB, Akt, FAK and Stat3 signaling in keratinocytes stimulated with Ang1 compared to keratinocytes stimulated with vehicle. *P < 0.05 vs vehicle alone.
To examine the molecular signaling events in keratinocytes following Ang1 stimulation, we used Western blotting approaches coupled with phosphorylation analyses of common kinase pathways (Figure 4B). Ang1 stimulated keratinocytes showed decreases in phosphorylation of MAPK, NFκB, Akt, FAK and Stat3 compared to keratinocytes stimulated with media alone (Figure 4B) and no expression of Tie2 protein.
Discussion
We sought to determine the effects of targeting Ang1 and VEGF in combination on diabetic and nondiabetic cutaneous wound healing outcomes in vivo and determined that K5-Ang1-VEGF mice heal more quickly than K5-Ang1, K5-VEGF and littermate controls under nondiabetic conditions only and that nondiabetic K5-Ang1-VEGF mice have significantly more Ang1 than diabetic K5-Ang1-VEGF mice suggesting a key role for Ang1 in promoting wound healing. In our efforts to better understand the cellular mechanisms mediating this Ang1-enhanced healing, we confirmed work by others demonstrating mice overexpressing Ang1 alone also healed more rapidly, and that this occurred concomitant with increases in granulation tissue formation [38-40] but in the presence of delayed re-epithelialization, a new observation. Our in vitro results confirmed the Ang1-mediated decrease in keratinocyte migration in an in vitro wound healing model and further examination of Ang1 signaling in keratinocytes identified decreases in MAPK, Akt, NFκB, FAK, and Stat3 signaling. Taken together, these results suggest that combined Ang1 and VEGF enhance wound healing under non-diabetic conditions only, perhaps as a result of having significantly more Ang1 compared to diabetic animals and that Ang1 enhances wound healing by increasing the levels of granulation tissue formation and angiogenesis, and by “holding back” keratinocyte migration, thus allowing secondary intention healing to occur more readily.
As expected, in all strains of mice (control, K5-Ang1, K5-VEGF and K5-Ang1-VEGF), diabetic animals healed more slowly than non diabetic animals, consistent with the well accepted finding that diabetes delays healing. However, in contrast to what others have published [11, 17, 23, 24, 33, 38-40], we failed to find significant increases in the rates of wound healing in diabetic animals expressing excess Ang1, VEGF, or Ang1 and VEGF compared to diabetic control mice. This most likely reflects differences in animal models as all of the wound healing literature evaluating effects of Angiopoietins have utilized Type II models of diabetes (db/db or ob/ ob mice) [38-40], and with the exception of one paper [48], the majority of wound healing evaluating effects of VEGF have also used the genetically diabetic mouse [11, 17, 23, 24, 33]. It is therefore possible that the positive effects previously reported for angiopoietins and VEGF may represent leptin interactions [49-51], rather than modification of the underlying causes of diabetic complications. Alternatively, the difference in findings may also represent duration of diabetes; expression levels of growth factor; route of administration of the growth factor; in addition to the type and location of the wound inflicted on the mouse itself as well as differences in background mouse strains.
Interestingly consistent increases in the rate of wound healing under nondiabetic conditions in animals overexpressing both Ang1 and VEGF (K5-Ang1-VEGF) were observed as early as 4 days after wounding. Our finding that diabetes attenuated this effect, coupled with the observation that Ang1 was significantly decreased in diabetic K5-Ang1-VEGF mice lead to the closer examination of the role of Ang1 in the wound healing process. Others have previously reported that exposure to excess Ang1-signaling enhances cutaneous wound healing [38-40], by increasing angiogenesis and granulation tissue formation; we observed similar outcomes on wound closure and granulation tissue responses. In addition, the improvement in wound healing in K5-Ang1 mice we observed in the current study may also reflect pre-existing anatomical and physiological changes in the skin present prior to wounding; including the increased presence of dermal vasculature, dermal and epidermal innervation, and perhaps increases in infiltrating immune cells [41], which may prime the skin to heal more readily.
However, we also report here that 7 days after wounding, K5-Ang1 animals show a time-specific delay in re-epithelialization of the wound at the same time point granulation tissue formation increases. Our in vitro analyses demonstrates similar effects on wildtype keratinocyte migration into a scratch wound bed following Ang1 stimulation, and also identified decreases in MAPK, NFκB, Akt, Fak and Stat3 signaling in these cells. These findings confirm a prior report showing Ang1-direct effects on keratinocytes occur in a Tie2 independent manner [47], and suggest the Ang1-mediated delay in re-epithelialization observed in vivo may occur via direct Ang1 effects on keratinocyte signaling and migration. The decreases we report here in Akt and MAPK signaling are opposite of what others have previously reported, and may reflect intrinsic differences between primary murine keratinocytes and the spontaneously immortalized human keratinocyte HaCAT cell line [47].
In summary, Ang1 overexpression appears to enhance wound healing not only by increasing angiogenesis and granulation tissue formation, but may also allow more rapid wound closure as a result of “holding back” the epidermis, allowing the wound to heal from the granulation tissue upward (i.e. improving secondary intention healing).
Acknowledgments
The work was funded by a grant to NLW from the Juvenile Diabetes Research Foundation (JDRF). We would like to thank Dr. Timothy Kern and Mr. Allen Lee for their assistance with diabetes induction in the mice.
References
- 1.American Diabetes Association. Economic costs of diabetes in the US In 2007. Diabetes Care. 2008;31:596–615. doi: 10.2337/dc08-9017. [DOI] [PubMed] [Google Scholar]
- 2.Martin A, Komada MR, Sane DC. Abnormal angiogenesis in diabetes mellitus. Med Res Rev. 2003;23:117–145. doi: 10.1002/med.10024. [DOI] [PubMed] [Google Scholar]
- 3.Gibran NS, Jang YC, Isik FF, Greenhalgh DG, Muffley LA, Underwood RA, Usui ML, Larsen J, Smith DG, Bunnett N, Ansel JC, Olerud JE. Diminished neuropeptide levels contribute to the impaired cutaneous healing response associated with diabetes mellitus. J Surg Res. 2002;108:122–128. doi: 10.1006/jsre.2002.6525. [DOI] [PubMed] [Google Scholar]
- 4.Wetzler C, Kampfer H, Stallmeyer B, Pfeilschifter J, Frank S. Large and sustained induction of chemokines during impaired wound healing in the genetically diabetic mouse: prolonged persistence of neutrophils and macrophages during the late phase of repair. J Invest Dermatol. 2000;115:245–253. doi: 10.1046/j.1523-1747.2000.00029.x. [DOI] [PubMed] [Google Scholar]
- 5.Lan CC, Liu IH, Fang AH, Wen CH, Wu CS. Hyperglycaemic conditions decrease cultured keratinocyte mobility: implications for impaired wound healing in patients with diabetes. Br J Dermatol. 2008;159:1103–1115. doi: 10.1111/j.1365-2133.2008.08789.x. [DOI] [PubMed] [Google Scholar]
- 6.Kampfer H, Pfeilschifter J, Frank S. Expressional regulation of angiopoietin-1 and -2 and the tie-1 and -2 receptor tyrosine kinases during cutaneous wound healing: a comparative study of normal and impaired repair. Lab Invest. 2001;81:361–373. doi: 10.1038/labinvest.3780244. [DOI] [PubMed] [Google Scholar]
- 7.Frank S, Hubner G, Breier G, Longaker MT, Greenhalgh DG, Werner S. Regulation of vascular endothelial growth factor expression in cultured keratinocytes. Implications for normal and impaired wound healing. J Biol Chem. 1995;270:12607–12613. doi: 10.1074/jbc.270.21.12607. [DOI] [PubMed] [Google Scholar]
- 8.Menke NB, Ward KR, Witten TM, Bonchev DG, Diegelmann RF. Impaired wound healing. Clinics in Dermatology. 2007;25:19–25. doi: 10.1016/j.clindermatol.2006.12.005. [DOI] [PubMed] [Google Scholar]
- 9.Stadelmann WK, Digenis AG, Tobin GR. Impediments to wound healing. Am J Surg. 1998;176:39S–47S. doi: 10.1016/s0002-9610(98)00184-6. [DOI] [PubMed] [Google Scholar]
- 10.Martin P. Wound Healing–Aiming for Perfect Skin Regeneration. Science. 1997;276:75–81. doi: 10.1126/science.276.5309.75. [DOI] [PubMed] [Google Scholar]
- 11.Kirchner LM, Meerbaum SO, Gruber BS, Knoll AK, Bulgrin J, Taylor RA, Schmidt SP. Effects of vascular endothelial growth factor on wound closure rates in the genetically diabetic mouse model. Wound Repair Regen. 2003;11:127–131. doi: 10.1046/j.1524-475x.2003.11208.x. [DOI] [PubMed] [Google Scholar]
- 12.Vranckx JJ, Yao F, Petrie N, Augustinova H, Hoeller D, Visovatti S, Slama J, Eriksson E. In vivo gene delivery of Ad-VEGF to full-thickness wounds in aged pigs results in high levels of VEGF expression but not in accelerated healing. Wound Repair Regen. 2005;13:51–60. doi: 10.1111/j.1067-1927.2005.130107.x. [DOI] [PubMed] [Google Scholar]
- 13.Stallmeyer B, Pfeilschifter J, Frank S. Systemically and topically supplemented leptin fails to reconstitute a normal angiogenic response during skin repair in diabetic ob/ob mice. Diabetologia. 2001;44:471–479. doi: 10.1007/s001250051645. [DOI] [PubMed] [Google Scholar]
- 14.Bohlen HG, Niggl BA. Adult microvascular disturbances as a result of juvenile onset diabetes in Db/Db mice. Blood Vessels. 1979;16:269–276. doi: 10.1159/000158215. [DOI] [PubMed] [Google Scholar]
- 15.Greenhalgh DG, Sprugel KH, Murray MJ, Ross R. PDGF and FGF stimulate wound healing in the genetically diabetic mouse. Am J Pathol. 1990;136:1235–1246. [PMC free article] [PubMed] [Google Scholar]
- 16.Sivan-Loukianova E, Awad OA, Stepanovic V, Bickenbach J, Schatteman GC. CD34+ blood cells accelerate vascularization and healing of diabetic mouse skin wounds. J Vasc Res. 2003;40:368–377. doi: 10.1159/000072701. [DOI] [PubMed] [Google Scholar]
- 17.Galiano RD, Tepper OM, Pelo CR, Bhatt KA, Callaghan M, Bastidas N, Bunting S, Steinmetz HG, Gurtner GC. Topical Vascular Endothelial Growth Factor Accelerates Diabetic Wound Healing through Increased Angiogenesis and by Mobilizing and Recruiting Bone Marrow-Derived Cells. Am J Pathol. 2004;164:1935–1947. doi: 10.1016/S0002-9440(10)63754-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wall SJ, Bevan D, Thomas DW, Harding KG, Edwards DR, Murphy G. Differential expression of matrix metalloproteinases during impaired wound healing of the diabetes mouse. J Invest Dermatol. 2002;119:91–98. doi: 10.1046/j.1523-1747.2002.01779.x. [DOI] [PubMed] [Google Scholar]
- 19.Loomans CJM, de Koning EJP, Staal FJT, Rookmaaker MB, Verseyden C, de Boer HC, Verhaar MC, Braam B, Rabelink TJ, van Zonneveld AJ. Endothelial Progenitor Cell Dysfunction: A Novel Concept in the Pathogenesis of Vascular Complications of Type 1 Diabetes. Diabetes. 2004;53:195–199. doi: 10.2337/diabetes.53.1.195. [DOI] [PubMed] [Google Scholar]
- 20.Sheetz MJ, King GL. Molecular understanding of hyperglycemia's adverse effects for diabetic complications. Jama. 2002;288:2579–2588. doi: 10.1001/jama.288.20.2579. [DOI] [PubMed] [Google Scholar]
- 21.Brown LF, Yeo KT, Berse B, Yeo TK, Senger DR, Dvorak HF, van de Water L. Expression of vascular permeability factor (vascular endothelial growth factor) by epidermal keratinocytes during wound healing. J Exp Med. 1992;176:1375–1379. doi: 10.1084/jem.176.5.1375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Altavilla D, Saitta A, Cucinotta D, Galeano M, Deodato B, Colonna M, Torre V, Russo G, Sardella A, Urna G, Campo GM, Cavallari V, Squadrito G, Squadrito F. Inhibition of Lipid Peroxidation Restores Impaired Vascular Endothelial Growth Factor Expression and Stimulates Wound Healing and Angiogenesis in the Genetically Diabetic Mouse. Diabetes. 2001;50:667–674. doi: 10.2337/diabetes.50.3.667. [DOI] [PubMed] [Google Scholar]
- 23.Corral CJ, Siddiqui A, Wu L, Farrell CL, Lyons D, Mustoe TA. Vascular endothelial growth factor is more important than basic fibroblastic growth factor during ischemic wound healing. Arch Surg. 1999;134:200–205. doi: 10.1001/archsurg.134.2.200. [DOI] [PubMed] [Google Scholar]
- 24.Galeano M, Deodato B, Altavilla D, Cucinotta D, Arsic N, Marini H, Torre V, Giacca M, Squadrito F. Adeno-associated viral vector-mediated human vascular endothelial growth factor gene transfer stimulates angiogenesis and wound healing in the genetically diabetic mouse. Diabetologia. 2003;46:546–555. doi: 10.1007/s00125-003-1064-1. [DOI] [PubMed] [Google Scholar]
- 25.Lim HS, Blann AD, Chong AY, Freestone B, Lip GYH. Plasma Vascular Endothelial Growth Factor, Angiopoietin-1, and Angiopoietin-2 in Diabetes: Implications for cardiovascular risk and effects of multifactorial intervention. Diabetes Care. 2004;27:2918–2924. doi: 10.2337/diacare.27.12.2918. [DOI] [PubMed] [Google Scholar]
- 26.Holash J, Maisonpierre PC, Compton D, Boland P, Alexander CR, Zagzag D, Yancopoulos GD, Wiegand SJ. Vessel cooption, regression, and growth in tumors mediated by angiopoietins and VEGF. Science. 1999;284:1994–1998. doi: 10.1126/science.284.5422.1994. [DOI] [PubMed] [Google Scholar]
- 27.Hanahan D. Signaling vascular morphogenesis and maintenance. Science. 1997;277:48–50. doi: 10.1126/science.277.5322.48. [DOI] [PubMed] [Google Scholar]
- 28.Lobov IB, Brooks PC, Lang RA. Angiopoietin-2 displays VEGF-dependent modulation of capillary structure and endothelial cell survival in vivo. Proceedings of the National Academy of Sciences of the United States of America. 2002;99:11205–11210. doi: 10.1073/pnas.172161899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pichiule P, LaManna JC. Angiopoietin-2 and rat brain capillary remodeling during adaptation and deadaptation to prolonged mild hypoxia. Journal of Applied Physiology. 2002;93:1131–1139. doi: 10.1152/japplphysiol.00318.2002. [DOI] [PubMed] [Google Scholar]
- 30.Puri MC, Partanen J, Rossant J, Bernstein A. Interaction of the TEK and TIE receptor tyrosine kinases during cardiovascular development. Development. 1999;126:4569–4580. doi: 10.1242/dev.126.20.4569. [DOI] [PubMed] [Google Scholar]
- 31.Jones N, Voskas D, Master Z, Sarao R, Jones J, Dumont DJ. Rescue of the early vascular defects in Tek/Tie2 null mice reveals an essential survival function. EMBO Rep. 2001;2:438–445. doi: 10.1093/embo-reports/kve093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Maisonpierre PC, Suri C, Jones PF, Bartunkova S, Wiegand SJ, Radziejewski C, Compton D, McClain J, Aldrich TH, Papadopoulos N, Daly TJ, Davis S, Sato TN, Yancopoulos GD. Angiopoietin-2, a natural antagonist for Tie2 that disrupts in vivo angiogenesis. Science. 1997;277:55–60. doi: 10.1126/science.277.5322.55. [DOI] [PubMed] [Google Scholar]
- 33.Jacobi J, Tam BY, Sundram U, von Degenfeld G, Blau HM, Kuo CJ, Cooke JP. Discordant effects of a soluble VEGF receptor on wound healing and angiogenesis. Gene Ther. 2004;11:302–309. doi: 10.1038/sj.gt.3302162. [DOI] [PubMed] [Google Scholar]
- 34.Thurston G. Complementary actions of VEGF and angiopoietin-1 on blood vessel growth and leakage. Journal of anatomy. 2002;200:575–580. doi: 10.1046/j.1469-7580.2002.00061.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Thurston G, Suri C, Smith K, McClain J, Sato TN, Yancopoulos GD, McDonald DM. Leakage-resistant blood vessels in mice transgenically overexpressing angiopoietin-1. Science. 1999;286:2511–2514. doi: 10.1126/science.286.5449.2511. [DOI] [PubMed] [Google Scholar]
- 36.Thurston G, Rudge JS, Ioffe E, Zhou H, Ross L, Croll SD, Glazer N, Holash J, McDonald DM, Yancopoulos GD. Angiopoietin-1 protects the adult vasculature against plasma leakage. Nature medicine. 2000;6:460–463. doi: 10.1038/74725. [DOI] [PubMed] [Google Scholar]
- 37.Jain RK, Munn LL. Leaky vessels? Call Ang1! Nature medicine. 2000;6:131–132. doi: 10.1038/72212. [DOI] [PubMed] [Google Scholar]
- 38.Bitto A, Minutoli L, Galeano MR, Altavilla D, Polito F, Fiumara T, Calo M, Lo Cascio P, Zentilin L, Giacca M, Squadrito F. Angiopoietin-1 gene transfer improves impaired wound healing in genetically diabetic mice without increasing VEGF expression. Clin Sci (Lond) 2008;114:707–718. doi: 10.1042/CS20070250. [DOI] [PubMed] [Google Scholar]
- 39.Cho CH, Sung HK, Kim KT, Cheon HG, Oh GT, Hong HJ, Yoo OJ, Koh GY. COMP-angiopoietin-1 promotes wound healing through enhanced angiogenesis, lymphangiogenesis, and blood flow in a diabetic mouse model. Proc Natl Acad Sci USA. 2006;103:4946–4951. doi: 10.1073/pnas.0506352103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Van Slyke P, Alami J, Martin D, Kuliszewski M, Leong-Poi H, Sefton MV, Dumont D. Acceleration of diabetic wound healing by an angiopoietin peptide mimetic. Tissue Eng Part A. 2009;15:1269–1280. doi: 10.1089/ten.tea.2007.0400. [DOI] [PubMed] [Google Scholar]
- 41.Ward NL, Hatala DA, Wolfram JA, Knutsen DA, Loyd CM. Cutaneous manipulation of vascular growth factors leads to alterations in immunocytes, blood vessels and nerves: Evidence for a cutaneous neurovascular unit. Journal of Dermatological Science. 2011;61:14–22. doi: 10.1016/j.jdermsci.2010.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wolfram JA, Diaconu D, Hatala DA, Rastegar J, Knutsen DA, Lowther A, Askew D, Gilliam AC, McCormick TS, Ward NL. Keratinocyte but not endothelial cell specific overexpression of Tie2 leads to the development of psoriasis. Am J Pathol. 2009;174:1443–1458. doi: 10.2353/ajpath.2009.080858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Caldelari R, Suter MM, Baumann D, De Bruin A, Muller E. Long-term culture of murine epidermal keratinocytes. J Invest Dermatol. 2000;114:1064–1065. doi: 10.1046/j.1523-1747.2000.00960-4.x. [DOI] [PubMed] [Google Scholar]
- 44.Nishiyama T, Kii I, Kashima TG, Kikuchi Y, Ohazama A, Shimazaki M, Fukayama M, Kudo A. Delayed re-epithelialization in periostin-deficient mice during cutaneous wound healing. PLoS One. 2011;6:e18410. doi: 10.1371/journal.pone.0018410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ward NL, Slyke PV, Dumont DJ. Functional inhibition of secreted angiopoietin: a novel role for angiopoietin 1 in coronary vessel patterning. Biochemical and Biophysical Research Communications. 2004;323:937–946. doi: 10.1016/j.bbrc.2004.08.185. [DOI] [PubMed] [Google Scholar]
- 46.Chen X, Fu W, Tung CE, Ward NL. Angiopoietin-1 induces neurite outgrowth of PC12 cells in a Tie2-independent, beta1-integrin-dependent manner. Neurosci Res. 2009;64:348–354. doi: 10.1016/j.neures.2009.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ismail NS, Pravda EA, Li D, Shih SC, Dallabrida SM. Angiopoietin-1 Reduces H2O2-Induced Increases in Reactive Oxygen Species and Oxidative Damage to Skin Cells. J Invest Dermatol. 2010;130:1307–1317. doi: 10.1038/jid.2009.431. [DOI] [PubMed] [Google Scholar]
- 48.Romano Di Peppe S, Mangoni A, Zambruno G, Spinetti G, Melillo G, Napolitano M, Capogrossi MC. Adenovirus-mediated VEGF(165) gene transfer enhances wound healing by promoting angiogenesis in CD1 diabetic mice. Gene Ther. 2002;9:1271–1277. doi: 10.1038/sj.gt.3301798. [DOI] [PubMed] [Google Scholar]
- 49.Cao R, Brakenhielm E, Wahlestedt C, Thyberg J, Cao Y. Leptin induces vascular permeability and synergistically stimulates angiogenesis with FGF-2 and VEGF. Proceedings of the National Academy of Sciences. 2001;98:6390–6395. doi: 10.1073/pnas.101564798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Garonna E, Botham KM, Birdsey GM, Randi AM, Gonzalez-Perez RR, Wheeler-Jones CPD. Vascular Endothelial Growth Factor Receptor-2 Couples Cyclo-Oxygenase-2 with Pro-Angiogenic Actions of Leptin on Human Endothelial Cells. PLoS ONE. 2011;6:e18823. doi: 10.1371/journal.pone.0018823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Cao Y. Angiogenesis modulates adipogenesis and obesity. The Journal of Clinical Investigation. 2007;117:2362–2368. doi: 10.1172/JCI32239. [DOI] [PMC free article] [PubMed] [Google Scholar]