Abstract
About 120 million people worldwide suffer from congenital (early-onset) hearing loss. Thirty percent of them have syndromic hearing loss and the remaining 70% have non-syndromic hearing loss. In addition, a large number of elderly people worldwide suffer from age-related (late-onset) hearing loss. c-Ret and c-RET have been shown to be essential for the development and maintenance of neurons including the enteric nervous system (ENS) in mice and humans. Impairments of endothelin receptor B (EDNRB) and SOX10 have been shown to cause a significantly increased risk of dominant sensorineural deafness in Hirschsprung disease (HSCR) patients. We have recently shown that impairments of tyrosine 1062 (Y1062) phosphorylation in c-Ret causes syndromic congenital deafness in mice and humans and non-syndromic age-related hearing loss with neurodegeneration of spiral ganglion neurons (SGNs) in mice. This review focuses on the pathogenesis of hearing loss caused by impairments of c-Ret.
Keywords: c-Ret, congenital deafness, age-related deafness, tyrosine kinase, spiral ganglion neuron, neurodegeneration
Introduction
Hearing level is affected by genetic, aging and environmental factors [1, 2]. This review focuses on genetic factor-mediated hearing loss. About 120 million people worldwide suffer from congenital (early-onset) hearing loss. Thirty percent of them have syndromic hearing loss and the remaining 70% have non-syndromic hearing loss. In addition, a large number of elderly people worldwide suffer from age-related (late-onset) hearing loss [3-5]. These hearing losses have been generally categorized as distinct diseases due to different pathogeneses [3, 4]. Inner ears have been morphologically analyzed to analyze the pathogeneses for these hearing losses. The inner ears consist of the organ of Corti and stria vascularis. The stria vascularis plays an important role in maintenance of endolymph potential. The organ of Corti, which contains two kinds of sensory cells [inner hair cells (IHCs) and outer hair cells (OHCs)], serves as mechanotransduction, by which sound stimuli are converted into electric stimuli. Auditory information from the sensory cells is transmitted to spiral ganglion neurons (SGNs) as the primary carriers, followed by eventual transmission to the auditory cortex in the cerebrum [3, 4]. In order to elucidate the pathogenesis for deafness caused by a target gene in mice, most of the recent studies on hearing have focused on the expression level of a target molecule rather than activities in inner ears [3, 4]. Meanwhile, there have been very few studies aimed at determining whether activity levels of a target molecule cause early- and late-onset hearing losses and syndromic- and non-syndromic hearing losses.
c-Ret and hirschsprung disease (HSCR)
The c-RET proto-oncogene encodes a receptor-tyrosine kinase, and glial cell line-derived neuro-trophic factor (GDNF) is one of the ligands for c-RET [6]. GDNF exhibits its effect on target cells by binding to a glycosyl phosphatidylinositol (GPI)-anchored cell surface protein (GFRα1). The binding leads to the formation of a complex with the receptor tyrosine kinase c-RET. Formation of this complex stimulates c-RET autophosphorylation as a trigger for c-RET-mediated signaling pathways to give positive effects for cell survival [6-9]. Previous studies have also indicated that there is a Ret-independent signaling pathway stimulated by GDNF [7, 10, 11]. Tyrosine 1062 (Y1062) in c-Ret is one of the most important autophosphorylation sites for its kinase activation and is a multi-docking site for several signaling molecules including SHC, a transmitter for c-Ret-mediated signaling pathways [10, 12, 13]. c-RET has been shown to be essential for the development and maintenance of the enteric nervous system (ENS) in both mice and humans [10, 12] and to be the most frequent causal gene of Hirschsprung disease (HSCR; megacolon disease) (in 20-25% of cases) in humans [14, 15]. As a matter of fact, severe HSCR with total intestinal agangliosis has been shown to develop in homozygous knock-in mice, in which Y1062 in c-Ret was replaced with phenylalanine (c-Ret-KIY1062F/Y1062F-mice), while heterozygous c-Ret Y1062F knock-in mice (c-Ret-KIY1062F/+-mice) have been reported to have no HSCR-linked phenotypes [8]. Thus, these results indicate that HSCR in mice is recessively developed [8], while HSCR in humans has been shown to dominantly develop due to RET mutations [16]. On the other hand, replacement of serine 697 (S697; a putative protein kinase A phosphorylation site) in c-Ret with alanine (c-Ret-KIS697A/S697A-mice) resulted in a mild HSCR phenotype (lack of ENS limited to the distal colon) [17].
c-Ret-mediated syndromic and non-syndromic hearing losses
Despite the fact that c-Ret and c-RET are responsible genes for HSCR as described above, there had been no direct evidence to link c-Ret and c-RET with deafness in mice and humans. Our recent results have shown that complete impairment of phosphorylated Y1062 in c-Ret without change in the expression level resulted in the development of congenital hearing loss in c-Ret-KIY1062F/Y1062F-mice [1], while partial impairment of phosphorylated c-Ret resulted in normal hearing development until 1 month of age but afterwards acceleration of age-related hearing loss in c-Ret-KIY1062F/+-mice [18] (summarized in Figure 1). Thus, impairments of c-Ret phosphorylation monogenetically cause not only early-onset syndromic hearing loss but also late-onset non-syndromic hearing loss. Our results partially correspond to results of previous studies showing that c-Ret, GFRα1 and GDNF are expressed in auditory neurons [19, 20] and that GDNF has a protective effect on antibiotics-induced ototoxicities [21-24]. In previous studies, mutations of cadherin 23 have been shown to correlate with early-onset syndromic hearing loss [25, 26] as well as non-syndromic hearing loss with different onsets [27-29] in mice and humans. Thus, if our data integrate the conclusions of those studies, different mutations in an identical gene can develop both early-onset syndromic and late-onset non-syndromic hearing losses. Our recent studies therefore provide a new concept for hearing research that early-onset syndromic hearing loss and late-onset non-syndromic hearing loss partially share a common pathogenesis that is monogenetically affected by a single point mutation (Y1062F) in c-Ret.
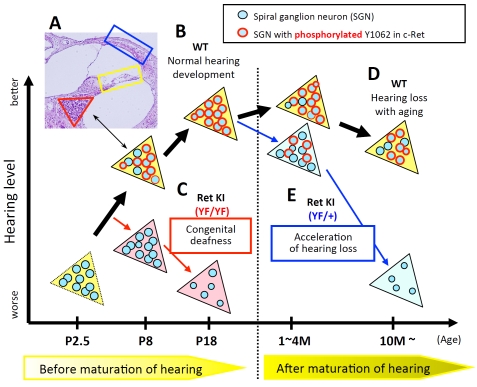
Figure 1.
Schematic summary of c-Ret-mediated SGNs loss. (A) Inner ear from a wild-type (WT) mouse at postnatal day (P) 14 stained with hematoxylin and eosin. Red triangle, yellow square and blue square contain spiral ganglion neurons (SGNs), inner- and outer-hair cells and the stria vascularis, respectively. Our morphological analyses showed almost no abnormalities in these areas besides SGNs from c-Ret-KIY1062F/Y1062F-mice. (B-E) Colored triangles in the schema represent Rosenthal's canals in WT (yellow triangles), homozygous c-Ret-KIY1062F/Y1062F(Ret KI (YF/YF), pink triangles) [1] and heterozygous c-Ret-KIY1062F/+-mice (Ret KI (YF/+), light blue triangles) [18]. Blue circles with a “black” line in the triangles represent SGNs. Blue circles with a “red” line in the triangles represent SGNs with “phosphorylated Y1062 in c-Ret". X-axis and Y-axis indicate age of mice and hearing levels, respectively. (B) WT mice showed that c-Ret protein was constantly expressed in SGNs (P1-18), while the numbers of Y1062-phosphorylated SGNs (blue circles with “red” line in yellow triangles in B) were sharply increased around P6-7, several days before the WT mice begin to acquire intact hearing levels (around P12∼) [1]. (C) c-Ret-KIY1062F/Y1062F-mice suffered from congenital deafness with decreased cell density of SGNs. c-Ret-KIY1062F/Y1062F-mice showed no Y1062-phosphorylated SGNs even on P8, although Y1062-phosphorylated SGNs began to appear in WT mice from P6 [1]. (D) WT mice (10 months old) showed hearing loss with aging. (E) c-Ret-KIY1062F/+-mice, in which the number of Y1062-phosphorylated SGNs was about half of that in WT mice (light blue triangles), suffered from acceleration of age-related hearing loss with decreased cell density of SGNs without functional and morphological abnormalities of hair cells [18]. Acceleration of age-related hearing loss in c-Ret-KIY1062F/+-mice was rescued by introducing constitutively activated RET [18].
c-Ret-mediated neurodegeneration of SGNs
Y1062 phosphorylation in c-Ret has been reported to mediate several biological responses, including survival of neuronal cells [10, 30]. Our recent studies showed that c-Ret-KIY1062F/Y1062F-mice had severe congenital deafness with neurodegeneration of SGNs that resulted in decreased numbers of SGNs on postnatal day (P) 8-18, while c-Ret-KIY1062F/Y1062F-mice showed a comparable number and comparable morphology of SGNs to those in WT mice on P2-3 [1] (summarized in Figure 1). The level of Y1062 phosphorylation in c-Ret of SGNs from WT mice on P2-3 was undetectable, while that on P8-18 sharply rose [1]. These results suggest that SGNs even from c-Ret-KIY1062F/Y1062F-mice developed normally at least until P3 after birth, when Y1062 phosphorylation in c-Ret of SGNs from WT mice was undetectable, but those from c-Ret-KIY1062F/Y1062F-mice no longer survived on P8 -18, when the level of Y1062 phosphorylation in c-Ret of SGNs from WT mice was high (summarized in Figure 1). On the other hand, partially impaired phosphorylation of Y1062 in c -Ret in SGNs facilitated age-related hearing loss with accelerated reduction of SGNs from 4 months of age, whereas it had almost no affect on hearing and cell density of SGNs at least until 1 month of age, when the auditory system had been maturated (summarized in Figure 1). The neurodegeneration of SGNs from c-Ret-KIY1062F/Y1062F-mice did not involve the hallmark of apoptotic signals [1]. Our results partially correspond to results of a previous study showing neurodegeneration of spiral ganglion neurons in endothelin receptor B (Ednrb) homozygously deleted mice [31] and postmigratory enteric neurons without apoptotic signals during the developmental stage in mice with conditional ablation of c-Ret [32].
c-Ret-mediated signaling pathway in spiral ganglion neurons
Results of a previous in vitro study showed that phosphorylated levels of Y1062 in c-RET modulate Akt and NF-κB activities in neural cells [30]. Our recent in vivo studies suggested that decreased levels of phosphorylated Y1062 in c-RET reduce Akt and NF-κB activities in SGNs [1, 18] (summarized in Figure 2). Furthermore, both congenital and age-related hearing losses with partial impairment of NF-κB in SGNs from c -Ret-KIY1062F/Y1062F- and c-Ret-KIY1062F/+-mice were rescued by introducing constitutively activated RET [1, 18]. A previous study demonstrated that mice with impaired function of NF-κB exhibited acceleration of age-related auditory nerve degeneration, resulting in age-related deafness [33]. Thus, these results suggest that impairments of the c-Ret-mediated signaling pathway involve decreased activities of Akt and NF-κB signaling, resulting in degeneration of SGNs.
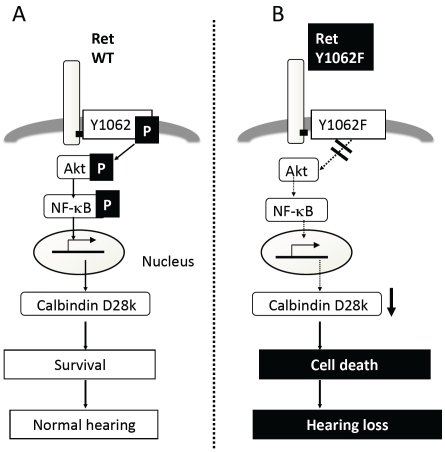
Figure 2.
Schematic summary of c-Ret-mediated signaling in SGNs. (A and B) Schematic illustration of a mechanistic model for c-Ret-mediated congenital deafness with neurodegeneration of SGNs in c-Ret-KI-mice. (A) Wild-type mice (WT) acquire normal hearing with phosphorylation of the c-Ret Y1062-mediated signaling pathway. P in A indicates phosphorylation. (B) Impairments of the c-Ret Y1062-mediated signaling pathway (blue double line and downward dashed arrows in B) cause decreased expression of calbindin D28k (blue arrow in B) via Akt/NF-κB signaling, resulting in auditory nerve degeneration in c-Ret-KIY1062F/Y1062F-mice (c-Ret Y1062F). This figure was drawn on the basis of results of previous studies [1, 18].
c-RET-mediated hearing loss in humans
The results obtained with c-Ret-KI mice showed that impairments of Y1062 phosphorylation in c -Ret cause early- and late-onset of hearing losses, while normal hearing is maintained with even complete impairment of S697 phosphorylation in c-Ret [1, 18]. In humans, we found hearing loss in three patients with severe HSCR, including two males with homozygous and heterozygous mutations at arginine 969 located in c-RET kinase domains and one male with a nucleotide deletion at RET codon 13 resulting in a frameshift and termination at codon 22. Meanwhile, no hearing impairments were found in patients with other heterozygous missense mutations at codons 30, 144, 489, 734, 897 and 942 [1]. Therefore, these results suggest that impairments of c-Ret and c-RET site-dependently cause hearing losses in mice and humans. Further study is needed to determine the correlation between mutational sites of c-Ret and c-RET and hearing losses in mice and humans.
Conclusions
Our studies provide direct evidence that c-Ret is a novel deafness-related molecule in humans and mice. These studies indicated the importance of considering the activity as well as the expression of the target molecule in order to elucidate the etiologies of hereditary deafness. The findings will be useful for the development of new diagnostic and therapeutic strategies targeting c-RET kinase against hearing losses.
Acknowledgments
We thank Yoko Kato and Harumi Ohno for their technical assistance. This study was supported in part by Grants-in-Aid for Scientific Research (B) (No. 19390168 and No. 20406003), Grant-in-Aid for Challenging Exploratory Research (No. 23650241) and Grants-in-Aid for Young Scientists (B) (No. 18790738 and No. 20791232) from the Ministry of Education, Culture, Sports, Science and Technology (MEXT), AA Science Platform Program from the Japan Society for the Promotion of Science (JSPS), Adaptable and Seamless Technology Transfer Program through Target-driven R&D, Japan Science and Technology Agency, COE Project (Health Science Hills) for Private Universities from MEXT and Chubu University (No. S0801055), Mitsui & Co., Ltd. Environment Fund (R08-C097), Research Grant from the Tokyo Biochemical Research Foundation (TBRF), the Naito Foundation Natural Science Scholarship, Research Foundation from the Institute of Science and Technology Research in Chubu University and Chubu University grants A, B and CG.
References
- 1.Ohgami N, Ida M, Shimotake T, Sakashita N, Sone M, Nakashima T, Tabuchi K, Hoshino T, Shimada A, Tsuzuki T, Yamamoto M, Sobue G, Jijiwa M, Asai N, Hara A, Takahashi M, Kato M. c -Ret-mediated hearing loss in mice with Hirschsprung disease. Proc Natl Acad Sci USA. 2010;107:13051–13056. doi: 10.1073/pnas.1004520107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ohgami N, Kondo T, Kato M. Effects of light smoking on extra-high-frequency auditory thresholds in young adults. Toxicol Ind Health. 2011;27:143–147. doi: 10.1177/0748233710382539. [DOI] [PubMed] [Google Scholar]
- 3.Lalwani AK, Gürtler N. Sensorineural Hearing Loss, The Aging Inner Ear, and Hereditary Hearing Impairment. In: Lalwani AK, editor. CURRENT Diagnosis & Treatment in Otolaryngology-Head & Neck Surgery. second edition. New York: McGraw-Hill; 2008. pp. 683–704. [Google Scholar]
- 4.Brown SD, Hardisty-Hughes RE, Mburu P. Quiet as a mouse: dissecting the molecular and genetic basis of hearing. Nat Rev Genet. 2008;9:277–290. doi: 10.1038/nrg2309. [DOI] [PubMed] [Google Scholar]
- 5.Gratton MA, Vázquez AE. Age-related hearing loss: current research. Curr Opin Otolaryngol Head Neck Surg. 2003;11:367–371. doi: 10.1097/00020840-200310000-00010. [DOI] [PubMed] [Google Scholar]
- 6.Kato M, Iwashita T, Takeda K, Akhand AA, Liu W, Yoshihara M, Asai N, Suzuki H, Takahashi M, Nakashima I. Ultraviolet light induces redox reaction-mediated dimerization and superactivation of oncogenic Ret tyrosine kinases. Mol Biol Cell. 2000;11:93–101. doi: 10.1091/mbc.11.1.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Trupp M, Scott R, Whittemore SR, Ibáñez CF. Ret-dependent and -independent mechanisms of glial cell line-derived neurotrophic factor signaling in neuronal cells. J Biol Chem. 1999;274:20885–20894. doi: 10.1074/jbc.274.30.20885. [DOI] [PubMed] [Google Scholar]
- 8.Jijiwa M, Fukuda T, Kawai K, Nakamura A, Kurokawa K, Murakumo Y, Ichihara M, Takahashi M. A targeting mutation of tyrosine 1062 in Ret causes a marked decrease of enteric neurons and renal hypoplasia. Mol Cell Biol. 2004;24:8026–8036. doi: 10.1128/MCB.24.18.8026-8036.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Drosten M, Pützer BM. Mechanisms of Disease: cancer targeting and the impact of oncogenic RET for medullary thyroid carcinoma therapy. Nat Clin Pract Oncol. 2006;3:564–574. doi: 10.1038/ncponc0610. [DOI] [PubMed] [Google Scholar]
- 10.Airaksinen MS, Saarma M. The GDNF family: signalling, biological functions and therapeutic value. Nat Rev Neurosci. 2002;3:383–394. doi: 10.1038/nrn812. [DOI] [PubMed] [Google Scholar]
- 11.Poteryaev D, Titievsky A, Sun YF, Thomas-Crusells J, Lindahl M, Billaud M, Arumäe U, Saarma M. GDNF triggers a novel ret-independent Src kinase family-coupled signaling via a GPI-linked GDNF receptor alpha1. FEBS Lett. 1999;463:63–66. doi: 10.1016/s0014-5793(99)01590-2. [DOI] [PubMed] [Google Scholar]
- 12.Heanue TA, Pachnis V. Enteric nervous system development and Hirschsprung's disease: advances in genetic and stem cell studies. Nat Rev Neurosci. 2007;8:466–479. doi: 10.1038/nrn2137. [DOI] [PubMed] [Google Scholar]
- 13.Kato M, Takeda K, Kawamoto Y, Iwashita T, Akhand AA, Senga T, Yamamoto M, Sobue G, Hamaguchi M, Takahashi M, Nakashima I. Repair by Src kinase of function-impaired RET with multiple endocrine neoplasia type 2A mutation with substitutions of tyrosines in the COOH-terminal kinase domain for phenylalanine. Cancer Res. 2002;62:2414–2422. [PubMed] [Google Scholar]
- 14.Moore SW, Johnson AG. Hirschsprung's disease: genetic and functional associations of Down's and Waardenburg syndromes. Semin Pediatr Surg. 1998;7:156–161. doi: 10.1016/s1055-8586(98)70011-3. [DOI] [PubMed] [Google Scholar]
- 15.Moore SW. The contribution of associated congenital anomalies in understanding Hirschsprung's disease. Pediatr Surg Int. 2006;22:305–315. doi: 10.1007/s00383-006-1655-2. [DOI] [PubMed] [Google Scholar]
- 16.Inoue K, Shimotake T, Iwai N. Mutational analysis of RET/GDNF/NTN genes in children with total colonic aganglionosis with small bowel involvement. Am J Med Genet. 2000;93:278–284. [PubMed] [Google Scholar]
- 17.Asai N, Fukuda T, Wu Z, Enomoto A, Pachnis V, Takahashi M, Costantini F. Targeted mutation of serine 697 in the Ret tyrosine kinase causes migration defect of enteric neural crest cells. Development. 2006;133:4507–4516. doi: 10.1242/dev.02616. [DOI] [PubMed] [Google Scholar]
- 18.Ohgami N, Ida-Etoh M, Sakashita N, Sone M, Nakashima N, Tabuchi K, Hoshino T, Shimada A, Tsuzuki T, Yamamoto M, Sobue G, Jijiwa M, Asai N, Hara A, Takahashi M, Kato M. Partial impairment of c-Ret at tyrosine 1062 accelerates age-related hearing loss in mice. Neurobiol Aging. 2012 doi: 10.1016/j.neurobiolaging.2011.04.002. in press. [DOI] [PubMed] [Google Scholar]
- 19.Stöver T, Gong TL, Cho Y, Altschuler RA, Lomax MI. Expression of the GDNF family members and their receptors in the mature rat cochlea. Brain Res Mol Brain Res. 2000;76:25–35. doi: 10.1016/s0169-328x(99)00328-9. [DOI] [PubMed] [Google Scholar]
- 20.Stöver T, Nam Y, Gong TL, Lomax MI, Altschuler RA. Glial cell line-derived neurotrophic factor (GDNF) and its receptor complex are expressed in the auditory nerve of the mature rat cochlea. Hear Res. 2001;155:143–151. doi: 10.1016/s0378-5955(01)00227-1. [DOI] [PubMed] [Google Scholar]
- 21.Suzuki M, Yagi M, Brown JN, Miller AL, Miller JM, Raphael Y. Effect of transgenic GDNF expression on gentamicin-induced cochlear and vestibular toxicity. Gene Ther. 2000;7:1046–1054. doi: 10.1038/sj.gt.3301180. [DOI] [PubMed] [Google Scholar]
- 22.Yagi M, Kanzaki S, Kawamoto K, Shin B, Shah PP, Magal E, Sheng J, Raphael Y. Spiral ganglion neurons are protected from degeneration by GDNF gene therapy. J Assoc Res Otolaryngol. 2000;1:315–325. doi: 10.1007/s101620010011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Liu Y, Okada T, Shimazaki K, Sheykholeslami K, Nomoto T, Muramatsu S, Mizukami H, Kume A, Xiao S, Ichimura K, Ozawa K. Protection against aminoglycoside-induced ototoxicity by regulated AAV vector-mediated GDNF gene transfer into the cochlea. Mol Ther. 2008;16:474–480. doi: 10.1038/sj.mt.6300379. [DOI] [PubMed] [Google Scholar]
- 24.Scheper V, Paasche G, Miller JM, Warnecke A, Berkingali N, Lenarz T, Stöver T. Effects of delayed treatment with combined GDNF and continuous electrical stimulation on spiral ganglion cell survival in deafened guinea pigs. J Neurosci Res. 2009;87:1389–1399. doi: 10.1002/jnr.21964. [DOI] [PubMed] [Google Scholar]
- 25.Di Palma F, Holme RH, Bryda EC, Belyantseva IA, Pellegrino R, Kachar B, Steel KP, Noben-Trauth K. Mutations in Cdh23, encoding a new type of cadherin, cause stereocilia disorganization in waltzer, the mouse model for Usher syndrome type 1D. Nat Genet. 2001;27:103–107. doi: 10.1038/83660. [DOI] [PubMed] [Google Scholar]
- 26.Bolz H, von Brederlow B, Ramírez A, Bryda EC, Kutsche K, Nothwang HG, Seeliger M, del C-Salcedó Cabrera M, Vila MC, Molina OP, Gal A, Kubisch C. Mutation of CDH23, encoding a new member of the cadherin gene family, causes Usher syndrome type 1D. Nat Genet. 2001;27:108–112. doi: 10.1038/83667. [DOI] [PubMed] [Google Scholar]
- 27.Astuto LM, Bork JM, Weston MD, Askew JW, Fields RR, Orten DJ, Ohliger SJ, Riazuddin S, Morell RJ, Khan S, Riazuddin S, Kremer H, van Hauwe P, Moller CG, Cremers CW, Ayuso C, Heckenlively JR, Rohrschneider K, Spandau U, Greenberg J, Ramesar R, Reardon W, Bitoun P, Millan J, Legge R, Friedman TB, Kimberling WJ. CDH23 mutation and phenotype heterogeneity: a profile of 107 diverse families with Usher syndrome and nonsyndromic deafness. Am J Hum Genet. 2002;71:262–275. doi: 10.1086/341558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Noben-Trauth K, Zheng QY, Johnson KR. Association of cadherin 23 with polygenic inheritance and genetic modification of sensorineural hearing loss. Nat Genet. 2003;35:21–23. doi: 10.1038/ng1226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schwander M, Xiong W, Tokita J, Lelli A, Elledge HM, Kazmierczak P, Sczaniecka A, Kolatkar A, Wiltshire T, Kuhn P, Holt JR, Kachar B, Tarantino L, Müller U. A mouse model for nonsyndromic deafness (DFNB12) links hearing loss to defects in tip links of mechanosensory hair cells. Proc Natl Acad Sci USA. 2009;106:5252–5257. doi: 10.1073/pnas.0900691106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hayashi H, Ichihara M, Iwashita T, Murakami H, Shimono Y, Kawai K, Kurokawa K, Murakumo Y, Imai T, Funahashi H, Nakao A, Takahashi M. Characterization of intracellular signals via tyrosine 1062 in RET activated by glial cell line-derived neurotrophic factor. Oncogene. 2000;19:4469–4475. doi: 10.1038/sj.onc.1203799. [DOI] [PubMed] [Google Scholar]
- 31.Ida-Eto M, Ohgami N, Iida M, Yajima I, Kumasaka MY, Takaiwa K, Kimitsuki T, Sone M, Nakashima T, Tsuzuki T, Komune S, Yanagisawa M, Kato M. Partial requirement of endothelin receptor B in spiral ganglion neurons for postnatal development of hearing. J Biol Chem. 2011;286:29621–29626. doi: 10.1074/jbc.M111.236802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Uesaka T, Nagashimada M, Yonemura S, Enomoto H. Diminished Ret expression compromises neuronal survival in the colon and causes intestinal aganglionosis in mice. J Clin Invest. 2008;118:1890–1898. doi: 10.1172/JCI34425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lang H, Schulte BA, Zhou D, Smythe N, Spicer SS, Schmiedt RA. Nuclear factor κB deficiency is associated with auditory nerve degeneration and increased noise-induced hearing loss. J Neurosci. 2006;26:3541–3550. doi: 10.1523/JNEUROSCI.2488-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]