Abstract
Xanthohumol, the major prenylated chalcone found in hops, is known for its anti-inflammatory properties. We have recently shown that xanthohumol inhibits hepatic inflammation and fibrosis in a murine model of nonalcoholic steatohepatitis. The aim of this study was to investigate the effect of xanthohumol in an acute model of liver injury. Carbon tetrachloride (CCl4), an industrial solvent, is a hepatotoxic agent and its administration is widely used as an animal model of toxin-induced liver injury. Xanthohumol was applied orally at a dose of 1 mg/g body weight 2 days prior as well as during and after exposure to CCl4. 72 h after a single CCl4 application histomorphology and serum levels of transaminases revealed considerable hepatocellular necrosis, which was accompanied by significantly enhanced hepatic expression of pro-inflammatory cytokines. Furthermore, elevated hepatic alpha-smooth muscle actin expression indicated activation of hepatic stellate cells, and in accordance, we detected enhanced hepatic expression levels of TGF-β and collagen type I reflecting a marked fibrogenic response to CCl4 exposure. While the degree of hepatocellular damage in response to CCl4 was similar in mice which received xanthohumol and the control group, pro-inflammatory and profibrogenic hepatic gene expression were almost completely blunted in xanthohumol fed mice. Furthermore, xanthohumol fed mice revealed decreased hepatic NFκB activity. These results suggest that the protective effects of xanthohumol in this toxic liver injury model involves direct mechanisms related to its ability to block both hepatic inflammation and the activation of hepatic stellate cells, presumable at least in part via decreasing NFκB activity. Thus, this study further indicates the potential of xanthohumol application to prevent or ameliorate the development and progression of liver fibrosis in response to hepatic injury.
Keywords: Xanthohumol, carbon tetrachloride, fibrosis, inflammation, acute liver injury
Introduction
Xanthohumol (XN) is the principal prenylated chalcone of the female inflorescences (hop cones, hops) of the hop plant Humulus lupulus L. and it has been shown to have several beneficial biological activities. Among them its chemopreventive and anti-inflammatory properties are the most extensively investigated [1, 2], and these are at least in part mediated via inhibition of the NFκB signaling pathway [3-6]. We have shown previously that XN inhibits hepatic inflammation and fibrosis in a murine model of non-alcoholic fatty liver disease (NAFLD) [6]. NAFLD is considered as the most frequent liver disease in Western countries [7-9]. It is characterized by hepatocellular lipid accumulation, on the ground of which inflammation and fibrosis may develop. The histological picture closely resembles alcoholic liver disease [10]. In nonalcoholic steatohepatitis (NASH) and alcoholic steatohepatitis (ASH) as well as in other chronic liver diseases like viral hepatitis, hepatic fibrosis is the peril that determines morbidity and mortality. Cirrhosis, as the end stage of hepatic fibrosis, is a major clinical issue for its high prevalence in the world and its tight relationship with hepatocellular carcinoma incidence [11-13]. Hepatic fibrosis is characterized by an excessive and aberrant deposition of extracellular matrix (ECM) proteins in the liver, the most abundant of which is collagen type I [14]. Activated hepatic stellate cells (HSC) are the cellular source of the excessive ECM deposition [15-17]. Normally, these cells are quiescent and produce only small amounts of ECM components, such as laminin and collagen type IV, during the formation of basement membrane [18]. However, in response to hepatic injury HSC get activated and transform into a myofibroblast-like phenotype, expressing alpha-smooth muscle actin (α-SMA), and dramatically increase the production of collagens [19]. Importantly, the transcription factor NFκB plays a crucial role in HSC activation [20, 21, 15, 22].
In addition to metabolic overload, alcohol or viral infection the liver is frequently exposed to various insults, including toxic chemicals [23, 24]. Liver damage caused by hepatotoxic chemicals induces compensatory hepatic hyperplasia after severe liver necrosis due to direct damage of hepatocytes and subsequent inflammation [25]. Carbon tetrachloride (CCl4), an industrial solvent, is a hepatotoxic agent and its administration is widely used as an animal model of toxin-induced liver injury that allows the evaluation of both necrosis and subsequent inflammation [26] as well as fibrosis [27]. In contrast to our previously used NASH-model which led to only mild hepatocellular damage and inflammation [6], CCl4 application results in excessive necrotic and apoptotic death of hepatocytes, which induces the activation of HSC.
To investigate the effect of XN on acute liver injury and to further study its role in liver fibrosis, we subjected mice, which were treated with and without XN at a dose of approximately 1 mg/g body weight, to acute CCl4-induced liver damage.
Methods
Chemicals and animal feeds
Carbon tetrachloride (CCl4) and olive oil were obtained from Sigma Pharmaceuticals (Hamburg, Germany). Xanthohumol (XN) was obtained from Alexis Biochemicals (Lausen, Switzerland) with a purity ≥ 98% determined by HPLC. All chows were prepared by Ssniff (Soest, Germany).
CCl4-induced acute liver injury
A single dose of CCl4 (1 μl/g body weight in olive oil) was intraperitoneally injected to 10 weeks old female BALB/c mice. 72 h after CCl4 injection, mice were killed by heart puncture under deep ketamine/xylazine (2:1) anesthesia, and liver tissue and blood samples were collected for further analysis. Livers and blood from olive oil treated animals served as controls.
Histology
For histological analysis murine liver tissue specimens were fixed for 24 h in 4% formalin at room temperature, dehydrated by graded ethanol and embedded in paraffin. Tissue sections (thickness 5 μm) were deparaffinized with xylene and stained with eosin/haematoxylin (H&E) as described [28].
Quantitative real time-PCR analysis
RNA isolation from liver tissue and reverse transcription were performed as described [29]. Quantitative real time-PCR was performed applying LightCycler technology (Roche, Mannheim, Germany) as described [30] applying the following pairs of primers: murine collagen-I (for: 5'-CGG GCA GGA CTT GGG TA; rev: 5'-CGG AAT CTG AAT GGT CTG ACT) and murine MCP-1 (for: 5'-TGG GCC TGC TGT TCA CA; rev: 5'-TCC GAT CCA GGT TTT TAA TGT A). All other mRNA expression analyses were performed using QuantiTect Primer Assays according to the manufacturer's instructions (Qiagen, Hilden, Germany). Amplification of cDNA derived from 18s rRNA (for: 5'-AAA CGG CTA CCA CAT CCA AG; rev: 5'-CCT CCA ATG GAT CCT CGT TA) was used for normalization.
Quantification of NFκB activity
Liver tissue extracts were obtained by homogenization of snap-frozen liver tissue in Cell Lysis Buffer from Cell Signaling (Danvers, MA, USA) supplemented with 1 mM PMSF and a protease inhibitor cocktail (cOmplete Mini Protease Inhibitor Cocktail Tablets from Roche Diagnostics, Mannheim, Germany) using a MICCRA D1 homogenizer (ART Prozess- & Labortechnik, Müllheim, Germany), and subsequent sonication (with a Sonopuls HD 70 from Bandelin electronics, Berlin, Germany) and centrifugation. Activated NFκB was quantified in liver tissue extracts via ELISA-technique using the PathScan Phospho-NFκB p65 (Ser536) Sandwich ELISA Antibody Pair from Cell Signaling following the manufacturer's protocols as described [6].
Statistical analysis
Values are presented as mean ± SEM. Comparison between groups was made using the Mann Whitney test. A p value <0.05 was considered statistically significant. All calculations were performed using the statistical computer package GraphPad Prism version 5.00 for Windows (GraphPad Software, San Diego, Ca, USA).
Results
Effect of xanthohumol on hepatocellular damage in toxin induced liver injury
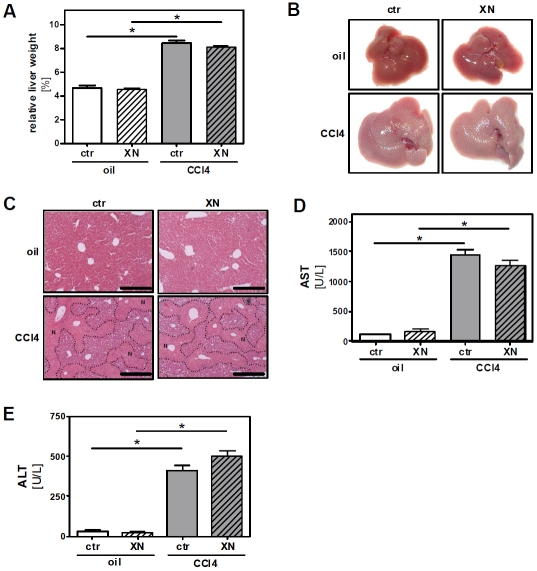
To assess the effect of xanthohumol (XN) in the model of carbon tetrachloride (CCl4) induced toxic liver injury mice were fed with control chow or a diet containing 0.5% (w/w) XN resulting in a daily dose of approximately 1 mg/g body weight (BW). After 2 days of XN-feeding, mice received a single dose of CCl4 (1 μl/g BW) or the same volume of the pure solvent (olive oil). Feeding of XN or control chow was continued, and 3 days after CCl4 application mice were sacrificed. At this time body weight did not differ significantly between control mice (18.2 ± 1.3 g) and XN-fed (18.3 ± 0.2 g) animals, and mice receiving CCl4 alone (16.4 ± 1.3 g) or CCl4 together with XN (16.8 ± 0.4 g). However, liver weight and liver to body weight ratio, respectively, were significantly elevated in CCl4-treated mice but did not differ between XN- and control chow-fed mice (Figure 1A). Macroscopically, livers of CCl4-treated mice were larger and revealed a pale and irregular surface indicative of severe hepatocellular damage (Figure 1B). Histopathological analysis confirmed large areas of necrotic tissue in the central zones of the livers of CCl4-treated mice with no significant differences between the XN-fed and control chow-fed group (Figure 1C). Hepatocellular damage in the CCl4 groups was also reflected by a marked increase of serum transaminases compared to both control groups, which was similar in XN-fed and control chow-fed mice (Figure 1D and 1E).
Figure 1.
Effect of xanthohumol on hepatocellular damage in toxin induced liver injury. Mice were fed either with control chow (ctr.) or with the same chow supplemented with 0.5% (w/w) xanthohumol (XN). After 2 days of feeding a single dose of CCl4 (1 μl/g body weight in olive oil) or olive oil alone (oil) was injected intraperitoneally to mice from both the control chow-fed and XN-fed group. 72 h after CCl4 or olive oil injection mice were sacrificed. (A) Liver-to-body-weight-ratio (*: p<0.05). (B) Representative macroscopic images of livers from the four treatment groups. (C) HE-staining of liver tissue. Necrotic areas are marked out by dotted lines. Black bars represent 0.5 mm. (D) AST and (E) ALT serum levels (*: p<0.05).
Effect of xanthohumol on hepatic inflammation and fibrosis in toxin induced liver injury
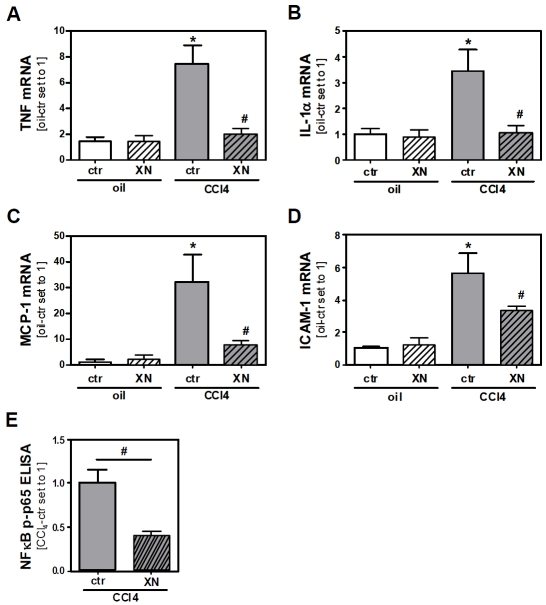
To determine whether XN affects the inflammatory response after acute CCl4-mediated liver injury we analyzed the expression of pro-inflammatory cytokines in injured livers using semi-quantitative real-time PCR. 72 h after CCl4-injection, hepatic mRNA expression levels of tumor necrosis factor (TNF) and interleukin-1 alpha (IL-1α) were significantly elevated in mice without XN-feeding. In contrast, inflammatory gene expression was almost completely blunted in CCl4-treated mice by XN-feeding (Figure 2A and 2B). Similar results were obtained when analyzing hepatic mRNA expression of monocyte chemoattractant protein-1 (MCP-1) and intercellular adhesion molecule-1 (ICAM-1) (Figure 2C and 2D), both genes which are strongly regulated by the transcription factor NFκB. In line with these and previous in vitro findings by our group [6, 31] and others [3-6] we found 72 h after CCl4-injection significantly lower hepatic NFκB activity in XN-fed mice compared to control chow-fed animals (Figure 2D).
Figure 2.
Effect of xanthohumol on hepatic inflammation in toxin induced liver injury. Mice were fed either with control chow (ctr) or with the same chow supplemented with 0.5% (w/w) xanthohumol (XN). After 2 days of feeding a single dose of CCl4 (1 μl/g body weight in olive oil) or olive oil alone (oil) was injected intraperitoneally to mice from both the control chow-fed and XN-fed group. 72 h after CCl4 or olive oil injection mice were sacrificed. Analysis of hepatic mRNA levels of (A) TNF, (B) IL-1α, (C) MCP-1 and (D) ICAM-1 by quantitative RT-PCR (*: p<0.05 compared to oil-ctr; #: p<0.05 compared to CCl4-ctr). (E) Comparison of NFκB activity in livers of control chow-fed and XN-fed mice 72 h after CCl4-injection via ELISA-based quantification of phospho-p65 (Ser536) (*: p<0.05).
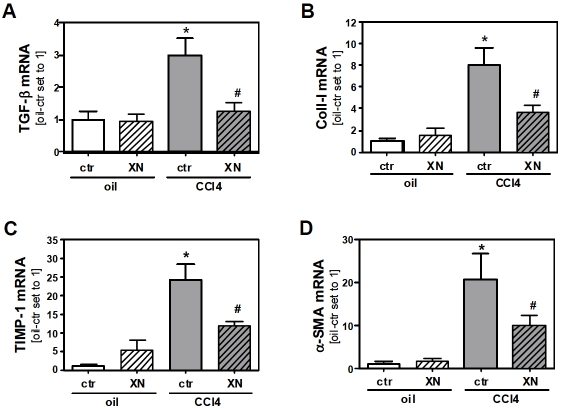
Next, we determined if the reduced inflammation seen in CCl4-treated and XN-fed mice is reflected by down-regulation of genes that mediate the fibrotic response. Levels of TGF-β mRNA were markedly induced in control chow-fed CCl4-treated mice while in XN-fed CCl4-treated mice expression levels of this cytokine, which plays a crucial pathophysiological role in liver fibrosis [32, 33], were not elevated compared to control mice (Figure 3A). In addition, expression of genes encoding collagen type I (Coll-I) and tissue inhibitor of matrix metalloproteinase-1 (TIMP-1) were induced after CCl4-treatment, but this increase was inhibited by XN application (Figure 3B and 3C). The expression of these genes reflects the onset of tissue remodeling processes, which can lead to fibrosis [34]. In line with this, a significant increase of alpha-smooth muscle actin (α-SMA) expression was observable in control chow-fed but not in XN-fed mice after CCl4-treatment, indicating a XN-mediated inhibition of injury-triggered HSC activation (Figure 3D). These data indicate that livers of XN-fed animals display a diminished pro-inflammatory and pro-fibrotic response after CCl4-induced injury.
Figure 3.
Effect of xanthohumol on toxin induced fibrogenic response of the liver. Mice were fed either with control chow (ctr) or with the same chow supplemented with 0.5% (w/w) xanthohumol (XN). After 2 days of feeding a single dose of CCl4 (1 μl/g body weight in olive oil) or olive oil alone (oil) was injected intraperitoneally to mice from both the control chow-fed and XN-fed group. 72 h after CCl4 or olive oil injection mice were sacrificed. Analysis of hepatic mRNA levels of (A) TGF-β, (B) Coll-I, (C) TIMP-1 and (D) α-SMA by quantitative RT-PCR (*: p<0.05 compared to oil-ctr; #: p<0.05 compared to CCl4-ctr).
Discussion
The aim of this study was to investigate the effect of xanthohumol (XN) in an acute model of liver injury and to further study its role in liver fibrosis. For this purpose we subjected mice fed with and without XN (at a dose of approximately 1 mg/g body weight) to acute CCl4-induced liver damage. Our results reveal a profound inhibitory effect of XN on pro-inflammatory and pro-fibrogenic hepatic gene expression in this model. Noteworthy, these effects occurred despite the fact that hepatocellular injury as reflected by serum levels of transaminases or histomorphological analysis was comparable between control mice and XN-fed mice 72 h after CCl4-injection. These findings suggest that the suppressive effect of XN against the progress of acute CCl4-induced hepatic fibrosis involves direct mechanisms related to its ability to block both hepatic inflammation and the activation of hepatic stellate cells (HSC). Actually, we have previously shown that XN exhibits direct anti-inflammatory effects on HSC and inhibits the activation of these cells, respectively, by inhibiting IκBα degradation and subsequent NFκB activation [6]. Notably, these anti-fibrogenic effects have been observed at concentrations as low as 5 μM. Previous studies could not detect (unmetabolized and unconjugated) XN in the systemic circulation upon oral application [35, 36]. However, the anatomical situation of the liver has to be considered. It can be expected that after oral intake the XN concentration in the portal vein is higher than in the systemic circulation. Further, HSC are located in the liver in the space of Disse (or perisinusoidal space), i.e. between the sinusoid and the hepatocytes. Herewith, HSC are directly exposed to XN concentration reaching the liver via the portal vein irrespective of (subsequent) metabolism in hepatocytes. Thus, XN concentrations reaching HSC in the space of Disse may be significantly higher than the levels in whole liver tissue.
In our previous study we have shown that XN inhibits hepatic inflammation and fibrosis in mice in a NASH model [6]. In this model hepatocellular lipid accumulation led to a mild inflammation and fibrogenic response after 3 weeks of feeding a NASH inducing diet. In contrast, the CCl4-model is used to induce extended hepatocellular death and inflammation, which in turn promotes fibrosis. The marked anti-fibrogenic effect of XN under these acute and harsh experimental conditions further advances the concept that XN is a promising natural substance with the potential to inhibit the development and progression of hepatic fibrosis in patients with (chronic) liver disease.
Acknowledgments
We want to thank Ruth Schewior and Marina Fink for excellent technical assistance. This work was supported by grants from the German Research Association (He 2458/14-1 to C.H.) and the Medical Faculty of the University of Re-gensburg (ReForM) to C.H. Further, this project was supported in part by an unrestricted research grant from the Joh. Barth & Sohn GmbH (Nuremberg, Germany). Financial relationships of the authors with Joh. Barth & Sohn GmbH are as follows: C.H. is a consultant, and C.D. is working in the laboratory of C.H. All authors had complete and independent control over the study design, analysis and interpretation of data, report writing, and publication, regardless of results.
References
- 1.Zanoli P, Zavatti M. Pharmacognostic and pharmacological profile of Humulus lupulus L. J Ethnopharmacol. 2008;116:383–396. doi: 10.1016/j.jep.2008.01.011. [DOI] [PubMed] [Google Scholar]
- 2.Stevens JF, Page JE. Xanthohumol and related prenylflavonoids from hops and beer: to your good health! Phytochemistry. 2004;65:1317–1330. doi: 10.1016/j.phytochem.2004.04.025. [DOI] [PubMed] [Google Scholar]
- 3.Albini A, Dell'Eva R, Vene R, Ferrari N, Buhler DR, Noonan DM, Fassina G. Mechanisms of the antiangiogenic activity by the hop flavonoid xanthohumol: NF-κB and Akt as targets. FASEB J. 2006;20:527–529. doi: 10.1096/fj.05-5128fje. [DOI] [PubMed] [Google Scholar]
- 4.Dell'Eva R, Ambrosini C, Vannini N, Piaggio G, Albini A, Ferrari N. AKT/NF-κB inhibitor xanthohumol targets cell growth and angiogenesis in hematologic malignancies. Cancer. 2007;110:2007–2011. doi: 10.1002/cncr.23017. [DOI] [PubMed] [Google Scholar]
- 5.Harikumar KB, Kunnumakkara AB, Ahn KS, Anand P, Krishnan S, Guha S, Aggarwal BB. Modification of the cysteine residues in IκBα kinase and NF-κB (p65) by xanthohumol leads to suppression of NF-κB-regulated gene products and potentiation of apoptosis in leukemia cells. Blood. 2009;113:2003–2013. doi: 10.1182/blood-2008-04-151944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dorn C, Kraus B, Motyl M, Weiss TS, Gehrig M, Scholmerich J, Heilmann J, Hellerbrand C. Xanthohumol, a chalcon derived from hops, inhibits hepatic inflammation and fibrosis. Mol Nutr Food Res. 2010;54:S205–213. doi: 10.1002/mnfr.200900314. [DOI] [PubMed] [Google Scholar]
- 7.Cobbold JF, Anstee QM, Taylor-Robinson SD. The importance of fatty liver disease in clinical practice. Proc Nutr Soc. 2010;69:518–527. doi: 10.1017/S0029665110001916. [DOI] [PubMed] [Google Scholar]
- 8.Clark JM, Brancati FL, Diehl AM. Nonalcoholic fatty liver disease. Gastroenterology. 2002;122:1649–1657. doi: 10.1053/gast.2002.33573. [DOI] [PubMed] [Google Scholar]
- 9.Vernon G, Baranova A, Younossi ZM. Systematic review: the epidemiology and natural history of non-alcoholic fatty liver disease and non-alcoholic steatohepatitis in adults. Aliment Pharmacol Ther. 2011;34:274–285. doi: 10.1111/j.1365-2036.2011.04724.x. [DOI] [PubMed] [Google Scholar]
- 10.Tannapfel A, Denk H, Dienes HP, Langner C, Schirmacher P, Trauner M, Flott-Rahmel B. Histopathological diagnosis of non-alcoholic and alcoholic fatty liver disease. Virchows Arch. 2011;458:511–523. doi: 10.1007/s00428-011-1066-1. [DOI] [PubMed] [Google Scholar]
- 11.Gines P, Cardenas A, Arroyo V, Rodes J. Management of cirrhosis and ascites. N Engl J Med. 2004;350:1646–1654. doi: 10.1056/NEJMra035021. [DOI] [PubMed] [Google Scholar]
- 12.Minguez B, Tovar V, Chiang D, Villanueva A, Llovet JM. Pathogenesis of hepatocellular carcinoma and molecular therapies. Curr Opin Gastroenterol. 2009;25:186–194. doi: 10.1097/MOG.0b013e32832962a1. [DOI] [PubMed] [Google Scholar]
- 13.Villanueva A, Newell P, Chiang DY, Friedman SL, Llovet JM. Genomics and signaling pathways in hepatocellular carcinoma. Semin Liver Dis. 2007;27:55–76. doi: 10.1055/s-2006-960171. [DOI] [PubMed] [Google Scholar]
- 14.Cutroneo KR. How is Type I procollagen synthesis regulated at the gene level during tissue fibrosis. J Cell Biochem. 2003;90:1–5. doi: 10.1002/jcb.10599. [DOI] [PubMed] [Google Scholar]
- 15.Lang A, Brenner DA. Gene regulation in hepatic stellate cell. Ital J Gastroenterol Hepatol. 1999;31:173–179. [PubMed] [Google Scholar]
- 16.Brenner DA, Waterboer T, Choi SK, Lindquist JN, Stefanovic B, Burchardt E, Yamauchi M, Gillan A, Rippe RA. New aspects of hepatic fibrosis. J Hepatol. 2000;32:32–38. doi: 10.1016/s0168-8278(00)80413-4. [DOI] [PubMed] [Google Scholar]
- 17.Kisseleva T, Brenner DA. Role of hepatic stellate cells in fibrogenesis and the reversal of fibrosis. J Gastroenterol Hepatol. 2007;22:S73–78. doi: 10.1111/j.1440-1746.2006.04658.x. [DOI] [PubMed] [Google Scholar]
- 18.Maher JJ, Bissell DM. Cell-matrix interactions in liver. Semin Cell Biol. 1993;4:189–201. doi: 10.1006/scel.1993.1023. [DOI] [PubMed] [Google Scholar]
- 19.Hautekeete ML, Geerts A. The hepatic stellate (Ito) cell: its role in human liver disease. Virchows Arch. 1997;430:195–207. doi: 10.1007/BF01324802. [DOI] [PubMed] [Google Scholar]
- 20.Lee KS, Buck M, Houglum K, Chojkier M. Activation of hepatic stellate cells by TGF α and collagen type I is mediated by oxidative stress through c-myb expression. J Clin Invest. 1995;96:2461–2468. doi: 10.1172/JCI118304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lang A, Schoonhoven R, Tuvia S, Brenner DA, Rippe RA. Nuclear factor κB in proliferation, activation, and apoptosis in rat hepatic stellate cells. J Hepatol. 2000;33:49–58. doi: 10.1016/s0168-8278(00)80159-2. [DOI] [PubMed] [Google Scholar]
- 22.Hellerbrand C, Jobin C, Iimuro Y, Licato L, Sartor RB, Brenner DA. Inhibition of NFκB in activated rat hepatic stellate cells by proteasome inhibitors and an IκB super-repressor. Hepatology. 1998;27:1285–1295. doi: 10.1002/hep.510270514. [DOI] [PubMed] [Google Scholar]
- 23.Grunhage F, Fischer HP, Sauerbruch T, Reichel C. Drug- and toxin-induced hepatotoxicity. Z Gastroenterol. 2003;41:565–578. doi: 10.1055/s-2003-39650. [DOI] [PubMed] [Google Scholar]
- 24.Zimmerman HJ, Lewis JH. Chemical- and toxin-induced hepatotoxicity. Gastroenterol Clin North Am. 1995;24:1027–1045. [PubMed] [Google Scholar]
- 25.Mehendale HM, Roth RA, Gandolfi AJ, Klaunig JE, Lemasters JJ, Curtis LR. Novel mechanisms in chemically induced hepatotoxicity. FASEB J. 1994;8:1285–1295. doi: 10.1096/fasebj.8.15.8001741. [DOI] [PubMed] [Google Scholar]
- 26.Huh CG, Factor VM, Sanchez A, Uchida K, Conner EA, Thorgeirsson SS. Hepatocyte growth factor/c-met signaling pathway is required for efficient liver regeneration and repair. Proc Natl Acad Sci USA. 2004;101:4477–4482. doi: 10.1073/pnas.0306068101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Iredale JP. Models of liver fibrosis: exploring the dynamic nature of inflammation and repair in a solid organ. J Clin Invest. 2007;117:539–548. doi: 10.1172/JCI30542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gabele E, Muhlbauer M, Dorn C, Weiss TS, Froh M, Schnabl B, Wiest R, Scholmerich J, Obermeier F, Hellerbrand C. Role of TLR9 in hepatic stellate cells and experimental liver fibrosis. Biochem Biophys Res Commun. 2008;376:271–276. doi: 10.1016/j.bbrc.2008.08.096. [DOI] [PubMed] [Google Scholar]
- 29.Hellerbrand C, Muhlbauer M, Wallner S, Schuierer M, Behrmann I, Bataille F, Weiss T, Scholmerich J, Bosserhoff AK. Promoterhypermethylation is causing functional relevant downregulation of methylthioadenosine phosphorylase (MTAP) expression in hepatocellular carcinoma. Carcinogenesis. 2006;27:64–72. doi: 10.1093/carcin/bgi201. [DOI] [PubMed] [Google Scholar]
- 30.Muhlbauer M, Bosserhoff AK, Hartmann A, Thasler WE, Weiss TS, Herfarth H, Lock G, Scholmerich J, Hellerbrand C. A novel MCP-1 gene polymorphism is associated with hepatic MCP-1 expression and severity of HCV-related liver disease. Gastroenterology. 2003;125:1085–1093. doi: 10.1016/s0016-5085(03)01213-7. [DOI] [PubMed] [Google Scholar]
- 31.Dorn C, Weiss TS, Heilmann J, Hellerbrand C. Xanthohumol, a prenylated chalcone derived from hops, inhibits proliferation, migration and interleukin-8 expression of hepatocellular carcinoma cells. Int J Oncol. 2010;36:435–441. [PubMed] [Google Scholar]
- 32.Gressner AM, Weiskirchen R. Modern pathogenetic concepts of liver fibrosis suggest stellate cells and TGF-β as major players and therapeutic targets. J Cell Mol Med. 2006;10:76–99. doi: 10.1111/j.1582-4934.2006.tb00292.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Brenner DA. Molecular pathogenesis of liver fibrosis. Trans Am Clin Climatol Assoc. 2009;120:61–368. [PMC free article] [PubMed] [Google Scholar]
- 34.Iredale JP, Benyon RC, Arthur MJ, Ferris WF, Alcolado R, Winwood PJ, Clark N, Murphy G. Tissue inhibitor of metalloproteinase-1 messenger RNA expression is enhanced relative to interstitial collagenase messenger RNA in experimental liver injury and fibrosis. Hepatology. 1996;24:176–184. doi: 10.1002/hep.510240129. [DOI] [PubMed] [Google Scholar]
- 35.Avula B, Ganzera M, Warnick JE, Feltenstein MW, Sufka KJ, Khan IA. High-performance liquid chromatographic determination of xanthohumol in rat plasma, urine, and fecal samples. J Chromatogr Sci. 2004;42:378–382. doi: 10.1093/chromsci/42.7.378. [DOI] [PubMed] [Google Scholar]
- 36.Bolca S, Li J, Nikolic D, Roche N, Blondeel P, Possemiers S, De KD, Bracke M, Heyerick A, van Breemen RB, Depypere H. Disposition of hop prenylflavonoids in human breast tissue. Mol Nutr Food Res. 2010;54:S284–294. doi: 10.1002/mnfr.200900519. [DOI] [PMC free article] [PubMed] [Google Scholar]