Abstract
The author investigated histopathology of 615 consecutive duodenal specimens in our pathology laboratory in Japan. A computer review of the duodenal specimens was done. In cases of malignancy, histological slides were reviewed. The duodenal specimens were composed of 567 benign lesions (92%) and 48 malignant lesions (8%). The 48 malignant lesions were composed of 20 cases (42%) of primary adenocarcinoma, 10 cases (21%) of primary adenocarcinoma of ampulla Vater, 4 cases (8%) of primary squamous cell carcinoma, 1 (2%) cases of primary spindle cell carcinoma, 4 (8%) cases of carcinoid tumors, 1 (2%) case of malignant lymphoma, and 8 cases (17%) of secondary carcinoma from the pancreatic carcinoma or bile duct carcinoma. The primary adenocarcinoma (n=20) was composed of well differentiated adenocarcinoma (n=9), papillary adenocarcinoma (n=1), moderately differentiated adenocarcinoma (n=6), and poorly differentiated adenocarcinoma (n=4). The primary adenocarcinoma of the ampulla of Vater (n=10) was composed of well differentiated adenocarcinoma (n=7) and moderately differentiated adenocarcinoma (n=3). The primary squamous cell carcinoma (n=4) showed proliferation of malignant squamous cells with keratinization and intercellular bridges. The spindle cell carcinoma (n=1) consisted of only malignant spindle cells immunohistochemistry positive for various cytokeratins and vimentin. The carcinoid tumor (n=4) was typical carcinoid and showed organoid, trabecular, and ribbon-like arrangements. The carcinoid tumor was immunohistochemically positive for neuroendocrine markers such as CD56, neuron-specific enolase and synaptophysin. The malignant lymphoma (n=1) was diffuse large B-cell lymphoma immunohistochemically positive for CD10, CD20, and CD79α. The secondary carcinoma (n=8) was adenocarcinoma invaded from the pancreatic adenocarcinoma (n=6) and extrahepatic bile duct adenocarcinoma (n=2).
Keywords: Duodenum, maligant lesions, pathology, consecutive duodenal specimens, immunohistochemistry
Introduction
Malignant lesions of the duodenum include carcinoma, carcinoid tumor, mixed carcinoid-adenocarcinoma, gastrointestinal stromal tumor (GIST), leiomyosarcoma, angiosarcoma, Ka-posi's sarcoma, malignant lymphoma, and secondary malignant tumors [1]. In the present study, 48 malignant duodenal lesions were described.
Materials and methods
The author reviewed, by a computer, the pathologic reports of 615 consecutive duodenal specimens in our pathology laboratory. In malignant cases, histological slides were reviewed. The duodenal specimens were composed of 567 benign lesions (92%) and 48 malignant lesions (8%) Clinical records were also reviewed on computer. The patients ranged from 25 years to 95 years with a mean of 53 years. Male to female ratio was 321:294. In appropriate cases, an immunohistochemical analysis for p53 protein (DO-7, Dako Corp, Glostrup, Denmark), pancytokeratin (AE1/AE3, polyclonal wide, Dako), pancytokeratin (CAM5.2, Becton Dickinson Corp., Ca, USA), cytokeratin (CK) 7, CK 20, vimentin (VIM, Dako), carcinoembryonic antigen (CEA) (polyclonal Dako), carbohydrate antigen 19-9 (CA19-9) (NA19-9, Kyowa, Tokyo, Japan), chromogranin (DAK-A3, Dako), synaptophysin (polyclonal, Dako), neuron-specific enolase (BBS/NC/VI-H14, Dako), CD56 (MOC-1, Dako), CD3 (M7193, Dako), CD10 (M0727, Dako), CD15 (M0733, Dako), CD30 (M0751, Dako), CD45 (M0855, DAKO), CD45RO (M0834, Dako), CD79α (M7050, Dako), CD57 (HNK-1, Santa Cruz, CA, USA), kappa light chain (polyclonal, Dako), lambda light chain (polyclonal, Dako), and Ki-67 antigens (MIB1, Dako) had been performed with the use of Dako Envision method (Dako), as previously described [2-6].
Results
The duodenal specimens were composed of 567 benign lesions (92%) and 48 malignant lesions (8%). The 48 malignant lesions were composed of 20 cases (42%) of primary adenocarcinoma, 10 cases (21%) of primary adenocarcinoma of ampulla Vater, 4 cases (8%) of primary squamous cell carcinoma, 1 (2%) cases of spindle cell carcinoma, 4 (8%) cases of carcinoid tumors, 1 (2%) case of malignant lymphoma, and 8 cases (17%) of secondary carcinoma from the pancreatic carcinoma or bile duct carcinoma.
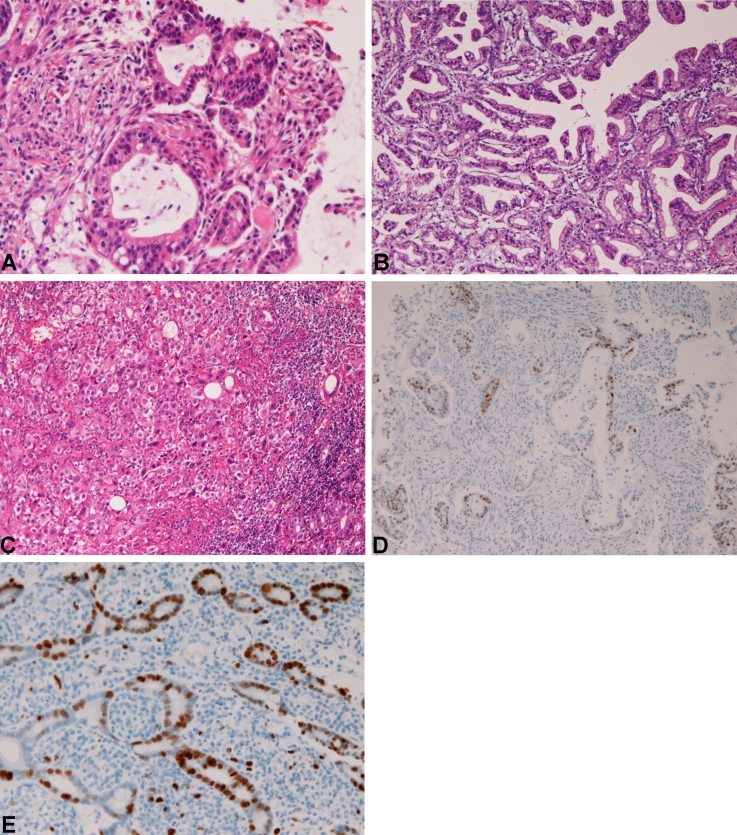
The primary adenocarcinoma (n=20) was composed of well differentiated adenocarcinoma (n=9) (Figure 1A), papillary adenocarcinoma (Figure 1B) (n=1), moderately differentiated adenocarcinoma (n=6), and poorly differentiated adenocarcinoma (n=4) (Figure 1C). Immunohistochemically, the adenocarcinoma cells were positive for pancytokeratin and p53 (Figure 1D) in all cases examined. CEA was positive in 5/7 cases, CA19-9 was positive in 3/4cases. The Ki-67 labeling ranged from 32% to 74% with a mean of 53% (Figure 1E). Grossly, the tumor was ulcerated in 15 cases, polypoid in 4 cases, and scirrhous in 1 cases. Clinically, the locations were the first portion in 5 cases, the second portion near the ampulla in 14 cases, and the third portion in 1 case.
Figure 1.
Duodenal adenocarcinoma. A. Well differentiated adenocarcinoma, HEx200 B. Papillary adenocarcinoma. HE x100 C. Poorly differentiated adenocarcinoma. HE, x200. D. p53 expression is positive. Immunostaining, x100. E. Ki-67 labeling is 70%. Immunostaining, x100.
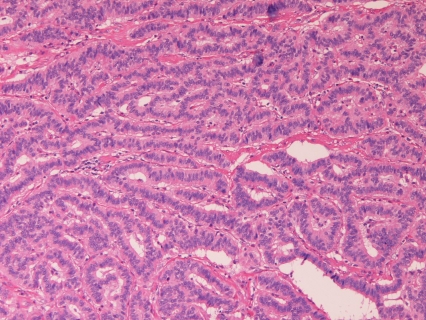
The primary adenocarcinoma of the ampulla of Vater (n=10) was composed of well differentiated adenocarcinoma (n=7) and moderately differentiated adenocarcinoma (n=3). Immunohistochemically, pancytokeratin were positive in 4/4 cases, cytokeratin 7 in 4/4 cases, p53 expression was in 4/4 cases, CEA in 2/3 cases, CA19-9 in 2/2 cases. The Ki-67 labeling ranged from 25% to 71% with a mean of 48%. Grossly, the carcinoma is erosive in 1 case, ulcerated in 7 cases, and polypoid in 2 cases.
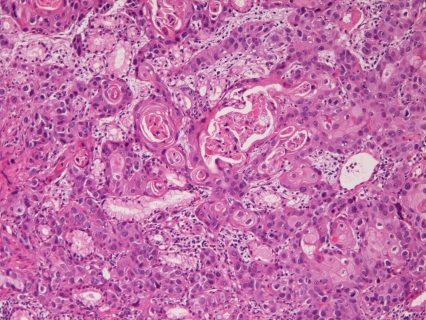
The primary squamous cell carcinoma (n=4) showed proliferation of malignant squamous cells with keratinization and intercellular bridges (Figure 2). Grossly, all cases were ulcerated tumor. Immunohistochemically, pancytokeratin was positive in 2/2 cases, CK7 in 2/2cases. CEA and CA19-9 were negative in all the 2 cases examined. p53 expression was recognized in 2/2 cases. The Ki-67 labeling ranged from 42% to 87% with a mean of 64%. Clinically, all cases were located in the second portion near the ampulla of Vater.
Figure 2.
Pure squamous cell carcinoma of the duodenum. Keratinization and intercellular bridges are seen. HE, x200.
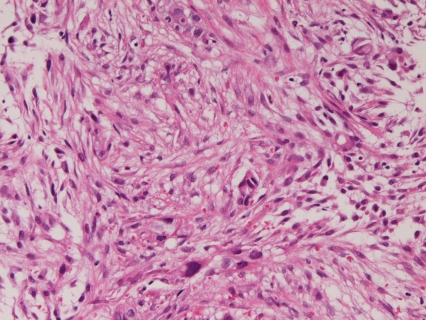
In the primary spindle cell carcinoma (n=1), the carcinoma cells were composed only of malignant spindle cell (Figure 3). Immunohistochemically, the tumor cells were positive for pancytokeratin, CK7, vimentin, p53 protein. CEA and CA19-9 was negative. The Ki-67 labeling was 67%. It is situated in the second portion near the ampulla.
Figure 3.
Pure spindle cell carcinoma of the duodenum. Malignant spindle cells are seen. HE, x200.
In the carcinoid tumor (n=4), all were typical carcinoids and composed of organoid, trabecular, or ribbon-like arrangements (Figure 4). The carcinoid tumor was immunohistochemically positive for pancytokeratins, and neuroendocrine markers such as CD56 (2/3), neuron-specific enolase (2/3) and synaptophysin (3/3) and chromogranin (0/3). p53 protein, CEA and CA19-9 were negative in all 3 cases examined. The Ki-67 labeling ranged from 5% to 12% with a mean of 7%. The carcinoid was located in the first portion in 2 cases and in the second portion in 2 cases. Grossly, all cases were submucosal tumor, the size of which was 1-2 cm.
Figure 4.
Carcinoid tumor of the duodenum. A trabecular arrangement is seen. HE, x200.
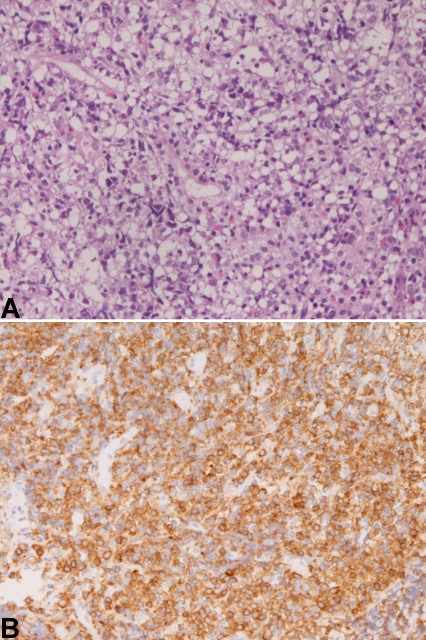
The malignant lymphoma (n=1) was a diffuse large B-cell lymphoma (Figure 5A) immunohistochemically positive for CD10, CD20, and CD79α (Figure 5B) and negative for CD3, CD45RO, CD46 and CD47. The lymphoma cells were positive for p53 protein, and negative for pancytokeratin and CK7. The Ki-67 labeling was 95%. The lymphoma was ulcerated tumor and located in the second portion.
Figure 5.
Diffuse large B-cell lymphoma of the duodenum. A. Proliferation of lymphoma cells are seen. HE, x200 B. The cells are positive for CD79α. Immunostaining, x200.
The secondary carcinoma (n=8) was adenocarcinoma invaded from the pancreatic adenocarcinoma (n=6) and extrahepatic bile duct adenocarcinoma (n=2).
Discussion
Primary adenocarcinoma of the duodenum is a rare malignancy with an incidence of 0.4 per 100,000 per year [1]. This carcinoma occurs most frequently in periampullary regions [7-9]. The prognosis is poor with a mean 5-year survival of 25%-31% [7-9]. No comprehensive pathologic study has been present, to the best of the author's knowledge. In the present study, the primary adenocarcinoma was composed of well differentiated adenocarcinoma (n=9) (Figure 1A), papillary adenocarcinoma (n=1), moderately differentiated adenocarcinoma, and poorly differentiated adenocarcinoma (n=4). The papillary carcinoma appears extremely rare. Immunohistochemically, the adenocarcinoma cells were positive for pancytokeratin, CK7, and p53 in all cases. CEA was positive in 15 cases, CA19-9 was positive in 13 cases. The Ki-67 labeling ranged from 32% to 74% with a mean of 53%. Grossly, the tumor was ulcerated in 15 cases, polypoid in 4 cases, and scirrhous in 1 cases. Clinically, the locations were the first portion in 5 cases, the second portion near the ampulla in 14 cases, and the third portion in 1 case.
The primary adenocarcinoma of the ampulla of Vater is also a rare disease. Several comprehensive pathological reports are present [10-13]. Seifelt et al. [10] demonstrated that most of ampullary carcinoma developed in adenoma. However, in the present study, no such cases were present. Komorowski et al. [11] mentioned an endoscopic pathology. Chu et al. [12] demonstrated that CDX2, CK17, MUC1 and MUC2 are useful marker of the adenocarcinoma of the ampulla of Vater. In the present study, frequent CK7 expression was noted. Zhou et al. [13] identified frequent expression of CK7, CEA, CA19-9, Ki-67 and p53 protein, being consistent with the present study. In the present study, ampullary adenocarcinoma was composed of well differentiated adenocarcinoma (n=7) and moderately differentiated adenocarcinoma, suggesting that most of ampullary adenocarcinoma is well differentiated and may be difficult to differentiate from adenoma.
In the present study, 4 cases of primary pure squamous cell carcinoma were recognized. Only 2 cases of duodenal squamous cell carcinoma have been reported in the English literature [14, 15]. Therefore, the present 4 cases of pure squamous cell carcinoma are of great value. Adenosquamous carcinoma is also very rare in the duodenum; only one case has been reported [16]. In the present 4 cases, malignant squamous cells were positive for keratinization and intercellular bridges and no other elements were recognized, implying that the present 4 cases are true pure squamous cell carcinoma of the duodenum. In the present study, all cases were ulcerated tumor. The preferential location was the second portion near the ampulla.
The present study revealed one case of the spindle cell carcinoma. The carcinoma cells were composed only of malignant spindle cells. Immunohistochemically, the tumor was positive for pancytokeratin, CK7, vimentin, p53 protein. CEA and CA19-9 was negative, confirming the diagnosis of spindle cell carcinoma. There have been no reports of spindle cell carcinoma of the duodenum in the literature. Further, WHO does not list spindle cell carcinoma in the WHO blue book [1]. Namely, the present case is the first case of spindle cell carcinoma of the duodenum, and is of great value.
Carcinoid tumors are prevalent in the duodenum. Carcinoid tumor is potentially malignant tumor. In the present study also, 4 case of typical carcinoid tumor were found (n=4). The present carcinoid tumors were immunohistochemically positive for pancytokeratin, and neuroendocrine markers such as CD56, neuron-specific enolase, and synaptophysin. P53 protein, CEA and CA19-9 were negative in all cases examined. The Ki-67 labeling ranged from 5% to 12% with a mean of 7%. No new findings were present in the present carcinoids.
Malignant lymphoma of the duodenum is rare. Most of duodenal lymphoma is MALT lymphoma or follicular lymphoma [17]. In the present series, one case of diffuse large B-cell lymphoma was recognized. This type is relatively rare in the duodenum [17], but no new findings are present.
In the present study, 8 cases of secondary carcinoma were noted. The primary site was pancreatic adenocarcinoma (n=6) and extrahepatic bile duct adenocarcinoma (n=2). The secondary carcinoma should be differentiated from primary carcinoma with the use of histopathology and imaging techniques.
Finally, most of malignant tumors of the duodenum tended to occurs in the ampulla or periampullary regions. This may be because these sites are irritated by bile and pancreatic juice, potent carcinogens.
References
- 1.Mamilton SR, Aaltonen LA, editors. Tumours of the small intestine. WHO Classification of tumours. Pathology and genetics of tumours of the digestive system. Lyon: IARC press; 2000. pp. 70–92. [Google Scholar]
- 2.Terada T, Kawaguchi M, Furukawa K, Sekido Y, Osamura Y. Minute mixed ductal-endocrine carcinoma of the pancreas with predominant intraductal growth. Pathol Int. 2002;52:740–746. doi: 10.1046/j.1440-1827.2002.01416.x. [DOI] [PubMed] [Google Scholar]
- 3.Terada T, Tanigichi M. Intraductal oncocytic papillary neoplasm of the liver. Pathol Int. 2004;54:116–123. doi: 10.1111/j.1440-1827.2004.01594.x. [DOI] [PubMed] [Google Scholar]
- 4.Terada T. Primary multiple extragastrointestinal stromal tumors of the omentum with different mutations of c-kit gene. Would J Gastroenterol. 2008;14:7256–7259. doi: 10.3748/wjg.14.7256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Terada T. Gastrointestinal stromal tumor of the uterus: A case report with genetic analyses of c-kit and PDGFRA genes. Int J Gynecol Pathol. 2009;28:29–34. doi: 10.1097/PGP.0b013e3181808000. [DOI] [PubMed] [Google Scholar]
- 6.Terada T. Large endocervical polyp with cartilaginous and osseous metaplasia: a hitherto unreported entity. Int J Gynecol Pathol. 2009;28:98–100. doi: 10.1097/PGP.0b013e31817eb796. [DOI] [PubMed] [Google Scholar]
- 7.Rose DM, Hochwald SN, Klimstra DS, Brennan MF. Primary duodenal adenocarcinoma: a ten-year experience with 79 patients. J Am Coll Surg. 1996;183:89–96. [PubMed] [Google Scholar]
- 8.Barnes G, Jr, Romero L, Hess KR, Curley SA. Primary adenocarcinoma of the duodenum: management and survival in 67 patients. Ann Surg Oncol. 1994;1:73–78. doi: 10.1007/BF02303544. [DOI] [PubMed] [Google Scholar]
- 9.Santore E, Sacchi M, Scutari F, Carboni F, Graziano F. Primary adenocarcinoma of the duodenum: treatment and survival in 89 patients. Hepatogastroenterology. 1997;44:1157–1163. [PubMed] [Google Scholar]
- 10.Seifelt E, Schulte F, Stolte M. Adenoma and adenocarcinoma of the duodenum and papilla of Vater: a clinicopatrhologic study. Am J Gastroenterol. 1992;87:37–42. [PubMed] [Google Scholar]
- 11.Komorowski RA, Beggs BK, Geenan JF, Venu RP. Assessment of ampulla of Vater pathology. An endocropic study. Am J Surg Pathol. 1991;15:1188–1196. doi: 10.1097/00000478-199112000-00010. [DOI] [PubMed] [Google Scholar]
- 12.Chu PG, Schwarz RE, Lau SK, Yen Y, Weiss LM. Immunohistochemical staining in the diagnosis of pancreaticobiliary and ampulla of Vater adenocarcinoma: application of CDX-2, CK17, MUC1, and MUC2. Am J Surg Pathol. 2005;29:359–367. doi: 10.1097/01.pas.0000149708.12335.6a. [DOI] [PubMed] [Google Scholar]
- 13.Zhou H, Schaefer N, Wolff M, Fisher HP. Carcinoma of the ampulla of Vater: comparative histochemical/immunohistochemical classification and follow-up. Am J Surg Pathol. 2004;28:875–882. doi: 10.1097/00000478-200407000-00005. [DOI] [PubMed] [Google Scholar]
- 14.Friedman E, Kwan MR, Cummins L. Squamous cell carcinoma of the transverse duodenum. Gastrointest Endosc. 1986;32:99–101. doi: 10.1016/s0016-5107(86)71766-5. [DOI] [PubMed] [Google Scholar]
- 15.von Delius S, Lersch C, Neu B, Huber B, Eckel F, Pitzl H, Fend F, Gaa J, Schmid RM. Squamous -cell carcinoma of the duodenum as a rare cause of upper gastrointestinal bleeding. Endoscopy. 2006;38:956. doi: 10.1055/s-2006-925161. [DOI] [PubMed] [Google Scholar]
- 16.de la Cruz A, de la Cruz E, Sanchez MJ, Ortiz S, Lobato A, Merino E. Adenosquamous carcinoma of the duodenum: an immunohistochemical study. Pathol Res Pract. 1993;189:481–485. doi: 10.1016/S0344-0338(11)80345-6. [DOI] [PubMed] [Google Scholar]
- 17.Yoshino T, Miyake K, Ichinomiya K, Mannami T, Ohara N, Hamazaki S, Akagi T. Increased incidence of follicular lymphoma in the duodenum. Am J Surg Pathol. 2000;24:688–693. doi: 10.1097/00000478-200005000-00007. [DOI] [PubMed] [Google Scholar]