Abstract
Aim
This study explored the cellular and biological interrelationships involved in Idiopathic Pulmonary Fibrosis (IPF) lung tissue remodelling using immunohistochemical analysis.
Methods and results
IPF and control lung tissues were examined for localisation of Epithelial Mesenchymal Transition (EMT), proliferation and growth factor markers assessing their relationship to key histological aberrations. E-cadherin was expressed in IPF and control (Alveolar type II) ATII cells (>75%). In IPF, mean expression of N-cadherin was scanty (<10%): however 4 cases demonstrated augmented expression in ATII cells correlating to histological disease status (Pearson correlation score 0.557). Twist was expressed within fibroblastic foci but not in ATII cells. Transforming Growth Factor- β (TGF-β) protein expression was significantly increased in IPF ATII cells with variable expression within fibroblastic foci. Antigen Ki-67 was observed within hyperplastic ATII cells but not in cells overlying foci. Collagen I and α-smooth muscle actin (α-SMA) were strongly expressed within fibroblastic foci (>75%); cytoplasmic collagen I in ATII cells was present in 3 IPF cases. IPF ATII cells demonstrated variable Surfactant Protein-C (SP-C).
Conclusions
The pathogenesis of IPF is complex and involves multiple factors, possibly including EMT. Histological analysis suggests TGF-β-stimulated myofib rob lasts initiate a contractile response within established fibroblastic foci while proliferating ATII cells attempt to instigate alveolar epithelium repair. Marker expression (N-cadherin and Ki-67) correlation with histological disease activity (as reflected by fibroblastic foci extent) may emerge as future prognostic indicators for IPF.
Keywords: Idiopathic Pulmonary Fibrosis, immunohistochemistry, epithelial-mesenchymal transition, tissue repair and remodelling, prognostic markers
Introduction
Idiopathic pulmonary fibrosis (IPF) is a devastating, progressive respiratory disease, with unknown aetiology. Mean survival is 2-3 years from initial diagnosis [1] and to date there is no effective treatment able to halt or reverse disease progression and improve clinical outcome.
Histological features of IPF, as defined by Usual Interstitial Pneumonia (UIP), include the diagnostic patchwork of normal unaffected lung alternating with remodelled fibrotic lung involving type I pneumocyte destruction, type II pneu-mocyte hyperplasia and areas of inactive collagen type scaring. Formation of fibroblastic foci is a key feature reflecting sites of active ongoing fibrogenesis. Increased numbers of fibroblastic foci have been associated with disease activity and a more rapid disease progression in IPF patients [2-4]. Within the context of the evolving tissue remodelling characteristic of IPF, this paper partly explores novel immunohistochemical markers as a tool for assessing histological disease status.
The pathogenesis of IPF is still to be fully elucidated; however current evidence suggests a failure or imbalance in a number of pathways eventually leading to the alveolar epithelial cell loss and differentiated fibroblast accumulation pathognomonic of this disease. Myofibroblasts, (which have contractile properties and express α-smooth muscle actin (α-SMA)) are responsible for the excessive collagen deposition and tissue remodelling seen in IPF [5]. There are 4 possible sources of myofibroblasts: (1) resident fibro-blast proliferation and differentiation [6]; (2) circulating fibrocytes attracted to regions of lung injury [7]; (3) endothelial-mesenchymal transition (EndMT) [8] and (4) epithelial-mesenchymal transition (EMT) [9-10].
EMT is a biological process whereby polarized epithelial cells undergo morphological changes to assume a mesenchymal phenotype [11]. EMT has been characterised by the switch from epithelial (E-cadherin) to mesenchymal (N-cadherin) calcium-ion-dependent adhesion accompanied by the gain of other markers such as α-SMA [12]. During the process of EMT cells become more motile, invasive and gain resistance to apoptosis [13]. EMT repairs damaged tissue via the production of fibroblasts and extracellular matrix (ECM), and has association with fibrosis in other organs [10, 13-16]. It is hypothesised that hyperplastic ATII cells, and/or lung endothelial cells in IPF, transform via EMT/ EndMT to produce the fibroblastic foci observed in IPF [17].
Several transcription and growth factors have been implicated in the pathogenesis of IPF and are recognised drivers of EMT. The transcription factor Twist, (not normally expressed in healthy human adult lung) inhibits proliferation and differentiation of cells and is proposed to drive EMT [18]. Overexpression of Twist results in increased N-cadherin which in turn leads to a decrease in E-cadherin. Hypoxia or mechanical stresses are known to induce Twist expression [19]. Transforming Growth Factor-β (TGF-β) is implicated in tissue remodelling, with involvement in differentiating fibroblasts to myofibroblasts [20] and inhibiting ATII cell proliferation [21]. TGF-β has also been reported to induce EMT [9] in cultured alveolar epithelial cells through Snail1, Snail2 and Smad-mediated signalling. In addition collagen I, which progressively accumulates within IPF lungs, has been shown to promote EMT in lung cancer cells via associated TGF-β signalling [22].
To support the hypothesis of EMT as a putative driver in IPF pathogenesis, and consequent lung remodelling, we would anticipate histological evidence of reduced E-cadherin in ATII cells overlying areas of established fibrosis combined with augmented N-cadherin and Twist expression. TGF-β would be upregulated, with associated expression of collagen I and α-SMA.
To date, much of the evidence presented on the potential source of fibroblasts and myofibroblasts has been based on cell culture and animal models of pulmonary fibrosis, which do not accurately reflect the disease. The present study uses immunohistochemical analysis to assess the presence and localisation of EMT markers in IPF patient lungs, and their relationship to regions of tissue remodelling. Cell proliferation associated antigen Ki-67 and cell cycle regulator p16INK4A were also evaluated to examine wound remodelling mechanisms via cell division. Furthermore, we sought to determine if there was any correlation between target markers and histological disease status.
Materials and methods
Research participants
The study population consisted of paraffin embedded lung tissue samples from 21 IPF patients and 19 control subjects; histologically-defined normal lung sections from subjects who had undergone lobectomy for cancer. IPF samples were reviewed by an independent pathologist to confirm a diagnosis of usual interstitial pneumonia (UIP) in line with recognised criteria [23].
Immunohistochemical analysis
Formalin-fixed, paraffin-embedded lung tissue samples were deparaffinised in Xylene and re-hydrated through a series of alcohols to water. Antigen retrieval was performed in citrate buffer (pH 6) microwaved for 20 minutes at 100% power. Antibodies were optimized using positive control tissue according to manufacturer's instructions (Table 1). Immunohistochemistry was performed using the En Vision system (Dako, Glostrup, Denmark). For dual immunohistochemistry staining an avidin biotin block was performed after completion of the first antibody labelled with Diaminobenzidine to prevent cross reaction with the En Vision kit. The second antibody was labelled with Very Intense Purple (VIP, Lab Vision, UK). In negative controls, the primary antibody was replaced with Tris-buffered saline. Sections were counterstained using either haematoxylin Z (CellPath, UK) or Alcian Blue with nuclear fast red.
Table 1.
Details of the antibodies, dilutions, incubation times and cellular localisation of markers used in this study.
| Antibody | Source | Dilution/incubation time | Localization/role |
|---|---|---|---|
| Pro-surfactant protein C | Abcam, UK | 1:1500, ON, 4°C | Confirmation of the localisation of type II pneumocytes [24]. |
| Collagen I | Abcam, UK | 1:500, 30 min, RT | Has been shown to promote EMT in lung cancer cells [22]. |
| TGF-β protein | Novocastra, UK | 1:20, 60min, RT | Affects cell growth, differentiation and extracellular matrix production [9,25]. |
| TGF-β receptor | Novocastra, UK | 1:40, 60min, RT | Membrane receptor for TGF-β protein. |
| α Smooth Muscle Actin (α-SMA) | Dako, UK | 1:800, 30 min, RT | Mesenchymal marker [26]. |
| E-cadherin | Vector Labs, UK | 1:50, 60 min, RT | Cell-cell adhesion marker. Loss associated with EMT [11]. |
| Antigen Ki-67 | Dako, UK | 1:50, 30 min, RT | Cell proliferation marker [27]. |
| p16INK4A | BD Bioscience, UK | 1:20, 30 min, RT | Cell cycle regulator [28]. |
| N-cadherin | Dako, UK | 1:50, 30 min, RT | Mesenchymal marker which leads to decreased E-cadherin [12]. |
| Twist | Abcam, UK | 1:50, 60min, RT | Transcription factor which inhibits proliferation and differentiation of cells [18]. |
RT, room temperature; ON, overnight.
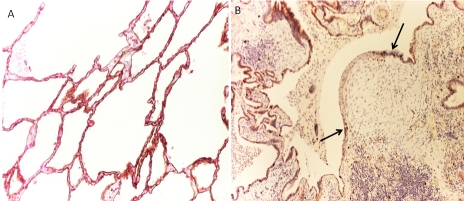
Histological disease status in IPF lungs was explored in relation to target marker expression levels in, (i) regions of hyperplastic ATII cells and (ii) cells within the fibroblastic foci. The number of established fibroblastic foci present in IPF lung tissue relates to disease activity and an increased rate of disease progression [2-4]. Based on the fibroblastic score, as devised by Nicholson et al [2], absence of fibroblastic foci was assigned a score of 0, with biopsy samples containing the highest number of fibroblastic foci scored as 6. Representative images shown in Figure 1.
Figure 1.
Representative histological images of IPF patient lung tissue stained with H&E. A. IPF lung tissue showing a high number of fibroblastic foci (score 6). B. IPF lung tissue very few fibroblastic foci (score 1). Fibroblastic foci are indicated with black arrows, magnification x100.
Semiquantitative analysis
Sections were reviewed by the lead investigator alongside an independent pathologist and scored by examining expression of markers at sites of fibroblastic foci and ATII cells in IPF and control samples. For the ATII cells, in IPF samples, 100 hyperplasic cells were counted and the number of cells expressing each marker was recorded. The same was performed on normal appearing ATII cells in the control samples. A semi-quantitative analysis was used to compare groups using a modified Allred scoring system [29] (Table 2).
Table 2.
Modified Allred scoring system forsemi-quantitative immunohistochemical analysis [29]
| Staining score | Positives staining cells (%) | Descriptive expression |
|---|---|---|
| 0 | 0 | No expression |
| 1 | <1 | Negligible expression |
| 2 | 1 to 10 | Scanty expression |
| 3 | 10 to 33 | Low-moderate expression |
| 4 | 33 to 66 | Moderate expression |
| 5 | >66 | Extensive expression |
Statistical analysis
A Mann Whitney U test was used for comparisons between IPF and control ATII cells. Results are presented as a mean ±SD score for each marker. Differences were considered significant if p≤0.05. Statistical analyses were performed using WinSTAT, (R. Fitch Software, Bad Krozingen, Germany). To determine correlations between target marker expression levels in hyperplastic ATII cells and cells within fibroblastic foci with disease severity, a Pearson correlation coefficient was calculated. The results were interpreted and categorised as no correlation, weak, moderate and strong correlation according to Cohen (1998) [30].
Results
Surfactant protein C (SP-C)
Cytoplasmic SP-C expression in the hyperplastic ATII cells in IPF lungs had a patchy pattern of distribution even in the same biopsy sample (mean expression score 3.42, 10-33%) (Figure 2A). However, cytoplasmic SP-C expression was uniform in control lungs (mean expression score 4.36, 33-75%) (Figure 2B). The degree of SP-C expression varied with the size of ATII cell, with hypertrophic cells exhibiting greater SP-C immunoreactivity (Figure 2C). SP-C expression was absent within the fibroblastic foci.
Figure 2.
Immunohistochemical analysis of IPF and control lungs for SP-C expression. A. Patchy expression of SP-C is observed in an area of ATII hyperpla-sia in IPF lung tissue. Black arrow SP-C expression, red arrow no SP-C expression. B. Representative histological image of SP-C expression in the cytoplasm of ATII cells in control lung tissue (arrow). C. Histological image of cytoplasmic SP-C expression in hyperplastic ATII cells in IPF lung tissue. Magnification x400.
E-cadherin
In all control lung samples, E-cadherin expression was evident in the cell membrane of ATII cells and bronchiolar epithelium (mean expression score 4.21, 33-75%) (Figure 3A and Figure 10). In IPF, increased E-cadherin expression was observed in cell membranes of hyperplastic ATII cells present in areas of interstitial fibrosis and overlying the fibroblastic foci (mean expression score 5, >75%) (Figure 3B). E-cadherin expression was absent within fibroblastic foci (Figure 11).
Figure 3.
Representative immunohistochemical images of E-cadherin expression in IPF and control lungs. A. Histological image of E-cadherin expressed around ATII cells in control lung tissue. B. Membrane expression of E-cadherin is seen in ATII cells overlying areas of fibroblastic foci in IPF lung tissue. Counterstained Alcian Blue with nuclear fast red. Magnification x200.
Figure 10.
Semi-quantitative analysis of target molecule expression within ATII cells of IPF and control lung samples. Data are presented as mean expression score ±SD. * = significant difference in expression between the control and IPF group p≤0.05.
Figure 11.
Semi-quantitative analysis of target molecule expression within the fibroblasticfoci of IPF lung tissue samples. Data are presented as mean expression score ±SD.
N-cadherin
Overall N-cadherin expression was scanty (mean expression score 1.71, <10%) in IPF hyperplastic ATII cells, and absent in control cases (p≤0.05) (Figure 4A). However 4 IPF cases demonstrated elevated N-cadherin expression with scores of 3 and above. The distribution of these cells was noticeable, with small clusters of hyperplastic ATII cells expressing N-cadherin interspersed with N-cadherin negative hyperplastic ATIIs. N-cadherin expression was not detected within fibroblastic foci (Figures 4B, Figure 10 and Figure 11).
Figure 4.
Dual-labelled immunohistochemistry of IPF and control lung samples for N-cadherin and Twist expression. A. Representative histological image of control lung tissue shows no N-cadherin and Twist. B. IPF lung tissue demonstrates Twist reactivity within the fibroblastic foci (arrow). Magnification A x100, B x400.
Twist
Nuclear expression of Twist was absent in ATII cells in both the control and IPF group (Figure 4A, Figure 4B and Figure 10). In contrast, moderate nuclear expression of Twist was detected within the fibroblastic foci, with a mean expression score of 3.71 (10-33%) (Figure 4B, Figure 10 and Figure 11).
α-smooth muscle actin (α-SMA)
Negligible α-SMA was expressed in hyperplastic ATII cells in IPF (mean expression score 0.28, <1%) compared to absent expression in control group ATII cells (p≤0.05, Figure 5A and Figure 10). Extensive expression of α-SMA was detected within IPF fibroblastic foci (mean expression score 5, >75%) consistent with a predominant myofibroblast phenotype (Figure 5B).
Figure 5.
Immunohistochemical analysis of IPF and control lungs for the mesenchymal marker α-SMA expression. A. Representative histological image of α-SMA expression around a blood vessel in control lung. B. α-SMA expression within fibroblastic foci in IPF lung tissue demonstrates the population of cells being composed of myofibroblasts. Magnification panel A x200, panel B x400.
Collagen I
Within the IPF group, 3 cases showed high cytoplasmic collagen I (expression score 4 33-75%) in ATII cells. The remaining IPF cases had scanty to no expression (overall mean expression score 0.61, <1%) compared to no collagen I expression in control group ATII cells (p≤0.05) (Figure 6A). Collagen I expression was markedly upregulated in IPF fibroblastic foci (mean expression score 5, >75%), (Figure 6B and Figure 10), and to a lesser extent within interstitial areas of IPF tissues.
Figure 6.
Immunohistochemical analysis of IPF and control lungs for collagen I expression. A. Representative histological image of collagen I immunoreactivity surrounding a vessel in control lung tissue. B. Collagen I immunoreactivity within a fibroblasticfoci is seen in IPF lung tissue. Magnification x200.
Transforming Growth Factor β protein and receptor (TGF-β and TGF-βR)
A significant increase in cytoplasmic TGF-β protein (mean expression score 5.0, >75%) was observed in IPF ATII cells compared with control cells (mean expression score 0.52, <1%) p≤0.05 (Figure 7A, Figure 7B and Figure 10). TGF-β receptor was only present in a small number (<1%) of ATII cells in both the IPF (mean expression score 0.47) and control group (mean expression score 0.57) (Figure 7C and Figure 7D). TGF-β protein expression within fibroblastic foci was variable, with mean expression scores ranging from 0 to 5 (0% - >75%, Figure 11) TGF-P receptor levels were negligible within fibroblastic foci (mean expression score 1, <1%).
Figure 7.
Immunohistochemical analysis of IPF and control lung samples labelled for either TGF- β protein or TGF- β receptor. A. Representative histological image showing no cytoplasmic TGF-β protein immunoreactivity in control lung tissue. B. Cytoplasmic TGF-β protein is seen in ATII cells overlyingfibroblastic foci in a representative histological image of IPF lung tissue. C. TGF-β receptor immunoreactivity is not observed in control lung tissue. D. TGF-β receptor immunoreactivity can be seen in lymphocytes in IPF lung tissue. Magnification A-C x200, D x400.
Antigen Ki-67
Antigen Ki-67 expression was negligible in the nuclei of control lung ATII cells (mean expression score 0.21, <1%) (Figure 8A) compared to the IPF group where expression was significantly elevated (mean expression score 1.76, 1% -10%) p≤0.05 (Figure 8B and Figure 10). Antigen Ki-67 in IPF lung ATII cells was localised within areas of interstitial fibrosis away from fibroblastic foci (Figure 8B). There was no evidence of antigen Ki-67 expression in ATII cells directly overlying fibroblastic foci. Myofibroblasts within fibroblastic foci showed negligible antigen Ki-67 expression (mean expression score 0.23, <1%).
Figure 8.
Immunohistochemical analysis of IPF and control lungs for the cell proliferation marker antigen Ki-67. A. Representative histological image shows antigen Ki-67 is not observed in control lung tissue. B. A representative histological image of IPF lung tissue shows antigen Ki-67 immunoreactivity is observed in the nucleus of ATII cells at the edges of fibroblastic foci (arrow) but not in the ATII cells directly overlying the area. Magnification x200.
p16INK4A
p16INK4A expression was absent or negligible in ATII cells of control samples (mean expression score 0.52, <1%) (Figure 9A and Figure 10). In IPF tissue samples cytoplasmic p16INK4A expression was observed (mean expression score 4.61, >75%) in ATII cells directly overlying fibroblastic foci (Figure 9B). p16INK4A was expressed to a lesser extent in ATII cells away from these areas. Overall a significant increase in ATII cells expressing p16INK4A was noted in IPF samples p≤0.05. No expression of p16INK4A was identified within fibroblastic foci (Figure 11).
Figure 9.
Immunohistochemical analysis of IPF and control lungs for p16INK4A . A. p16INK4A reactivity is not observed in control lung tissue in this representative histological image. B. Cytoplasmic p16INK4A reactivity is seen in the ATII cells overlying fibroblastic foci (arrow) in a representative histological image of IPF lung tissue. Magnification x400.
Correlation of markers with disease activity
N-cadherin expression within ATII cells had a strong positive correlation with disease activity (Pearson correlation co-efficient 0.557). Ki-67 expression in ATII cells had a moderate negative correlation (Pearson correlation co-efficient -0.366). Within the fibroblastic foci we demonstrated moderate positive correlations for Twist (Pearson correlation co-efficient 0.402), Ki-67 (0.32) and Collagen I (Pearson correlation coefficient 0.468). All other markers showed weak or no correlation (Table 3).
Table 3.
Correlation of the expression levels of target markers and disease severity in ATII cells and fibroblastic foci. For cases where all expression scores were the same a Pearson correlation coefficient could not be calculated and is recorded as not applicable (n/a).
| Target Molecule | Pearson correlation coefficient | |
|---|---|---|
| Hyperplastic ATII cells | Fibroblastic Foci | |
| SP-C | -0.023 | n/a |
| E-cadherin | n/a | n/a |
| N-cadherin | 0.557 | n/a |
| Twist | n/a | 0.402 |
| α-SMA | 0.080 | n/a |
| Collagen I | 0.242 | 0.468 |
| TGF-β protein | n/a | 0.195 |
| TGF-β receptor | 0.181 | 0.292 |
| Antigen Ki-67 | -0.366 | 0.232 |
| p16INK4A | -0.156 | 0.225 |
Discussion
Fibroblastic foci represent the leading edge of IPF disease development, however, the source of these clusters of myofibroblasts remains to be elucidated with conflicting evidence as to the role of EMT [7, 17,18,22,31,32]. Histological analysis of ATII cells in IPF tissue showed E-cadherin, TGF-β and p16INK4A were highly expressed. Consistent expression of α-SMA, collagen I and Twist, together with varied TGF-β expression was observed within fibroblastic foci of IPF cases. Some of these markers correlated to disease status; significant, although patchy, upregulation of N-cadherin in IPF ATII cells demonstrated a strong positive correlation, with Ki-67 having a moderate negative correlation with disease activity. Expression of Twist, Ki-67 and collagen I within fibroblastic foci revealed a moderate positive correlation with disease status.
SP-C is a protein produced by functional ATII cells with a role in replacing damaged type I pneumocytes in an attempt to repair damaged alveolar epithelium. Previous reports have found genetic mutations in SP-C are found infrequently in IPF patients and rarely in cases of sporadic IPF [33]. This is the first report of reduced and varied SP-C expression observed throughout human IPF lung tissue samples. This variation may be linked to the maturity of the cell, as it appears the larger ATII cells secrete SP-C. The atrophic ATII cells, observed in the IPF group, may be transforming into type I pneumocytes following injury with the associated loss of SP-C production. When associated with increased p16INK4A levels and negligible antigen Ki-67 expression the varied SP-C expression is suggestive of a stress-induced senescence-like response in ATII cells in IPF. However we cannot rule out the possibility that these cells are undergoing EMT. These results corroborate findings from SP-C deficient mice, which were predisposed to the development of pulmonary fibrosis [34].
The level of N-cadherin expression was upregulated in IPF (particularly in 4 cases), substantiating previous reports where a heterogeneous pattern of N-cadherin expression was noted [18]. We also observed concomitant high levels of E-cadherin in hyperplasic ATII cells in all IPF cases, suggesting no significant loss of cell-cell adhesion in the IPF tissue at the time of biopsy, a finding also reported by Yamada et al [32].
Key markers of a myofibroblast phenotype (α-SMA and collagen I) were highly expressed in the fibroblastic foci; however negligible expression was detected in the cytoplasm of the overlying hyperplastic ATII cells in IPF cases. Taken together with E-cadherin and N-cadherin expression our findings suggest EMT may not be a key driver of IPF pathogenesis, but may be involved at an alternate time point, or play a prominent role in a subset of patients. This may explain the discrepancy in the literature with those that do and do not find EMT in IPF [7, 17,18,22,31,32].
This study also evaluated cell proliferation and senescence. An absence of proliferation in ATII cells in direct contact with areas of fibroblastic foci, combined with elevated cell stress marker p16INK4A and varied SP-C expression, suggests these cells could be undergoing cell-cycle arrest, a theory supported by previous work by Chilosi et al [35]. Myofibroblasts within the fibroblastic foci were not observed to be actively dividing, as substantiated by the presence Twist and minimal expression of antigen Ki-67. Proliferation of hyperplastic ATII cells away from areas of fibroblastic foci may suggest an attempt at lung repair to unknown micro-insults. We propose a theory, supported by the pattern of Ki-67 expression, that α-SMA expressing myofibroblasts may provide a scaffold for ATII cells, (dividing at the edge of the insult) to migrate over the foci surface, rather than transforming into myofibroblasts, via EMT. Increased TGF-β expression and lack of N-cadherin expression may lead to a contractile myofibroblast population within the fibroblastic foci [36]. These unbalanced signals could contribute to the production of abnormal mesenchymal components [35], and progressive architectural distortion of tissue remodelling in IPF.
Collagen I has been shown to promote EMT in non-small cell lung cancer lines via TGF-β signalling [22]. We observed significantly increased expression of TGF-β protein in the ATII cells of IPF cases, varied expression in the fibroblastic foci with negligible and equivocal expression of the TGF-β receptor in ATII cells of both the IPF and control group. Increased TGF-β protein may have multiple roles in the pathogenic development of IPF by promoting fibroblast to myofibro-blast differentiation [37], driving EMT via Smad proteins [9 31], or suppressing epithelial cell proliferation [37]. Our findings of N-cadherin expression in some ATII cells, resulting in loss of cell-cell adhesion, combined with consistent expression of TGF-β in ATII cells and varied expression in myofibroblasts supports a notion that myofibroblasts are invading through the alveolar basement wall via a TGF-β mediated mechanism.
Correlating target marker expression with histological disease status, as previously defined [2-4], has revealed novel putative markers for predicting disease activity and progression in IPF at the time of biopsy. It is logical that a negative correlation of the cell proliferation marker Ki-67 in ATII cells was linked with disease activity; this implies a decline in ATII cell proliferation in late stage disease and reduced capacity for lung repair. Twist and collagen I expression within the fibroblastic foci demonstrated a moderate positive correlation with disease activity. Twist inhibits proliferation of myofibroblasts within these foci, while it follows that a more rapidly progressing active disease would result in increased deposition of collagen I. A novel finding of this study was the strong correlation between increased ATII cell N-cadherin expression and increased disease activity. This would indicate that as disease progresses so does the possibility of EMT occurring, resulting in faster decline of lung function. A recent study identified Epstein-Barr virus (EBV), in conjunction with TGF-β protein, may induce EMT in cell culture studies of lung epithelial cells and notes that patients with EBV infection have a poorer prognosis [38]. The four cases demonstrating N-cadherin expression in ATII cells identifies a subgroup of IPF patients whose pathogenesis may be influenced by additional factors, such as EMT or viral infection that worsens prognosis.
Our results suggest that tissue remodelling in IPF is a complex processes involving multiple cellular and biological factors within a dynamic cascade in which EMT may be one component. Biopsy material represents a snapshot in the IPF lung and it maybe that to conclusively indicate EMT patients need to be sampled and monitored longitudinally over time. This is a major challenge in proving the existence of EMT in human IPF lung. Elucidating the origin of the myofibroblasts within the fibroblastic foci may help unravel critical targets for development of novel therapeutic agents. The association of N-cadherin to histological disease activity, combined with increased expression of markers for Twist and collagen I, and decreased expression of antigen Ki-67, may provide a useful panel for predicting the rate of disease progression in IPF patients. A larger study, including case note follow up, is now required to assess the predictive value of this panel via immunohistochemical analysis of biopsy samples.
Acknowledgments
We are grateful to Dr Daniel Gey van Pittius, consultant histopathologist at the University Hospital of North Staffordshire for his assistance with histological analysis. N Lomas is supported by a grant from the Institute of Biomedical Science and a Keele University Acorn Studentship.
References
- 1.Gribben J, Hubbard RB, Le-Jeune I, Smith CJ, West J, Tata LJ. Incidence and mortality of idiopathic pulmonary fibrosis and sarcoidosis in the UK. Thorax. 2006;61:980–985. doi: 10.1136/thx.2006.062836. 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nicholson AG, Fulford LG, Colby TV, du Bois RM, Hansell DM, Wells AU. The relationship between individual histologic features and disease progression in IPF. Am J Respir Crit Care Med. 2002;166:173–177. doi: 10.1164/rccm.2109039. 15. [DOI] [PubMed] [Google Scholar]
- 3.King TE, Jr, Schwarz MI, Brown K, Tooze JA, Colby TV, Waldron JA, Jr, Flint A, Thurlbeck W, Cherniak RM. Am J Respir Crit Care Med. 2001;164(6):1025–1032. doi: 10.1164/ajrccm.164.6.2001056. [DOI] [PubMed] [Google Scholar]
- 4.Barlo NP, van Moorsel CH, van den Bosch JM, Grutters JC. Predicting prognosis in idiopathic pulmonary fibrosis. Sarcoidosis Vasc Diffuse Lung Dis. 2010;27(2):85–95. [PubMed] [Google Scholar]
- 5.Maher TM, Wells AU, Laurent GJ. Idiopathic pulmonary fibrosis: multiple causes and mechanisms? Eur Respir J. 2007;30(5):835–839. doi: 10.1183/09031936.00069307. [DOI] [PubMed] [Google Scholar]
- 6.Phan SH. The myofibroblast in pulmonary fibrosis. Chest. 2002;122(6) suppl:286s–289s. doi: 10.1378/chest.122.6_suppl.286s. [DOI] [PubMed] [Google Scholar]
- 7.Lama VN, Phan SH. The extra pulmonary origin of fibroblasts: stem/progenitor cells and beyond. Proc Am Thorac Soc. 2006;3:373–376. doi: 10.1513/pats.200512-133TK. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hashimoto N, Phan SH, Imaizumi K, Matsuo M, Nakashima H, Kawabe T, Shimokata K, Hasegawa Y. Endothelial-mesenchymal transition in bleomycin-induced pulmonary fibrosis. Am J Respir Cell Mol Biol. 2010;43(2):161–172. doi: 10.1165/rcmb.2009-0031OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Willis BL, Liebler JM, Luby-Phelps K, Nicholson AG, Crandall ED, du Bois RM, Borok Z. Induction of epithelial-mesenchymal transition in alveolar epithelium cells by transforming growth factor-beta 1: potential role in idiopathic pulmonary fibrosis. Am J Pathol. 2005;166:1321–1332. doi: 10.1016/s0002-9440(10)62351-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kim KK, Kulger MC, Wolters PJ, Robillard L, Galvez MG, Brumwell AN, Sheppard D, Chapman HA. Alveolar epithelial cell mesenchymal transition develops in vivo during pulmonary fibrosis and is regulated by the extracellular matrix. Proc Natl Acad Sci U.S.A. 2006;103:13180–13185. doi: 10.1073/pnas.0605669103. 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hay ED. An overview of epithelial-mesenchymal transition. Act Anat (Basel) 1995;154:8–20. doi: 10.1159/000147748. [DOI] [PubMed] [Google Scholar]
- 12.De Wever O, Westbroek W, Verloes A, Bloemen N, Bracke M, Gespach C, Brugneel E, Mareel M. Critical role of N-cadherin in myofibroblast invasion and migration in vitro stimulated by colon-cancer-cell-derived TGF-β or wounding. J Cell Sci. 2004;117:4691–4703. doi: 10.1242/jcs.01322. 15. [DOI] [PubMed] [Google Scholar]
- 13.Willis BC, Borok Z. TGF-β induced EMT: mechanisms and implications for fibrotic lung disease. Am J Physiol Lung Cell Mol Physiol. 2007;10:1152. doi: 10.1152/ajplung.00163.2007. [DOI] [PubMed] [Google Scholar]
- 14.Potenta S, Zeisberg E, Kalluri R. The role of endothelial-to-mesenchymal transition in cancer progression. Br J Cancer. 2008;99:1275–1279. doi: 10.1038/sj.bjc.6604662. 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zeisberg Em, Tarnavski D, Zeisberd M, Dorfman AL, Mcmullen JR, Gustafsson E, Chandraker A, Yuan X, Pu WT, Roberts AB, Neilson EG, Sayegh MH, Izumo S, Kalluri R. Endothelial-to-mesenchymal transition contributes to cardiac fibrosis. Nat Med. 2007;13:952–961. doi: 10.1038/nm1613. 29. [DOI] [PubMed] [Google Scholar]
- 16.Zeisberg M, Yang C, Martino M, Duncan MB, Rieder F, Tanjore H, Kalluri R. Fibroblasts derive from hepatocytes in liver fibrosis via epithelial to mesenchymal transition. J Biol Chem. 2007;282:23337–23347. doi: 10.1074/jbc.M700194200. 10. [DOI] [PubMed] [Google Scholar]
- 17.Harada T, Nabeshima K, Hamasaki M, Uesugi N, Watanabe K, Iwasaki H. Epithelialmesenchymal transition in human lungs with usual interstitial pneumonia: quantitative immunohistochemistry. Pathol Int. 2010;60(1):14–21. doi: 10.1111/j.1440-1827.2009.02469.x. [DOI] [PubMed] [Google Scholar]
- 18.Pozhaeskaya V, Torres-Gonzalez E, Rojas M, Gal A, Amin M, Dollard S, Roman J, Stecenko AA, Mora AL. TWIST: A regulator of epithelial-mesenchymal transition in lung fibrosis. PLos ONE. 2009;4(10):e7559. doi: 10.1371/journal.pone.0007559. 23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sun S, Ning X, Zhang Y, Lu Y, Nie Y, Han S, Liu L, Du R, Xia L, He L, Fan D. Hypoxia-inducible factor-1 alpha induces Twist expression in tubular epithelial cells subjected to hypoxia, leading to epithelial-to-mesenchymal transition. Kidney Int. 2009;75(12):1278–1287. doi: 10.1038/ki.2009.62. [DOI] [PubMed] [Google Scholar]
- 20.Desmouliere A, Geinoz A, Gabbiani F. Transforming growth factor-beta 1 induces alpha -smooth muscle actin expression in granulation tissue myofibrob lasts and in quiescent and growing cultured fibroblasts. J Cell Biol. 1993;122(1):103–111. doi: 10.1083/jcb.122.1.103. 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bartram U, Speer CP. The role of transforming growth factor beta in lung development and disease. Chest. 2004;125(2):754–765. doi: 10.1378/chest.125.2.754. [DOI] [PubMed] [Google Scholar]
- 22.Shintani Y, Maeda M, Chaika N, Johnson KR, Wheelock MJ. Collagen 1 promotes epithelial-to-mesenchymal transition in lung cancer cells via Transforming Growth Factor-β signaling. Am J Respir Cell Mol Biol. 2008;38:95–104. doi: 10.1165/rcmb.2007-0071OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Raghu G, Collard HR, Egan JJ, Martinez FJ, Behr J, Brown KK, Colby TV, Cerdier JF, Flaherty KR, Lasky JA, Lynch DA, Ryu JH, Swigriss JJ, Wells AU, Ancochea J, Bouros D, Carvalho C, Costabel U, Ebina M, Hansell DM, Johkah T, Kim DS, King TE, Jr, Kondoh Y, Myers J, Mülliner NL, Nicholson AG, Richeldi L, Selman M, Dudden RF, Griss BS, Protzko SL, Schünemann HJ. An official ATS/ERS/JRS/ ACAT statement: Idiopathic Pulmonary Fibrosis: Evidence-based guidelines for diagnosis and management. Am J Respir Crit Care Med. 2011;183:788–824. doi: 10.1164/rccm.2009-040GL. 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhou L, Lim L, Costa RH, Whitsett JA. Thyroid transcription factor-1 hepatocyte nuclear fctor-3 beta, surfactant protein B, C and Clara cells secretory protein in developing mouse lung. Am J Pathol. 1994;145:114–125. doi: 10.1177/44.10.8813084. [DOI] [PubMed] [Google Scholar]
- 25.Godin I, Whlie CC. TGF beta 1 inhibits proliferation and has a chemotropic effect on mouse primordial germ cells in culture. Development. 1991;113(4):1451–1457. doi: 10.1242/dev.113.4.1451. [DOI] [PubMed] [Google Scholar]
- 26.Zhang K, Rekhter MD, Gorden D, Phan SH. Myofibroblasts and their role in lung collagen gene expression during pulmonary fibrosis: A combined immunohistochemical and in situ hybridization study. Am J Pathol. 1994;145:114–125. [PMC free article] [PubMed] [Google Scholar]
- 27.Bullwinkel J, Baron-Luhr B, Ludemann A, Wohlenberg C, Gerdes J, Scholzen J. Ki67 protein is associated with ribosomal RNA transcription in quiescent and proliferating cells. J Cell Physiol. 2006;206:624–635. doi: 10.1002/jcp.20494. [DOI] [PubMed] [Google Scholar]
- 28.Serrano M, Hannon GJ, Beach D. A new regulatory motif in cell-cycle control causing specific inhibition of cyclin D/CDK4. Nature. 1993;336:704–707. doi: 10.1038/366704a0. 13. [DOI] [PubMed] [Google Scholar]
- 29.Harvey J M, Clark GM, Allred DC. Estrogen receptor status by immunohistochemistry is superior to the ligand-binding assay for predicting response to adjuvant endocrine therapy in breast cancer. J Clin Oncol. 1999;17(5):1474–1481. doi: 10.1200/JCO.1999.17.5.1474. [DOI] [PubMed] [Google Scholar]
- 30.Cohen J. Statistical power analysis for the behavioral sciences. New Jersey: Lawrence Erlbaum Ass; 1988. [Google Scholar]
- 31.Kim JH, Jang YS, Eom KS, Hwang Y II, Kang HR, Jang SH, Kim CH, Park YB, Lee MG, Hyun IG, Jung K, Kim D. Transforming growth factor β1 induces epithelial-to-mesenchymal transition of A549 cells. J Korean Med Sci. 2007;22:898–904. doi: 10.3346/jkms.2007.22.5.898. 31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yamada M, Kuwano K, Maeyama T, Hamada N, Yoshimi M, Nakanishi Y, Kasper M. Dual immunohistochemistry provides little evidence for epithelial-mesenchymal transition in pulmonary fibrosis. Histochem Cell Biol. 2008;129:453–462. doi: 10.1007/s00418-008-0388-9. [DOI] [PubMed] [Google Scholar]
- 33.Lawson WE, Grant SW, Ambrosini V, Womble KE, Dawson EP, Lane KB, Markin C, Renzoni E, Lymphany E, Thomas AQ, Roldon J, Scott TA, Blackwell TS, Phillips JA, 3rd, Loyd JE, du Bois RM. Genetic mutations in surfactant protein C are a rare cause of sporadic cases of IPF. Thorax. 2004;59:977–980. doi: 10.1136/thx.2004.026336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lawson WE, Polosukhin VV, Stathopoulos GT, Zoia O, Han W, Lane KB, Li B, Donnelley EF, Holburn GE, Lewis KG, Collins RD, Hull WM, Glasser SW, Whitsett JA, Blackwell TS. Increased and prolonged pulmonary fibrosis in surfactant protein C-deficient mice following intratrachealbleomycin. Am J Pathol. 2005;167:1267–1276. doi: 10.1016/S0002-9440(10)61214-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chilosi M, Poletti V, Murer B, Lestani M, Montagna L, Piccoli P, Pedron S, Bertaso M, Scarpa A, Murer B, Cancellieri A, Maestro R, Semenzato G, Dogliono C. Abnormal re-repithelialization and lung remodeling in Idio-pathic Pulmonary Fibrosis: The role of ΔN-p63. Lab Invest. 2002;82:1335–1345. doi: 10.1097/01.lab.0000032380.82232.67. [DOI] [PubMed] [Google Scholar]
- 36.Hinz B, Pittet P, Smith-Clerc J, Chaponnier C, Meister J-J. Myofibroblast development is characterized by specific cell-cell adherens junctions. Mol Biol Cell. 2004;15:4310–4320. doi: 10.1091/mbc.E04-05-0386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chambers RC, Leoni P, Kaminski N, Laurent GJ, Heller RA. Global expression profiling of fibroblast responses to transforming growth factor-beta1 reveals the induction of inhibitor of differentiation-1 and provides evidence of smooth muscle cell phenotype switching. Am J Pathol. 2003;162:533–546. doi: 10.1016/s0002-9440(10)63847-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sides MD, Klingsberg RC, Shan B, Gordon KA, Nquyen HT, Lin Z, Takahashi T, Flemmington EK, Lasky JA. The Epstein-Barr virus latent membrane protein 1 and transforming growth factor-β1 synergistically induce epithelial-mesenchymal transition in lung epithelial cells. Am J Respir Cell Mol Biol. 2011;44:852–862. doi: 10.1165/rcmb.2009-0232OC. [DOI] [PMC free article] [PubMed] [Google Scholar]