Abstract
We present a case of a 70-year-old HIV negative man with a five-year history of progressive dysnomia and new onset right extremity numbness, dysarthria, and blurry vision. On magnetic resonance imaging (MRI), an infiltrative enhancing tumor was noted. Follow up brain biopsy results revealed a small lymphocytic infiltrate with scattered plasma cells in a predominantly perivascular growth pattern. Flow-cytometric findings revealed a lambda monotypic B-cell population. The morphology and the flow cytometric findings were consistent with involvement by a low grade B-cell lymphoma. Subsequent positron emission tomography (PET) studies along with bone marrow biopsy and serum protein electrophoresis showed no evidence of systemic disease. The above findings are consistent with involvement by a non-dural extranodal marginal zone B-cell lymphoma (MZBCL) primary to the central nervous system (CNS). This is the first reported case of a primary CNS MZBCL with flow cytometric analysis. A review of literature on this rare entity is also included.
Keywords: Primary Non-Dural Central Nervous System Low grade B-cell lymphoma, extranodal marginal zone lymphoma
Introduction
Primary central nervous system lymphomas (PCNSL) are rare non-Hodgkin lymphomas that can be found in the brain, leptomeninges, eyes, or spinal cord, and are mostly intracerebral. PCNSLs constitute 3-4% of primary brain tumors, and most cases are diffuse large B-cell lymphomas (DLBCL) [1]. PCNSLs in HIV patients are associated with EBV co-infection [2]. Interestingly, the incidence of PCNSL has been increasing at a rate higher than peripheral non-Hodgkin lymphomas (NHL) and glial tumors [3].
Excluding dural lymphomas, low-grade PCNSLs are extremely rare entities that may be less aggressive compared to their DLBCL counterparts. Although therapy for these entities is not well defined, less aggressive therapy may be sufficient to control low-grade PCNSL [3]. Hence, clinical awareness of this entity and rendering an accurate diagnosis is essential.
Materials and methods
After procurement of a small portion of the fresh excised tissue for flow cytometric analysis (see below), the remaining tissue was fixed in 10% neutral formalin, embedded in paraffin and stained with hematoxylin-eosin (H&E). Immunohistochemistry was performed on the tissue sections using the following antibodies: kappa (DAKO), lambda (DAKO), Ki67 (Ventana), BCL2 (DAKO), CD3 (LEICA), CD5 (LEICA), C10 (LEICA), CD20 (DAKO), CD138 (DAKO), CyclinD1 (BIOCARE), CD38 (VENTANA), CD56 (VENTANA), CD23 (VENTANA), IgA (CELLMARQUE), IgD (CELLMARQUE), IgG (CELLMARQUE), or IgM (CELLMARQUE). Ki67, BCL2, CD3, CD5, CD10, CD20, CD138, CyclinD1, CD38, CD56, and CD23 are monoclonal antibodies. Kappa, lambda, IgA, IgD, IgG, and IgM are polyclonal.
For the flow cytometry study, the tissue was macerated and exposed to flow buffer NH4CI (Acros Organics), Potassium Bicarbonate (Sigma), Ethylenediamine and Tetra sodium salt (Sigma). Cell suspensions were centrifuged, suspended in flow buffer, and added to individual tubes with different antibodies. Four color analysis was performed by flow cytometry (FC; BD FacsCalibur) with the following antibodies: CD45, CD5, CD10, CD19, CD20, CD23, CD38, kappa, lambda both surface and cytoplasmic (BD biosciences).
Clinical history
The patient is a 70-year-old HIV negative male with a five-year history of progressively worsening dysnomia. A few months prior to admission, optometristic examination revealed borderline nerve cupping in the left eye. At admission, he presented with right arm and leg numbness, slurred speech and blurry vision. His past medical history includes bilateral hearing loss which he attributed to work-related exposure to airplane jet engines, and left facial cellulitis and abscess.
The neurological examination demonstrated bilaterally intact cranial nerves, normal motor strength and sensation, as well as a normal gait. The patient had dysnomia in addition to slow and dysarthric speech with normal comprehension and naming. MRI imaging of the brain revealed a 3.5 × 2.9 cm infiltrative enhancing tumor centered in the left posterior putamen, left posterior subinsular region, and adjacent left mid temporal region with extensive associated vasogenic edema involving most of the left temporal lobe white matter and subcentimeter cysts (Figure 1). Mild mass effect on the left ventricle and a 2-mm left to right midline shift was also noted. Computed tomography (CT) of the thorax, abdomen and pelvis showed no evidence of malignancies.
Figure 1.
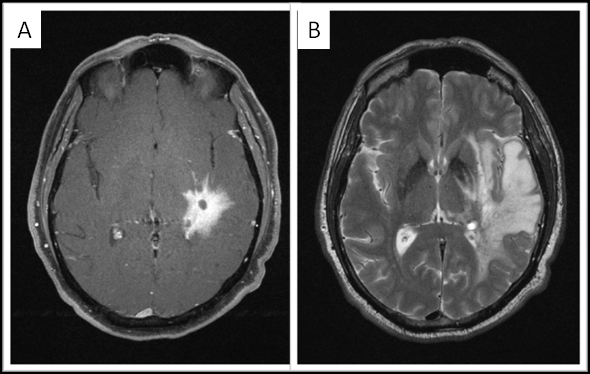
3 Tesla MRI imaging of the brain. A. Axial fat-saturated postcontrast Tl-weighted image shows the 3.5 × 2.9 cm infiitrative enhancing tumor centered in the left posterior putamen, left posterior subinsular region, and adjacent left mid temporal region. B. Axial T2-weighted image shows extensive associated vasogenic edema, with relatively little mass effect.
A postsurgical PET scan did not demonstrate malignant lymphadenopathy. An ultrasound of the scrotum demonstrated only bilateral varicoceles. Repeat MRI two years after the diagnosis demonstrated stable enhancing tumor consistent with the diagnosed malignancy.
A follow up frozen section biopsy was performed, which demonstrated an infiltrate of small mature-looking lymphocytes in a perivascular configuration. Histologic examination of permanent H&E sections showed a multifocal infiltrate comprised of small to intermediatesized lymphocytes with surrounding plasma cells mainly in an angiocentric distribution with associated background reactive astrocytes and Rosenthal fibers (Figure 2). The lymphocytic component was comprised of small to intermediate-sized cells with some nuclear membrane irregularities, moderately dispersed chromatin and inconspicuous nucleoli. Some of the lymphocytes showed an increased amount of cytoplasm and monocytoid features. Additionally, necrosis or increase in mitotically active cells were not noted. Also seen were numerous bland plasma cells, some with Russell bodies, but no definite Dutcher bodies were detected. The scattered background plasma cells showed no definite immature features and binucleate or multinucleated forms were not identified. On immunohistochemistry the abnormal small to intermediate-sized lymphocytes were positive for CD20 (Figure 2) and PAX-5 (consistent with a B-cell lineage) with subset expressing BCL2 but lacking CD5, CD10, CD23, and cyclin D1 expression (not shown). Additionally, a subset of these lymphocytes expressed IgM and IgA with a minor subpopulation that was IgD positive (not shown). Concordant with the morphology, the B-cell population showed a Ki-67 proliferation index of less than 10% (Figure 2), compatible with a low grade lymphoproliferative B-cell neoplasm. The plasma cells were positive for plasma cell markers (CD38 and CD138, not shown) with an excess expression of lambda light chains (Figure 2).
Figure 2.
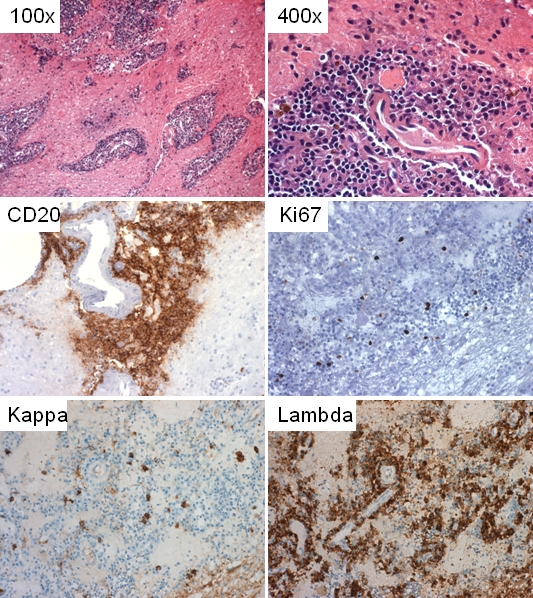
Microscopic and selected immunohistochemicai features. Multifocal and angiocentric lymphoplasmacytic infiltrate at 1OOx and 400x; CD20 reactive lymphocytes; There is lambda light chain restriction; Less than 10% of the cells are reactive with Ki67.
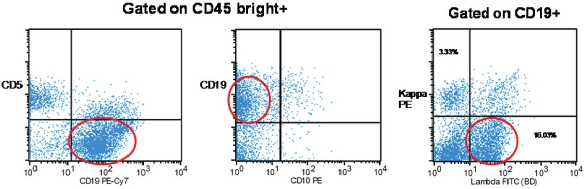
Flow cytometry of the fresh specimen showed 52% lymphoid cells. Fifty eight percent of these (24% of the total cells) were B-cells that expressed CD19 and were lambda restricted (kappa:kambda ratio of 1:5) (Figure 3). These cells were also positive for CD20 (not shown). Similar to what was noted by immunohistochemistry, these cells did not express CD5, CD10 (Figure 3) or CD23 (not shown). A population of CD45 positive, CD38 bright positive, CD19 negative plasma cells with lambda excess (kappa:lambda ratio of 1:2) was also noted by flow cytometry (not shown). Follow-up cytogenetic analysis on the specimen showed a normal male karyotype (46, XY). Further molecular studies were not done due to paucity of material.
Figure 3.
Flow cytometry. Monotypic CD19 positive B-cells that have a kappa:lambda ratio of 1:5 (Note: Lambda restricted B-cells are highlighted within the red circle).
The patient's stereotactic biopsy results along with the flow cytometry findings are consistent with involvement by a low grade B-cell lymphoma. Given the immunophenotype by flow cytometry and immunohistochemistry, the differential diagnosis included extranodal marginal zone lymphoma primary to the CNS and lymphoplasmacytic lymphoma (LPL) secondarily involving the CNS. Subsequent serum protein electrophoresis and immunofixation electrophoresis showed no evidence of a clonal population. Follow up bone marrow biopsy showed no evidence of lymphoma. The above findings and the PET results were not in favor of secondary involvement by a LPL. Hence, this lesion is best characterized as an extranodal MZBCL primary to the CNS.
The patient was started on dexamethasone, temozolamide and rituximab. On two year follow up, his symptoms remained unchanged, and repeat MRI showed no change in the residual tumor.
Discussion
The most common PCNSLs are DLBCLs which are mostly intraparenchyma I [4]. Of note, the low grade B-cell lymphomas of the CNS typically involve the dura and spare the cerebral tissue [4]. Our case is very rare in that there was no dural involvement and the lesion was exclusively intraparenchymal. Furthermore, this low grade B-cell PCNSL presented in an immunocompetent patient. The vast majority of PCNSLs in immunocompetent patients are diffuse large B-cell lymphomas [5]. Non-DLBCLs PCNSLs in the immunocompetent population are very rare and consist of T-cell lymphomas and low grade B-cell lymphomas [6].
The morphologic and immunohistochemical findings in our case were initially consistent with a low grade B-cell lymphoma with a differential diagnosis that included a non-dural primary CNS MZBCL or secondary involvement by a LPL. However, in the absence of bone marrow involvement, negative PET scan, and no clinical history of Waldenstrom's macroglobulinemia or clonal immunoglobulins by serum protein electrophoresis and immunofixation electrophoresis, involvement by LPL is unlikely and the findings are most consistent with a non-dural” primary CNS Mucosa Associated Lymphoid Tissue (MALT) lymphoma (MZBCL).
The largest case series of CNS low grade lymphomas reported forty cases which include eight T-cell lymphomas and thirty two B-cell lymphomas. The low grade B-cell lymphomas described in this series were predominantly non-classifiable small lymphomas but eleven cases of LPL and one case of follicular lymphoma were noted [7]. We have also reviewed thirteen other reported cases and small case series of “non-dural” Primary CNS low grade B-cell lymphomas in which the diagnoses included (per the authors reports) three “lymphoplasmacytoid, immunocytoma, pleomorph", one “lymphoplasmacytoid", one “diffuse B cell malignant non-hodgkin lymphoma, lymphoplasmacytoid type Wandenstrom like", one small lymphocytic B-cell lymphoma, three lymphoplasmacytic lymphomas, four MALT lymphomas, and one unclassified low grade B-cell lymphoma [4, 6, 8-14] (Table 1). Cumulatively, there was no gender predilection noted in the above reported cases and the age range was 5-78 years. Unfortunately, it is impossible to draw reliable conclusions regarding the frequency of these non-dural based low grade B -cell lymphoma subtypes mainly because of the small number of cases, the use of old diagnostic criteria (pre-WHO 2008 classification criteria), and the unknown HIV status of many of these patients.
Table 1.
Case reports and case series of patients reported with PCNSL without dural involvement
| Reference | Demographics | Location | Diagnosis | HIV |
|---|---|---|---|---|
| Jahnke et al 2006 | F(n=22)/M(n=18): Age range 19-78 | Multiple | 8 T-cell lymphomas and thirty two B-cell lymphomas including 20 non-classifiable, 11 LPL, 1 follicular lymphoma (grade 1) | Negative |
| Bogdahn et al 1986 | M/60 | "Pontocerebellar edema" | "Unclassified low grade" | Unknown |
| F/60 | Left parietal | "lymphoplasmacytoid immunocytoma pleomorph" | Unknown | |
| F/57 | Right cerebellar | "lymphoplasmacytoid immunocytoma pleomorph" | Unknown | |
| M/68 | Left pontocerebellar | "lymphoplasmacytoid immunocytoma pleomorph" | Unknown | |
| F/27 | Diffuse | "Lymphoplasmacytoid" | Unknown | |
| Mikol et al 1987 | M/59 | Frontocallosal | "Diffuse B cell malignant non-hodgkin lymphoma, lymphoplasmacytoid type Wandenstrom like" | Negative |
| Kai Y et al 1998 | M/5 | Left cerebellum | Small lymphocytic B-cell lymphoma | Unknown |
| Braks et al 2000 | F/42 | Right lateral ventricle | LPL* | Unknown |
| Itoh 2001 | F/28 | CP angle | MZBCL* | Unknown |
| Shenkier et al 2005 | F/47 | Corpus callosum, basal ganglia, thalamus, internal capsule | LPL* | Unknown |
| Tu et al 2005 | M/66 | R frontal cortex | MZBCL* | Unknown |
| Park et a1 2008 | M/18 | Left basal ganglia | MZBCL* | Negative |
| Carrasco et al 2010 | F/49 | Pituitary | LPL* | Negative |
| Papanicolau et al 2011 | M/70 | left posterior putamen, posterior subinsular region, and adjacent mid-temporal region | MZBCL* | Negative |
MZBCL (Marginal Zone B-Cell Lymphoma); LPLflymphoplasmacytic Lymphoma)
For low grade lymphomas involving the CNS, MALT lymphoma and LPL constitute the majority of cases in immunocompetent patients. MALT lymphoma and LPL may have a similar appearance and can be characterized by malignant mature lymphocytes in addition to background plasma cells or plasmacytoid cells and may also share a very similar immunophenotypic profile. In contrast to MALT lymphoma, LPLs typically involve the bone marrow and may be seen in association with Waldenstrom's macroglobulinemia [15]. It has been theorized that MALT-type tissue may be formed under inflammatory conditions within CNS, providing a site for the development of MALT-lymphoma [4].
Considering the limited amount of brain tissue available, it was fortuitous in our case that there was enough tissue available for a flow cytometric study which facilitated the diagnosis of this low grade B-cell lymphoma. Therefore if possible, when confronted with similar morphologic findings (during an intraoperative evaluation) as in this case, a follow up flow cytometry can be very helpful in distinguishing a reactive process from a neoplastic one. This case emphasizes the utility of intraoperative morphologic evaluation of CNS tumors, and the need for additional studies such as flow cytometry to facilitate the diagnosis of unusual entities such as this non-dural based CNS MALT lymphoma. Additionally, differentiating between a low grade CNS B-cell lymphoma from a DLBCL or a reactive phenomenon is essential both from a therapeutic and prognostic standpoint.
References
- 1.Commins DL. Pathology of primary central nervous system lymphoma. Neurosurg Focus. 2006;21:E2. doi: 10.3171/foc.2006.21.5.3. [DOI] [PubMed] [Google Scholar]
- 2.Camilleri-Broët S, Davi F, Feuillard J, Seilhean D, Michiels JF, Brousset P, Epardeau B, Navratil E, Mokhtari K, Bourgeois C, Marelle L, Raphäel M, Hauw JJ. AIDS-related primary brain lymphomas: histopathologic and immunohistochemical study of 51 cases. The French Study Group for HIV-Associated Tumors. Hum Pathol. 1997;28:367–374. doi: 10.1016/s0046-8177(97)90137-4. [DOI] [PubMed] [Google Scholar]
- 3.Razaq W, Goel A, Amin A, Grossbard ML. Primary central nervous system mucosa-associated lymphoid tissue lymphoma: case report and literature review. Clin Lymphoma Myeloma. 2009;9:E5–9. doi: 10.3816/CLM.2009.n.052. [DOI] [PubMed] [Google Scholar]
- 4.Tu PH, Giannini C, Judkins AR, Schwalb JM, Burack R, O'Neill BP, Yachnis AT, Burger PC, Scheithauer BW, Perry A. Clinicopathologic and genetic profile of intracranial marginal zone lymphoma: a primary low-grade CNS lymphoma that mimics meningioma. J Clin Oncol. 2005;23:5718–5727. doi: 10.1200/JCO.2005.17.624. [DOI] [PubMed] [Google Scholar]
- 5.Camilleri-Broët S, Martin A, Moreau A, Angonin R, Hénin D, Gontier MF, Rousselet MC, Caulet-Maugendre S, Cuillière P, Lefrancq T, Mokhtari K, Morcos M, Broët P, Kujas M, Hauw JJ, Desablens B, Raphaël M. Primary central nervous system lymphomas in 72 immunocompetent patients: pathologic findings and clinical correlations. Groupe Ouest Est d'étude des Leucénies et Autres Maladies du Sang (GOELAMS) Am J Clin Pathol. 1998;110:607–612. doi: 10.1093/ajcp/110.5.607. [DOI] [PubMed] [Google Scholar]
- 6.Shenkier TN. Unusual variants of primary central nervous system lymphoma. Hematol Oncol Clin North Am. 2005;19:651–664. doi: 10.1016/j.hoc.2005.05.001. [DOI] [PubMed] [Google Scholar]
- 7.Jahnke K, Korfel A, O'Neill BP, Blay JY, Abrey LE, Martus P, Poortmans PM, Shenkier TN, Batchelor TT, Neuwelt EA, Raizer JJ, Schiff D, Pels H, Herrlinger U, Stein H, Thiel E. International study on low-grade primary central nervous system lymphoma. Ann Neurol. 2006;59:755–762. doi: 10.1002/ana.20804. [DOI] [PubMed] [Google Scholar]
- 8.Bogdahn U, Bogdahn S, Mertens HG, Dommasch D, Wodarz R, Wünsch PH, Kühl P, Richter E. Primary non-Hodgkin's lymphomas of the CNS. Acta Neurol Scand. 1986;73:602–614. doi: 10.1111/j.1600-0404.1986.tb04607.x. [DOI] [PubMed] [Google Scholar]
- 9.Mikol J, Wassef M, Galian A, Woimant F, Thurel C. Primary malignant non-Hodgkin's lymphomas of the central nervous system. Report of two cases with a predominant callosal localisation. In: Chatel M, Darcel F, Pecker J, editors. Brain Oncology. Dordrecht: Martinus Nijhoff Publishers; 1987. pp. 257–266. [Google Scholar]
- 10.Kai Y, Kuratsu J, Ushio Y. Primary malignant lymphoma of the brain in childhood. Neurol Med Chir. 1998;38:232–237. doi: 10.2176/nmc.38.232. [DOI] [PubMed] [Google Scholar]
- 11.Braks E, Urbach H, Pels H, Träber F, Block W, Schild HH. Primary central nervous system immunocytoma: MRI and spectroscopy. Neuroradiology. 2000;42:738–741. doi: 10.1007/s002340000392. [DOI] [PubMed] [Google Scholar]
- 12.Itoh T, Shimizu M, Kitami K, Kamata K, Mitsumori K, Fujita M, Ohnishi A, Nagashima K. Primary extranodal marginal zone B-cell lymphoma of the mucosa-associated lymphoid tissue type in the CNS. Neuropathology. 2001;21:174–180. doi: 10.1046/j.1440-1789.2001.00392.x. [DOI] [PubMed] [Google Scholar]
- 13.Park I, Huh J, Kim JH, Lee SW, Ryu MH, Kang YK. Primary central nervous system marginal zone B-cell lymphoma of the Basal Ganglia mimicking low-grade glioma: a case report and review of the literature. Clin Lymphoma Myeloma. 2008;8:305–308. doi: 10.3816/CLM.2008.n.043. [DOI] [PubMed] [Google Scholar]
- 14.Carrasco CA, Rojas ZD, Chiorino R, Gonzalez G. Primary pituitary lymphoma in immunocompetent patient: diagnostic problems and prolonged follow-up. Pituitary. 2010 doi: 10.1007/s11102-010-0219-6. [DOI] [PubMed] [Google Scholar]
- 15.Owen RG, Treon SP, Al-Katib A, Fonseca R, Greipp PR, McMaster ML, Morra E, Pangalis GA, San Miguel JF, Branagan AR, Dimopoulos MA. Clinicopathological definition of Waldenstrom's macroglobulinemia: consensus panel recommendations from the Second International Workshop on Waldenstrom's Macroglobulinemia. Semin Oncol. 2003;30:110–115. doi: 10.1053/sonc.2003.50082. [DOI] [PubMed] [Google Scholar]