Abstract
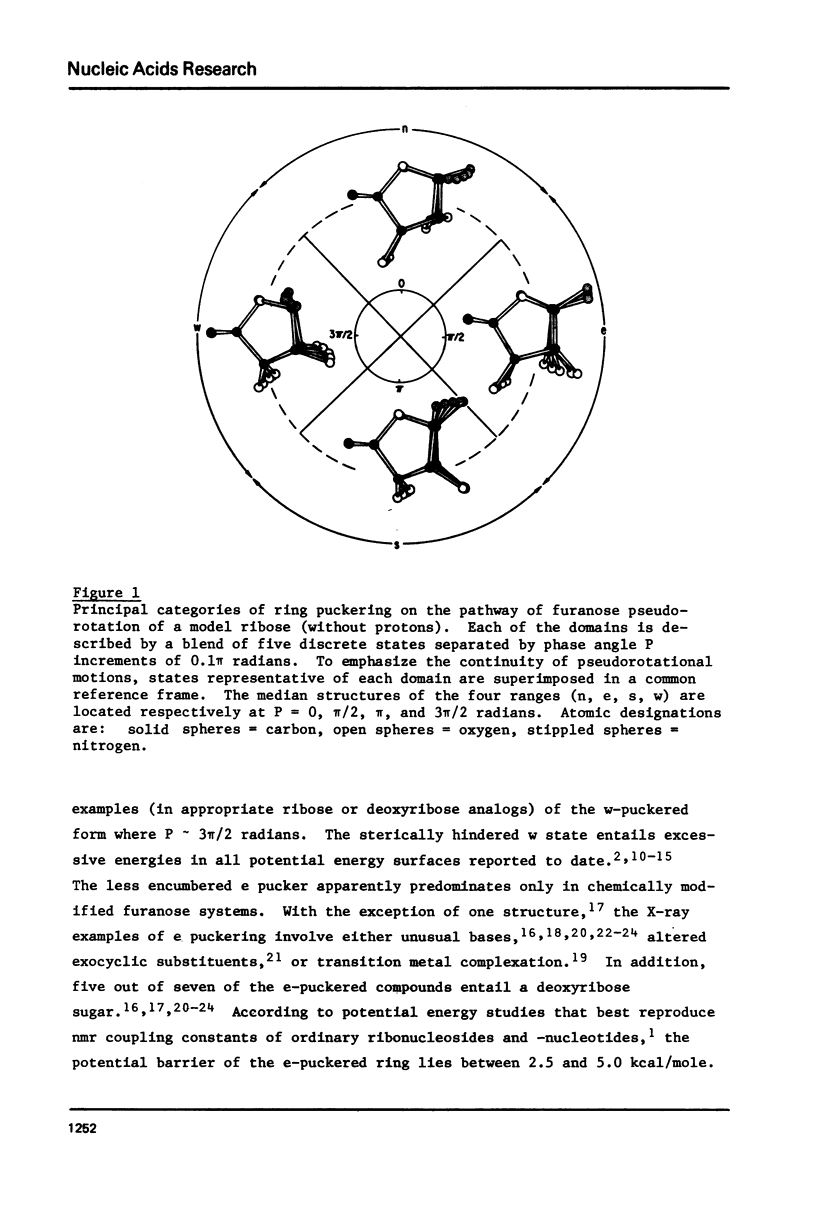
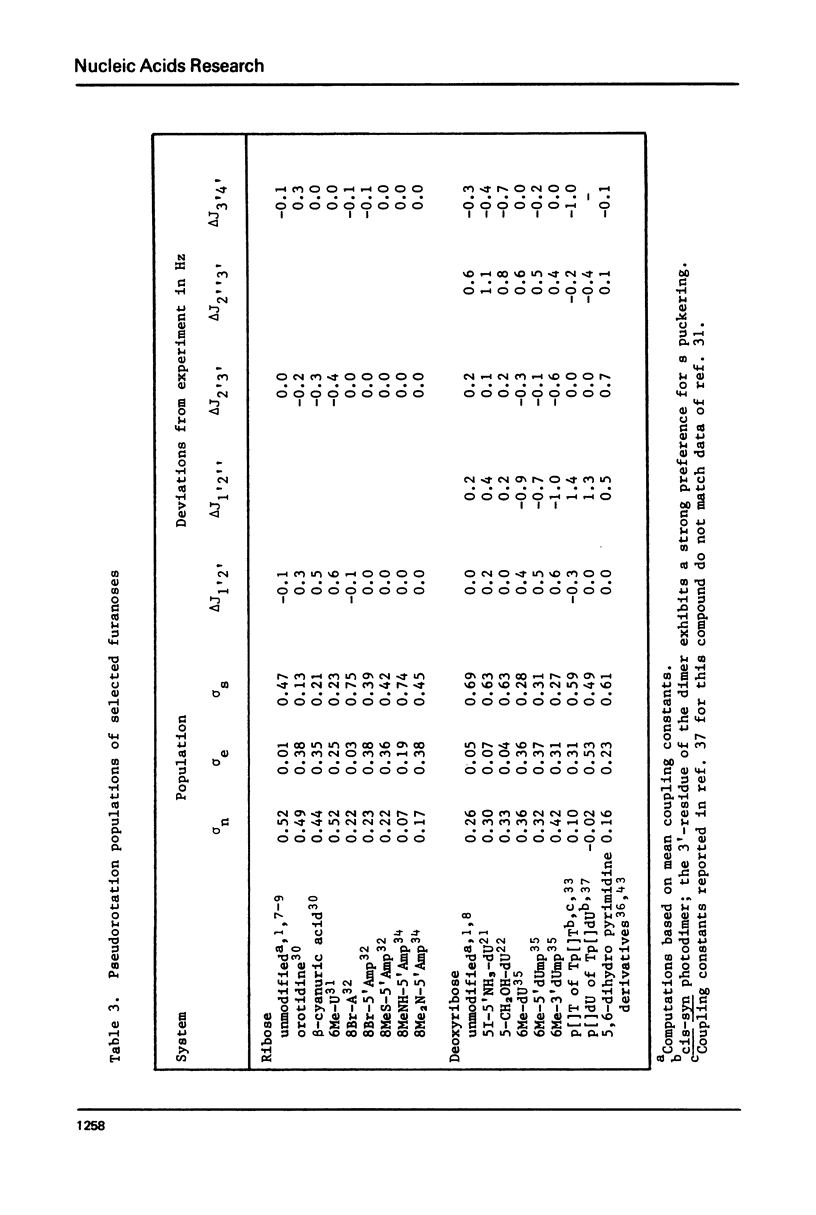
A general procedure is described to treat the pseudorotation of the furanose ring in terms of a three-state conformational equilibrium. In addition to the principal n (C3'-endo) and s (C2'-endo) puckering domains, the unusual e (01'-endo) intermediate is included in the analysis. Each of these three conformational categories is represented by a blend of five closely related puckered forms rather than by a single rotational isomeric state. Using this model together with experimentally measured nmr coupling constants, the puckering populations of various nucleic acid analogs are estimated. The conventional two-state n/s equilibria is confirmed in ordinary ribose and deoxyribose systems. The e domain, however, is found to be of major importance in several chemically modified furanoses including certain pyrimidine deoxynucleosides damaged by radiation and various nucleosides and nucleotides forced by bulky substituents on the base into unusual syn glycosyl arrangements. The "free" pseudorotation of these modified systems is not detected by conventional two-state puckering analyses.
Full text
PDF









Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Altona C., Sundaralingam M. Conformational analysis of the sugar ring in nucleosides and nucleotides. A new description using the concept of pseudorotation. J Am Chem Soc. 1972 Nov 15;94(23):8205–8212. doi: 10.1021/ja00778a043. [DOI] [PubMed] [Google Scholar]
- Altona C., Sundaralingam M. Conformational analysis of the sugar ring in nucleosides and nucleotides. Improved method for the interpretation of proton magnetic resonance coupling constants. J Am Chem Soc. 1973 Apr 4;95(7):2333–2344. doi: 10.1021/ja00788a038. [DOI] [PubMed] [Google Scholar]
- Cadet J., Ducolomb R., Hruska F. E. Proton magnetic resonance studies of 5,6-saturated thymidine derivatives produced by ionizing radiation. Conformational analysis of 6-hydroxylated diastereoisomers. Biochim Biophys Acta. 1979 Jun 20;563(1):206–215. doi: 10.1016/0005-2787(79)90021-2. [DOI] [PubMed] [Google Scholar]
- Cheng D. M., Sarma R. H. Intimate details of the conformational characteristics of deoxyribodinucleoside monophosphates in aqueous solution. J Am Chem Soc. 1977 Oct 26;99(22):7333–7348. doi: 10.1021/ja00464a038. [DOI] [PubMed] [Google Scholar]
- Cross B. P., Schleich T. Determination of the molecular conformation of beta-D-O2,2'-cyclouridine in aqueous solution by proton magnetic resonance spectroscopy. Biopolymers. 1973;12(10):2381–2389. doi: 10.1002/bip.1973.360121016. [DOI] [PubMed] [Google Scholar]
- Davies D. B., Danyluk S. S. Nuclear magnetic resonance studies of 5'-ribo- and deoxyribonucleotide structures in solution. Biochemistry. 1974 Oct 8;13(21):4417–4434. doi: 10.1021/bi00718a027. [DOI] [PubMed] [Google Scholar]
- Evans F. E., Kaplan N. O. 8-Alkylaminoadenyl nucleotides as probes of dehydrogenase interactions with nucleotide analogs of different glycosyl conformation. J Biol Chem. 1976 Nov 10;251(21):6791–6797. [PubMed] [Google Scholar]
- Govil G., Fisk C., Howard F. B., Miles H. T. Structure of poly 8-bromoadenylic acid; conformational studies by CPF energy calculations. Nucleic Acids Res. 1977 Aug;4(8):2573–2592. doi: 10.1093/nar/4.8.2573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howard F. B., Frazier J., Miles H. T. Poly(8-bromoadenylic acid): synthesis and characterization of an all-syn polynucleotide. J Biol Chem. 1975 May 25;250(10):3951–3959. [PubMed] [Google Scholar]
- Hruska F. E. Molecular conformation of orotidine, a naturally occurring nucleoside, in the syn conformation in aqueous solution. J Am Chem Soc. 1971 Apr 7;93(7):1795–1797. doi: 10.1021/ja00736a046. [DOI] [PubMed] [Google Scholar]
- Konnert J., Karle I. L., Karle J. The structure of dihydrothymidine. Acta Crystallogr B. 1970 Jun 15;26(6):770–778. doi: 10.1107/s0567740870003102. [DOI] [PubMed] [Google Scholar]
- Lee C. H., Sarma R. H. Aqueous solution conformation of rigid nucleosides and nucleotides. J Am Chem Soc. 1976 Jun 9;98(12):3541–3548. doi: 10.1021/ja00428a026. [DOI] [PubMed] [Google Scholar]
- Liu F. T., Yang N. C. Photochemistry of cytosine derivatives. 1. Photochemistry of thymidylyl-(3' leads to 5')-deoxycytidine. Biochemistry. 1978 Nov 14;17(23):4865–4876. doi: 10.1021/bi00616a003. [DOI] [PubMed] [Google Scholar]
- Lugovskoi A. A., Dashevskii V. G. Conformations of -D-ribose. Mol Biol. 1972 May-Jun;6(3):354–360. [PubMed] [Google Scholar]
- Narayanan P., Berman H. M. A crystallographic determination of a chemical structure: 6-amino-10-(beta-D-ribofuranosylamino)pyrimido-[5,4-d]pyrimidine, an example of an unusual D-ribose conformation. Carbohydr Res. 1975 Nov;44(2):169–180. doi: 10.1016/s0008-6215(00)84161-0. [DOI] [PubMed] [Google Scholar]
- Nelson J. H., Grunberger D., Cantor C. R., Weinstein I. B. Modification of ribonucleic acid by chemical carcinogens. IV. Circular dichroism and proton magnetic resonance studies of oligonucleotides modified with N-2-acetylaminofluorene. J Mol Biol. 1971 Dec 14;62(2):331–346. doi: 10.1016/0022-2836(71)90431-1. [DOI] [PubMed] [Google Scholar]
- Olson W. K. Syn-anti effects on the spatial configuration of polynucleotide chains. Biopolymers. 1973;12(8):1787–1814. doi: 10.1002/bip.1973.360120808. [DOI] [PubMed] [Google Scholar]
- Schweizer M. P., Banta E. B., Witkowski J. T., Robins R. K. Determination of pyrimidine nucleoside syn, anti conformational preference in solution by proton and carbon-13 nuclear magnetic resonance. J Am Chem Soc. 1973 May 30;95(11):3770–3778. doi: 10.1021/ja00792a049. [DOI] [PubMed] [Google Scholar]
- Swaminathan V., Sundaralingam M. The crystal structures of metal complexes of nucleic acids and their constituents. CRC Crit Rev Biochem. 1979;6(3):245–336. doi: 10.3109/10409237909102565. [DOI] [PubMed] [Google Scholar]


