Abstract
This study examined the genetic and environmental architecture underlying aggressive behavior measured by the Life History of Aggression Questionnaire (LHA; Coccaro et al. 1997a). Following preliminary phenotypic factor analysis procedures, multivariate behavioral genetics models were fit to responses from 2,925 adult twins from the PennTwins cohort on five LHA items assessing lifetime frequency of temper tantrums, indirect aggression, verbal aggression, fighting, and physical assault. The best-fitting model was a 2-factor common pathway model, indicating that these five aggressive behaviors are underpinned by two distinct etiological factors with different genetic and nonshared environmental influences. Although there was evidence of significant sex differences, the structure of the two factors appeared to be quite similar in males and females, where General Aggression and Physical Aggression factors emerged. Heritability of these factors ranged from .37 to .57, and nonshared environmental effects ranged from .43 to .63. The results of this study highlight the heterogeneous nature of the aggression construct and the need to consider differences in genetic and environmental influences on individual aggressive behaviors in a multivariate context.
Keywords: Aggressive behavior subtypes, Multivariate behavioral genetics, Genetic factor structure, Heritability, Sex differences
Human aggressive behavior has long been a focus of study across various disciplines, owing to its considerable cost to society and pervasiveness among people of all ages, ethnicities and socioeconomic status. Consistent relations have been found between aggression and other forms of pathology, including violent and suicidal behavior, depressive symptomatology, and somatic illnesses (Asberg 1994; Caspi et al. 1998; Friedman and Booth-Kewley 1987; Goodman et al. 2008; Riley et al. 1989; Swanson et al. 1990). Aggressive behavior is a key component of many psychiatric illnesses, including conduct disorder, adult antisocial personality disorder, and borderline personality disorder, and may further be comorbid with others, such as substance use disorders and bipolar disorder. Both impulsive and premeditated aggression present the potential for significant physical and psychological harm to the individual, to the targets of the individual, and to society in general. Given these and other associated negative outcomes, much effort has been devoted to increasing our understanding of the etiology of aggressive behavior in the hopes of mitigating its effects. In particular, as aggression tends to show early onset and high stability over time (Loeber and Hay 1997), and is found to aggregate in families (Cadoret 1978; Frick 1994), several researchers in this area have attempted to delineate the genetic and environmental factors that influence their development and manifestation.
Heterogeneity of aggression
While genetic and environmental influences emerge reliably in behavior genetic studies of aggression, their magnitude can vary widely across individual studies. This inconsistency can be attributed at least in part to methodological differences across studies. Meta-analyses by Miles and Carey (1997) and Rhee and Waldman (2002) clearly show that estimates of genetic and environmental effects vary across mode of measurement or operationalization of the aggression construct (e.g., different informants, or type of aggressive behavior assessed). Additionally, the heterogeneous nature of the aggression construct adds complexity to the estimation of etiological effects across studies. Various subtypes have been identified in the literature alongside the development of many different measures of aggressive behavior (Suris et al. 2004), and there is little consensus regarding the definition of the aggression construct. Distinctions have been made between reactive (usually impulsive, affective aggressive behavior in response to provocation) and proactive (goal-directed, pre-meditated) aggression (e.g., Poulin and Boivin 2000; Vitaro et al. 1998), overt and covert aggression (e.g., Björkqvist et al. 1994), and many other forms.
Attempts to establish discriminant validity between subtypes have included factor analyses of aggressive behavior measures and identification of different predictors and outcomes. Physical aggression has most often been studied, and there have been many attempts to distinguish it from other forms of aggression. For instance, physical and social (or indirect) aggression have been found to be two factorially distinct but correlated types of aggressive behavior (Vaillancourt et al. 2003) with contrasting profiles. Björkqvist and colleagues posit that early aggressive behavior shown by young children is usually physical in nature, and indirect, or social, aggression is expressed later, when verbal and sociocognitive abilities are more developed (Björkqvist et al. 1992). In addition, these two types of aggression, while highly correlated, have been linked to disparate outcomes. Physical aggression in children is consistently associated with negative outcomes, such as peer rejection (Dodge 1983) and later delinquency and externalizing behaviors (Coie and Dodge 1998; Pulkkinen 1992; Stouthamer-Loeber and Loeber 1989) whereas indirect social aggression has in fact been linked to positive outcomes in some studies, such as greater popularity among peers (Vitaro et al. 2006), and positive teacher-rated characteristics (Xie et al. 2002a, b). Similarly, in adults, research has sought to clarify the distinction between various aggressive subtypes, including impulsive (reactive) and nonimpulsive (or premeditated) aggression (e.g., Berkowitz 1974; Linnoila et al. 1983). For instance, compared to nonimpulsive aggression, impulsive aggression is more strongly related to anger (Barratt et al. 1999), guilt or remorseful feelings (Barratt et al. 1999). In addition, Schwartz et al. (1998) found that impulsive aggression in children was more strongly related to hostility of attributional bias.
Genetic studies of aggression
Converging evidence from numerous univariate twin and adoption studies indicates that both genetic and environmental influences make substantial contributions to aggressive and antisocial behavior. Recent reviews and meta-analyses have reported overall heritability estimates ranging from .32 to .50 (Mason and Frick 1994; Miles and Carey 1997; Rhee and Waldman 2002). Significant nonshared environmental influences are also consistently found. Shared environmental influences tend to be small, as is often the case in studies of personality traits and disorders (Jang et al. 1996; Plomin and Daniels 1987; Torgersen 2005). While exceptions are few (e.g., Plomin et al. 1981), some studies do indicate that estimates of heritability and environmental variance may depend on the types of aggression or subpopulations studied. For example, Coccaro et al. (1997b) found significant additive or nonadditive genetic influences on the Indirect Aggression, Verbal Aggression, Irritability, and Assault subscales of the Buss-Durkee Inventory (BDHI; Buss and Durkee 1957) in their sample of males, whereas Cates et al. (1993) found significant heritability for Indirect Aggression, Verbal Aggression, and Irritability, but not Assault in their sample of females. Univariate analyses such as these help to determine whether the heritability of aggressive behavior varies across different constructs, but are limited in that they do not elucidate the extent to which aggressive behaviors that appear phenotypically differentiable have shared or independent etiological influences, a question raised by several researchers (e.g., DiLalla 2002).
Multivariate behavior genetic studies can shed some light on this debate by delineating the extent to which genetic and environmental influences on individual subtypes overlap with one another, though surprisingly few such genetic studies have been published. Cholesky modeling has been used in a handful of studies. In their study of the BDHI subcales, Coccaro et al. (1997b) found that patterns of genetic and environmental influence differed across behaviors; for example, the phenotypic correlation (r) between physical assault and indirect aggression was .36, with a genetic correlation (rg) of .42 and a nonshared environmental correlation (re) of .58. In contrast, the same correlations for physical assault and verbal aggression were r = .50, rg = .16 and re = .84. Coccaro et al.’s findings suggest that phenotypically related aggressive behaviors or subtypes are likely to be influenced by common genetic and environmental influences, but also by etiological factors specific to each behavior. Moreover, the proportions of these influences will differ widely depending on which specific aggressive behaviors are investigated.
Saudino and Hines (2007) examined the overlap of genetic and environmental influences on the use of physical and psychological aggression (as measured by the Conflict Tactics Scales; Straus et al. 1996) in 185 adult same-sex twin pairs. The two types of aggression showed a moderate phenotypic correlation (r = .38), a high genetic correlation (rg = .74), and a much lower nonshared environmental correlation (re = .30). Thus, while physical and psychological aggression shared most of their genetic etiology and a small amount of their nonshared environmental influences, Saudino and Hines (2007) concluded that etiological differences between the two are a function of their nonoverlapping nonshared environmental influences.
In a study of 6-year-old twins, Brendgen et al. (2005) found that the significant phenotypic correlation between teacher ratings of physical and social aggression (r = .43) was underpinned by a strong genetic correlation (rg = .79) and a lower nonshared environmental correlation (re = .31). Similar results were obtained for peer ratings of aggressive behavior, though the re in this case was non-significant (r = .41, rg = .31, re = .12). Shared environmental influences were negligible for both teacher and peer ratings. This pattern of a strong overlap of genetic and weak overlap of nonshared environmental influences underlying a moderate phenotypic correlation in children resembles the pattern of results found by Saudino and Hines (2007) in adults.
In another child study, Baker et al. (2008) recently investigated the etiological distinction between reactive and proactive aggression in a large sample of 9-10 year old twins (N = 1219). While they found strong evidence to distinguish the two subtypes in a phenotypic factor analysis, results from the bivariate genetic analyses were less straightforward. When measured by parent- and teacher-report, genetic and shared environment correlations for reactive and proactive aggression were large and significant. Confidence intervals included 1.0, indicating complete overlap of these etiological factors. However, for child self-report, genetic and environmental correlations were significantly greater than 0 but smaller and significantly less than unity, indicating some independence of the two forms of aggression. The contradiction in these findings highlights the challenges faced when attempting to elucidate the etiology of a construct with such great methodological and substantive heterogeneity.
Alternatively, some researchers have attempted to address the multivariate aspect of aggression by first performing a phenotypic factor analysis on several aggressive behaviors, and then examining the genetic and environmental etiology of the resulting factor(s). For example, in a study of child aggressive behavior, Ligthart et al. (2005) employed principle components analysis to first derive two phenotypic factors, ‘relational’ (similar to social, or indirect) and ‘direct’ (similar to physical) aggression, from the Aggression subscale of the Child Behavior Checklist (Achenbach 1991). They found that these two factors were underpinned by the same shared environmental influences, but by partially independent genetic influences, a result that contradicts the abovementioned findings of Brendgen et al. (2005), which found a high genetic correlation between physical and social aggression, and negligible effects of shared environment.
In a study of adults, Vernon et al. (1999) employed principal components analysis on 18 measures of aggression, elucidating a general aggression factor (which received significant loadings from all 18 measures), and three correlated factors: spontaneous aggression (which received strong loadings from indices of physical aggression, as well as some indices of verbal aggression); aggressive attitudes (comprising measures of impulsivity, affective instability, hostility and anger), and verbal aggression. Univariate analyses revealed a heritability of .50 for the general aggression factor, and heritabilities of .44 to .54 for each sub-factor (with nonshared environment accounting for the remaining variance), indicating substantial genetic influence. Moreover, genetic correlations among the sub-factors were between .31 and .51. Though the confidence intervals were not presented, the magnitudes of the rg estimates suggest that there are both shared and independent genetic influences acting on each of the sub-factors.
A more informative methodology that can be used to delineate the etiology of aggressive behaviors may be to perform a genetic factor analysis on various subtypes; the present study employs this approach. In contrast to the procedure used by Ligthart et al. (2005) and Vernon et al. (1999), which first groups aggressive behaviors together at the phenotypic level and subsequently models their etiological structure, this approach allows one to examine how different behaviors group together based on their etiological commonalities. Sluyter et al. (2000) used this methodology in a twin study of testosterone and the aggression-hostility-anger (AHA) syndrome in adult males. They examined seven subscales of the BDHI (physical assault, indirect aggression, irritability, negativism, resentment, suspicion and verbal aggression; Buss and Durkee 1957). Multivariate model fitting yielded a 2-factor solution where irritability and resentment loaded highly onto one common genetic factor, and assault, negativism and verbal aggression loaded onto a second common genetic factor. Indirect aggression and suspicion crossloaded onto both common factors. While two nonshared environmental influences emerged, most variables loaded moderately onto both. Consistent with most studies of aggression, shared environmental influences were negligible. Sluyter et al. (2000) interpreted their results as suggesting that the emotional AHA and behavioral AHA components have different sets of genetic influences and overlapping nonshared environmental influences.
Sex differences
Another important issue to consider is whether etiological influences on aggression differ in males and females. Early research on sex differences largely asserted that males were more aggressive than females (see Eagly and Steffen 1986; Frodi et al. 1977). More recently, this finding has held up in studies of physical aggression (e.g., Archer 2004) but is less true of nonphysical forms such as relational aggression (Crick and Grotpeter 1995). Coupled with research on differential parenting and socialization effects in boys and girls (e.g., Smetana 1989; Zahn-Waxler 1993), these findings suggest that female aggression is not uncommon, but tends to be nonphysical in nature.
In the behavioral genetic context, the nature of sex differences is somewhat unclear. Differences in the etiological make-up of aggression may well depend on the type of aggression examined; for example, the influence of environmental factors such as socialization may serve to reduce heritability estimates of physical types of aggressive behavior in women due to a restriction of variance, whereas nonphysical types such as verbal aggression may be more likely to show genetic variance (Cates et al. 1993). Indeed, various studies employing different aggression measures have found genetic influences on aggression to be greater in males than females (e.g., Silberg et al. 1994; Van den Oord et al. 1994), others find greater genetic influences on aggression in females than in males (e.g., Eley et al. 1999), and yet others conclude that they are equivalent between sexes (Cadoret et al. 1995; Finkel and McGue 1997). The Miles and Carey (1997) meta-analysis found that overall heritability was slightly greater in males than females across 24 studies. In addition, Rhee and Waldman (2002) found that, while estimates differed between sexes when all 51 studies were examined, this difference was attenuated when studies of only one sex were excluded. More specificity regarding the aggression measure employed may provide some clarity regarding sex differences in genetic and environmental influences.
The present study
The purpose of the present endeavor was to explore the genetic and environmental architecture of aggressive behaviors over the lifespan, as assessed in adulthood via the Life History of Aggression Questionnaire (LHA; Coccaro et al. 1997a). The LHA is a self-report measure developed using modified items from the Brown et al. (1979) life history rating assessment and new items written by EFC. In addition to 3 a priori subscales (Aggression, Self-Directed Aggression, and Consequences/Antisocial Behavior), a total aggression score may be created by summing across all items. Initial psychometrics indicated that the performance of the Aggression subscale (the LHA-AGG), comprising temper tantrums, fighting, verbal aggression, physical assault, and indirect aggression1 items, was nearly identical to that of the total score, leading the authors to suggest that it be used on its own as a primary measure of life history aggression (Coccaro et al. 1997a). The LHA-AGG has since been used as a stand-alone measure of aggression in several studies. Coccaro et al. (2003) found that levels of a plasma free norepinephrine metabolite correlated inversely with LHA-AGG (but not with impulsivity) in personality disordered participants. Another study by Boyle et al. (2008) found higher LHA total and antisocial subscale scores in generally violent men as opposed to partner-only violent men, and also reported a Cronbach’s alpha reliability of .87 for the LHA-AGG. Though originally conceptualized as a single a priori subscale, closer consideration of the LHA-AGG subscale suggests some conceptual heterogeneity among the individual behaviors (items) it assesses. In particular, some of the items assess behaviors that are more physical in nature (i.e., fighting, assault) than others (tantrums, verbal aggression).
Prior to the focal behavior genetic analyses, preliminary phenotypic factor analyses were performed on the 5 items to confirm whether they are best described by a 1- or 2-factor structure. After these factor analysis procedures, multivariate genetic factor models were applied to determine the genetic and environmental architecture that underlies the variance and covariance relationships among the items of the LHA-AGG. Sex differences in genetic and environmental influences as well as in the genetic factor structure were also explored.
Methods
Participants
Participants were taken from the PennTwins Cohort, a population-based sample of all twins born in Pennsylvania between 1959 and 1978. Full details of the cohort development can be found in Coccaro and Jacobson (2006). In brief, beginning in 1996, an initial list of 77,012 individuals who were likely to be part of a twin pair was extracted from computerized birth records kept by the Division of Vital Statistics at the Pennsylvania Department of Health. This list was then cross-referenced with active driving license records on file with the Pennsylvania Department of Transportation, resulting in an address list of 30,801 individuals. Of these 30,801 individuals, 9,341 (40.8% of those who had valid addresses and were eligible to participate) returned consent-to-contact forms.
Effect size comparisons between individuals who consented to participate and those who were unresponsive or could not be located were made on age, race (Caucasian, African American, Hispanic and other), marital status (never married, currently married, and other), unemployment rate, educational attainment (without high school degree, high school degree only, and some college), household income, per capita income, psychiatric diagnoses, and medical diagnoses. This demographic information was extrapolated via geocode analysis [see Coccaro and Jacobson (2006) for details of this process]. While some differences between the participants and the non-participants emerged, all Cohen’s d statistics were .25 or below (mean Cohen’s d = .14), and none of the comparisons represented discrepancies of practical significance (Coccaro and Jacobson 2006). Thus, any differences between participating and nonparticipating twins were considered negligible and nonsystematic.
Procedure
Twins who consented to participate in the PennTwins cohort were mailed a brief zygosity questionnaire containing basic demographic questions as well as standard twin similarity items, with a response rate of ~76%. Of the 7,282 twins who returned a zygosity questionnaire, slightly more than one-half (51.4%) were female. The majority of twins (N = 5,409; 74.3%) were from same-sex twin pairs, with 1,798 twins (24.7%) from opposite-sex pairs. A small number of twins (N = 75, ~1%) were not assigned a zygosity because there was insufficient information to determine whether they were from same- or opposite-sex pairs. Approximately 60% of the overall sample were twin pairs in which both twins returned zygosity questionnaires (65% of twins from same-sex pairs and 50% of twins from opposite-sex pairs).
Zygosity was established via genotyping in a subsample of 169 twin pairs (N = 338 individuals), using 12 microsatellite markers from 12 different chromosomes. Twin pairs were considered monozygotic (MZ) if they matched on all 12 markers, otherwise they were considered dizygotic (DZ). Zygosity of non-genotyped twins from same-sex pairs was established through a discriminant function analysis (DFA) of five standard twin similarity questions (Lykken 1978): (1) whether the twin believed they were MZ or DZ; (2) whether they and their twin were as alike as peas in a pod, or of only ordinary family resemblance as children; and how often it was difficult for (3) parents; (4) teachers; and (5) strangers to tell the twins apart as children. Of the 5,409 same-sex twins, the majority (97.7%) had non-missing data on all five twin similarity items. A small number (N = 70, 1.3%) were missing data on only one twin similarity item. These twins were given scores of ‘2’ for the missing item and were included in the DFA. Twins with more than one missing item (N = 55, 1.0%) were eliminated from the DFA.
For the 1,536 twin pairs (N = 3,072 individual twins) in which both twins returned zygosity questionnaires, the DFA was based on both of their responses to the 5 zygosity questions (i.e., all 10 individual items), using 163 genotyped pairs who also had complete data on all 10 items. For the 1,949 twins whose cotwins did not return zygosity questionnaires, the DFA was based on only their responses (i.e., 5 individual items), using all 333 genotyped individuals with non-missing twin similarity data as the comparison. Slightly more than half (63%) of genotyped twin pairs were MZ. The error rate of the DFA based on the genotyped twins was 6.7% among the paired twins, and 8.7% among unpaired twins, consistent with other studies (Baker et al. 2007; Torgersen 1979). Assignment of zygosity was based on an 80/20 split, where twins with probability of monozygosity greater than 80% were assigned MZ status, and twins with probability of monozygosity <20% were assigned DZ status. Twins who fell between 20 and 80% were not assigned a zygosity. Among the 3,072 same-sex twins from complete pairs, 69.9% were assigned MZ, 26.6% were assigned DZ, and 3.5% could not be assigned a zygosity. Percentages among twins in which cotwins did not participate were 53.0% MZ, 39.6% DZ, and 7.4% unassigned.
A second survey, the Behavioral Health Questionnaire (BHQ), was initially sent to all twin pairs in which both twins had returned the zygosity questionnaire. This survey contained a battery of personality and behavioral measures, including the Lifetime History of Aggression Questionnaire, which is examined in this study. The response rate to the BHQ was between 70 and 75%. BHQ data are currently available for 3,065 individual twins. Males comprised 41.5% of the sample. In a small number of cases, zygosity data was either missing (N = 55) or zygosity could not be established with certainty from the DFA analyses (N = 70), resulting in a possible total sample of 2,940 twins. The average age of twins at the time they completed the BHQ was 33.14 (SD = 6.02), with 80% of the sample between the ages of 26 and 42.
Measures
Lifetime History of Aggression Questionnaire: Aggression subscale
The content of the five items that make up the LHA-AGG are given in Table 1. Each item is rated on a 5-point scale, based on the total number of occurrences of a given behavior since age 18 (0 = no occurrences, 1 = one occurrence, 2 = two or three occurrences, 3 = four to nine occurrences, 4 = 10 or more occurrences, and 5 = more occurrences than can be counted). Initial development of the LHA-AGG indicated good concurrent validity, internal consistency (α = .87), inter-rater reliability (intraclass correlation coefficient = .94), and test–retest reliability (r = .80; Coccaro et al. 1997a).
Table 1.
LHA Aggression subscale items
| Item name | |
|---|---|
| Since you were 18 years old, how many times had you … | |
| Temper tantrums | …thrown a temper tantrum (for example: screaming, slamming doors, throwing things when frustrated to the “breaking point”) |
| Verbal aggression | …gotten into verbal fights or arguments with other people |
| Indirect aggression | …deliberately struck or deliberately broken objects (for example: windows, dishes, etc.) in anger |
| Fighting | …gotten into physical fights with other people |
| Physical assault | …deliberately hit another person (or an animal) in anger |
Complete data on all 5 LHA-AGG items was available for the majority of the sample (N = 2,899, 98.6%), and a small number of twins had missing data on 1–4 items (N = 26). The few individuals who had no valid LHA data (N = 15) were excluded from the analyses. Thus, the present analyses are based on a sample of 2,925 individual twins, including 2,290 paired twins, comprising 538 monozygotic males, 824 monozygotic females, 192 same-sex dizygotic males, 318 same-sex dizygotic females, 209 opposite-sex dizygotic males, and 209 opposite-sex dizygotic females. Of the 635 unpaired twins in the sample, 259 were male and 376 were female.
Statistical analyses
We first applied factor analytic procedures to the 5 items of the LHA-AGG subscale in order to determine whether a single latent factor underlies the subscale. Using the MPLUS v.5.21 software program (Muthén and Muthén 1998–2007), exploratory factor analysis (EFA) was first performed on one of the twins in each pair (e.g., the Twin 1 subgroup). The results of the EFA were then cross-validated in the second subgroup (e.g., Twin 2) via confirmatory factor analysis (CFA) procedures.
Subsequently, we employed a twin design that relies on the different levels of genetic relatedness between MZ twin pairs, who share 100% of their genes, and DZ twin pairs, who share only 50% of their segregating alleles on average. This difference allows for estimation of the genetic and environmental influences on individual differences in a given phenotype. In this study, phenotypic variation was assumed to be due to three latent factors: additive genetic effects (A); shared environmental effects (C); and nonshared environmental effects (E). Shared environmental effects are those environmental factors which contribute to similarities among family members (but may differ across families), such as SES or parental education. Nonshared environmental effects include uncorrelated errors of measurement and those environmental factors that serve to differentiate family members such as different peer groups or accidents that affect only one twin. Comparison of the within-pair covariances for MZ and DZ pairs allows for estimation of the contribution of the additive genetic, shared, and nonshared environmental influences to the phenotypic variance. Models are readily extended to multivariate analyses, which also estimate genetic and environmental influences on covariation across measures.
In the present study, the maximum-likelihood structural equation modeling program Mx (Neale et al. 2003) was used to estimate genetic and environmental influences on the variances and covariances across items. Prior to fitting a series of multivariate factor models, fully saturated and full baseline Cholesky decomposition models were run. Because full Cholesky models perfectly estimate the genetic and environmental covariance structure of the data, they are not very useful in testing theoretically driven hypotheses about the sources of covariance across measures on their own. To accomplish such tests, we fitted a series of genetic factor models based on the common pathway (CP) model.2 Figure 1 presents a 2-factor CP model. Latent factors (F1 and F2 in Fig. 1) are defined by manifest, phenotypic variables (V1 through V5) via factor loadings (f11 through f51 on latent factor F1, and f12 through f52 on F2). Genetic (A1 and A2) and environmental (C1, C2, E1, and E2), influences operate through the latent factors. While covariances among variables act through the latent factors, the CP model also allows for specific influences on individual variables which do not contribute to the covariance between phenotypes. These are denoted by the subscript ‘s’ in Fig. 1. For example, as1, cs1 and es1 are, respectively, the genetic, shared environmental and nonshared environmental influences unique to V1. Correlations between factors operate through correlations between the common latent influences, depicted by the dashed lines with double-headed arrows.3
Fig. 1.
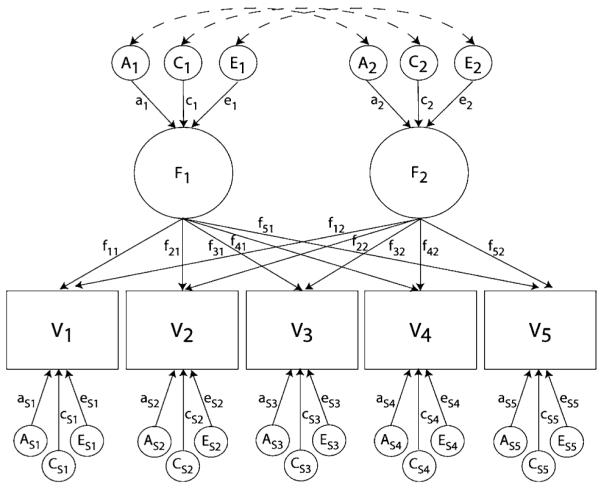
2-Factor ACE common pathway model
Hypotheses about model structure can be tested by dropping specific parameter estimates to form simplified or theoretically derived models. Similarly, sex differences are examined by specifying a model in which parameters are constrained to be equal across males and females, or by a model which specifies different factor structures in males and females.
Quantitative comparisons between models are performed via nested model comparisons. The difference in −2LL between nested models (the likelihood ratio test, or LRT) follows a χ2 distribution, and thus a critical value can be determined based on the difference in degrees of freedom between the models. A significant difference between models indicates a significant degradation in fit between the original model and the modified model. Model fit is also determined by examination of other indices, including the Akaike’s Information Criterion (AIC; Akaike 1987) and the corrected Bayesian Information Criterion (BIC; Schwarz 1978), where lower values indicate better model fit.
Results
Means and phenotypic correlations
Raw means, standard deviations, and within-person phenotypic correlations for the 5 LHA-AGG items are given in Table 2 for males and females. Males scored significantly higher than females on indirect aggression, fighting, and physical assault. We also tested whether LHA-AGG items differed among twins whose cotwins participated versus twins whose cotwins did not participate, using SAS PROC MIXED to control for correlated observations within twin pairs (not shown). None of the ANOVAs on the individual LHA-AGG items were statistically significant (all p-values >.08), indicating that non-response, as measured indirectly through cotwin response, was not related to level of aggression. Within-person phenotypic correlations ranged from .26 to .57 in males, and .29 to .50 in females. All correlations were significant (p < .05). Some evidence of non-normality was found for three items: fighting, physical assault, and indirect aggression (skewness = 1.25–1.78, kurtosis = .83–3.01), thus a log transformation was applied to these variables (after a constant of 1 was added to their values).
Table 2.
Raw means, standard deviations, and within-person phenotypic correlations for the five LHA-AGG items
| Temper tantrums | Verbal aggression | Indirect aggression | Fighting | Physical assault | |
|---|---|---|---|---|---|
| Temper tantrums | … | .50 | .49 | .35 | .40 |
| Verbal aggression | .43 | … | .29 | .29 | .34 |
| Indirect aggression | .57 | .35 | … | .37 | .40 |
| Fighting | .26 | .33 | .29 | … | .50 |
| Physical assault | .29 | .32 | .36 | .53 | … |
| Mean (SD)—males | 2.20 (1.59)b | 3.14 (1.34)b | 1.20 (1.32)a,b | .83 (1.11)a,b | .90 (1.23)a,b |
| Mean (SD)—females | 2.17 (1.62) | 3.11 (1.41) | .72 (1.16) | .42 (.85) | .59 (1.05) |
Note: Male correlations in lower diagonal, females in upper diagonal. All correlations significant at p < .05. Nmales = 1198, Nfemales = 1727
Significant mean differences between genders, p < .001
Significant variance differences between genders, p < .05
Exploratory factor analysis (EFA)
Unweighted least squares extraction with oblique geomin rotation was conducted on the 5 LHA-AGG items. One- and two-factor solutions were extracted as theoretically possible structures. Results are presented in Table 3. Examination of the root mean square error of approximation (RMSEA), standardized root mean square residual (SRMR), Comparative Fit (CFI) and Tucker-Lewis Index (TLI) values indicated that the one factor solution did not fit well (χ2(5) = 216.32, p < .001, RMSEA = .17, SRMR = .05, CFI = .87, TLI = .75). The fit indices indicated that the 2-factor solution provided a much better fit to the data, (χ2(1) = .16, p = .69, RMSEA = .00, SRMR = .00, CFI = 1.00, TLI = 1.00). Factor loadings of this solution are given in Table 3. The pattern of loadings indicates that the LHA-AGG subscale splits into two; temper tantrums, verbal aggression, and indirect aggression form a single factor and fighting and physical assault form a separate factor.
Table 3.
Factor loadings from the 2-factor result of the EFA and CFA on LHA-AGG items
| EFA |
CFA |
|||
|---|---|---|---|---|
| Item | Factor 1 Nonphysical aggression |
Factor 2 Physical aggression |
Factor 1 Nonphysical aggression |
Factor 2 Physical aggression |
| 1. Temper tantrums | 1.04 | .00 | .74 | … |
| 3. Verbal aggression | .35 | .29 | .59 | … |
| 5. Indirect aggression | .35 | .33 | .67 | … |
| 2. Fighting | −.03 | .72 | … | .72 |
| 4. Physical assault | .01 | .74 | … | .70 |
Confirmatory factor analysis (CFA)
The 2-factor solution that resulted from the EFA was subsequently tested via CFA in the Twin 2 subsample (see Table 3). It showed an adequate fit to the data; χ2(1,4) = 80.56, p < .001, RMSEA = .10, SRMR = .04, CFI = .95, TLI = .91, and the two factors yielded a correlation of .68. All loadings were significant and all residuals were positive.
From these analyses, the 2-factor result suggests that the a priori LHA-AGG subscale can be taken to index 2 latent factors, a Nonphysical Aggression factor4 (temper tantrums, verbal aggression and indirect aggression), and a Physical Aggression factor (fighting and physical assault items).
Multivariate genetic analyses
Results of the multivariate twin models are presented in Table 4. Prior to running substantive models, model testing indicated that a saturated model constraining means equal within same-sex twin pairs and across MZ and DZ pairs within gender did not show a significant decrease in fit compared to a fully saturated model (LRT = 33.06, df = 40, p = .77), and thus was used as the saturated comparison model (Model I) in Table 4. In addition, given the mean differences between males and females on three of the five LHA-AGG items, all means were allowed to vary between sexes in the following genetic analyses. The ACE Cholesky decomposition (Model II) fit the data well compared to the means-equal saturated model, indicating no significant differences in variance or phenotypic covariance across Twin 1 and Twin 2, or across MZ and DZ twins within sex. CE Cholesky (Model III) and AE Cholesky (Model IV) models indicated that shared environmental influences could be eliminated from the model, but genetic influences could not be dropped. The full 2-factor ACE CP model (Model V) also fit the data well, and did not fit the data significantly more poorly than the ACE Cholesky model. Similar to results from the Cholesky, the 2-factor AE CP model (Model VII) was the best fitting model of the three 2-factor CP models. Finally, the 1-factor ACE CP (Model VIII) model fit the data very poorly, indicating that a two-factor structure was necessary to explain the covariance among the different aggressive behaviors.
Table 4.
Selected results of multivariate behavior genetic model fitting of the LHA-AGG items
| Model | −2LL | df | p | AIC | BIC | Δ χ 2 | Δ df | p | Comparison model |
|---|---|---|---|---|---|---|---|---|---|
| I. Saturated, means equal | 56494.67 | 14310 | … | 27874.67 | −2572.38 | ||||
| II. ACE Cholesky | 56649.78 | 14495 | .95 | 27659.78 | −2893.27 | 155.11 | 185 | .95 | I |
| III. CE Cholesky | 56707.21 | 14525 | .54 | 27657.21 | −2929.17 | 57.43 | 30 | .00 | II |
| IV. AE Cholesky | 56668.24 | 14525 | .98 | 27618.24 | −2948.65 | 18.46 | 30 | .95 | II |
| V. ACE CP 2-factor full | 56689.33 | 14527 | .86 | 27635.32 | −2942.41 | 39.56 | 32 | .17 | II |
| VI. CE CP 2-factor full | 56727.40 | 14541 | .46 | 27645.40 | −2953.52 | 38.07 | 14 | .00 | V |
| VII. AE CP 2-factor full | 56693.48 | 14541 | .94 | 27611.48 | −2970.49 | 4.15 | 14 | .99 | V |
| VIII. ACE CP 1-factor full | 57077.78 | 14541 | .00 | 27995.78 | −2778.34 | 388.45 | 14 | .00 | V |
Note:CP = common pathway model; AE = model in which no shared environment parameters are estimated, CE = model in which no additive genetic parameters are estimated; AIC = Akaike’s Information Criterion; BIC = corrected Bayesian Information Criterion
Further model testing was undertaken to simplify the structure of the common factors in the best-fitting 2-factor AE CP model (Model VII in Table 4). First, we tested whether parameters could be equated across sex. This model fit the data significantly more poorly compared to the model with parameters estimated freely for males and females (−2LL = 56863.77, df = 14563, AIC = 27737.77, BIC = −2932.73, LRT = 170.29, df = 22, p < .001). Next, we tested a correlated 2-factor AE CP model that corresponded to the phenotypically-derived factor structure. The temper tantrums, verbal aggression and indirect aggression items were estimated to load only on one factor, and fighting and physical assault were estimated to load only on a second factor (with equality constraints on the factor loadings for the second factor). This model also allowed for correlations across factors via the common A and E influences. The fit of this model was also significantly poorer than the 2-factor AE CP model (−2LL = 56812.06, df = 14549, AIC = 27714.06, BIC = −2928.42, LRT = 118.58, df = 8, p < .001). As a final simplification, we dropped all nonsignificant parameters from the 2-factor AE CP model (the temper tantrum factor loading on the second latent factor, and the specific genetic influences on temper tantrums and physical assault, for both males and females). This model resulted in a nonsignificant change in fit (−2LL = 56695.22, df = 14547, AIC = 27601.22, BIC = −2982.53, LRT = 1.74, df = 6, p = .94). Despite what appeared to be very similar patterns of parameter estimates, this model still could not be equated across sexes (−2LL = 56864.40, df = 14566, AIC = 27732.40, BIC = −2938.89, LRT = 169.14, df = 19, p < .001; details available from author).
Further exploration of the significant sex differences revealed that while the genetic and environmental influences on the two latent factors could be equated across males and females (LRT = .21, df = 2, p = .90), the standardized factor loadings5 could not be equated across sex for either latent factor (LRT = 37.68, df = 5, p < .001; LRT = 15.04, df = 4, p < .01). In addition, there was some evidence for sex differences on the standardized behavior-specific genetic and/or nonshared environmental parameters (LRT = 14.65, df = 8, p = .06). Extensive posthoc analyses testing significant sex differences by behavior revealed significant differences for temper tantrums, indirect aggression, fighting, and physical assault, but not verbal aggression. Specifically, standardized factor loadings could not be equated for any of the above four variables (results available from author). In addition, either the behavior-specific genetic and/or the nonshared environmental parameters could not be equated across sex for temper tantrums or indirect aggression. Thus, the most parsimonious model could equate across sex: (a) the genetic and environmental influences on the latent factors, (b) the factor loadings for verbal aggression, (c) the behavior-specific genetic factors for verbal aggression and fighting; and (d) the behavior-specific nonshared environmental factors for verbal aggression, fighting, and physical assault (−2LL = 56701.38, df = 14555, AIC = 27591.38, BIC = −2996.69). Standardized parameter estimates from this final model are shown in Fig. 2, and Table 5 presents the variance components for each of the five aggression behaviors based on this model.
Fig. 2.
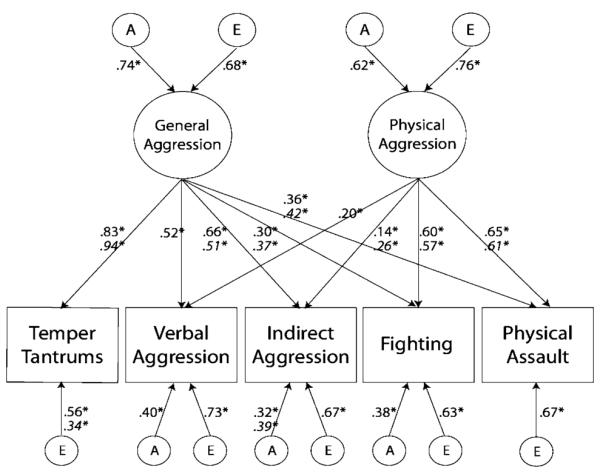
2-Factor AE common pathway model showing standardized estimates (when parameters could not be equated between sexes, male parameters appear above and females appear below in italics). * Denotes significant parameters based on a 95% confidence interval
Table 5.
Heritability estimates (and 95% CIs) from best-fitting model
| Total heritability |
Proportion due to PhysAgg (%) |
Proportion due to GenAgg (%) |
Proportion due to As (%) |
Total e2 | Proportion due to PhysAgg (%) |
Proportion due to GenAgg (%) |
Proportion due to Es (%) |
|
|---|---|---|---|---|---|---|---|---|
| Temper tantrums | ||||||||
| Males | .37 (.31, .43) | 0.0 | 100.0 | 0.0 | .63 (.57, .69) | 0.0 | 49.9 | 50.1 |
| Females | .48 (.42, .53) | 0.0 | 100.0 | 0.0 | .52 (.47, .58) | 0.0 | 78.1 | 21.9 |
| Verbal aggression | ||||||||
| Males | .32 (.27, .37) | 4.7 | 45.4 | 49.9 | .68 (.63, .73) | 3.6 | 18.5 | 77.9 |
| Females | .32 (.27, .37) | 4.7 | 45.4 | 49.9 | .68 (.63, .73) | 3.6 | 18.5 | 77.9 |
| Indirect aggression | ||||||||
| Males | .35 (.29, .42) | 2.3 | 68.6 | 29.1 | .66 (.58, .74) | 2.0 | 31.0 | 67.1 |
| Females | .32 (.25, .39) | 8.3 | 43.7 | 48.0 | .68 (.61, .75) | 6.4 | 18.0 | 75.6 |
| Fighting | ||||||||
| Males | .33 (.28, .39) | 42.0 | 14.6 | 43.4 | .67 (.61, .72) | 33.8 | 6.3 | 59.9 |
| Females | .34 (.28, .39) | 36.2 | 21.3 | 42.5 | .66 (.61, .72) | 30.1 | 9.4 | 60.5 |
| Physical assault | ||||||||
| Males | .23 (.17, .29) | 70.2 | 29.8 | 0.0 | .77 (.71, .83) | 33.5 | 7.6 | 58.9 |
| Females | .24 (.19, .29) | 59.8 | 40.2 | 0.0 | .76 (.71, .81) | 29.8 | 10.7 | 59.5 |
Note: PhysAgg = Physical Aggression latent factor. GenAgg = General Aggression latent factor. As = behavior-specific genetic influences. Es = behavior-specific nonshared environmental influences
Figure 2 shows that, in both sexes, a General Aggression (GenAgg) factor emerged, with significant loadings from all five aggressive behavior items. For both sexes, genetic factors accounted for roughly half (53.9%; 95% CI = 46.5–60.7%) of the variance in GenAgg, with nonshared environment accounting for the remaining 46.1% (95% CI = 39.3–53.5%). The proportion of an item’s variance accounted for by the latent factor can be calculated by squaring the corresponding factor loading. Thus, across sexes, the GenAgg factor accounted for 68.9–88.4% of temper tantrums, 27.0% of verbal aggression, 26.0–43.6% of indirect aggression, 9.0–13.7% of fighting, and 13.0–17.6% of physical assault.
The second independent factor accounted for 32.5–36.0% of the variance in fighting and 37.2–42.3% of the variance in physical assault. Loadings on this second factor were statistically significant for verbal aggression and indirect aggression, as well, but the factor accounted for only 4.0% and 2.0–6.8% of their respective variances. This factor was thus named Physical Aggression (PhysAgg), as the main contributing aggression items index fighting and assault. Genetic influences explained 38.3% of the variance in PhysAgg (95% CI = 26.8–49.4%), with nonshared environmental influences accounting for the remaining 61.7% (95% CI = 50.6–73.2%).
As shown in Table 5, total heritabilities ranged from .23 (physical assault) to .37 (temper tantrums) in males, and from .24 (physical assault) to .48 (temper tantrums) in females. With the exception of temper tantrums, total heritabilities were nearly identical for males and females. Behavior-specific genetic factors were significant for three of the five behaviors: fighting, verbal assault, and indirect aggression. Moreover, these specific genetic factors accounted for 42.5–49.9% of the genetic variance in fighting and verbal assault (see Table 5). Behavior-specific genetic factors also accounted for nearly half (48.0%) of the genetic variance of indirect aggression in females, but just over one-quarter (29.1%) of the genetic variance in males (Table 5). Consistent with the pattern of factor loadings for the GenAgg and PhysAgg latent factors, genetic influences that operated through GenAgg accounted for relatively larger proportions of the genetic variance in temper tantrums, verbal aggression, and indirect aggression, while between 36.2 and 70.2% of the total genetic variance on fighting and physical assault came from genetic influence on the PhysAgg factor. Nonshared environmental influences accounted for between 52.3 and 77.1% of the variance in each measure, with the majority of nonshared environmental influence coming from behavior-specific factors for all behaviors except temper tantrums in females.
Overall, the significant sex differences resulted in relatively subtle differences in patterns for males and females. Temper tantrums loaded more strongly on the GenAgg factor for females than males. Conversely, behavior-specific nonshared environment accounted for more variation in males (31%) than females (12%). The overall heritability of temper tantrums was also higher in females (.48) than in males (.37). While fighting and physical assault loaded more strongly on the PhysAgg factor than the GenAgg factor for both males and females, the GenAgg factor accounted for a slightly higher proportion of the variance in fighting and physical assault in females compared to males (14 vs. 9% for fighting; 18 vs. 13% for physical assault), and the PhysAgg factor accounted for slightly more variance in fighting and physical assault in males compared to females (36 vs. 33% for fighting; 42 vs. 37% for males). Similarly, although the total heritabilities for fighting (.33–.34) and physical assault (.23–.24) were similar for males and females, the PhysAgg factor accounted for a greater proportion of the genetic variance in males (42 vs. 36% for fighting; 70 vs. 60% for physical assault), while the GenAgg factor accounted for a higher proportion of the genetic variance in females (21 vs. 15% for fighting; 40 vs. 30% for physical assault). Finally, while indirect aggression loaded very weakly on the PhysAgg factor for both males and females, the GenAgg factor accounted for a greater proportion of overall variance in indirect aggression among males (.44) compared to females (.26). Conversely, behavior-specific genetic and nonshared environmental influences accounted for more variation in indirect aggression among females compared to males (.15 vs. .10 for specific genetic influence; .50 vs. .44 for specific nonshared environmental influence).
Age effects
The sample ranged in age from 24 to 55. Based on moderated means analyses using Mx, significant associations with age were found for temper tantrums in males (r = .08, p = .02), fighting in males (r = .07, p = .03) and females (r = .10, p = .02), and physical assault in females (r = .08, p = .04). Subsequent univariate genetic analyses tested whether age moderated the genetic and environmental variance components of each item; χ2 difference tests with df = 6 indicated no such moderation of the ACE components for any of the 5 items (p = .17–.99). Further, to ensure that the age range of our sample was not biasing the multivariate results, the models described in Table 4 were re-run allowing for age-moderated means; results were virtually unchanged. Thus, though age was significantly related to three of the LHA-AGG behaviors, there was no evidence of its moderation of the genetic and environmental architecture of the LHA-AGG.
Discussion
The present study is one of only a handful of behavioral genetic studies investigating genetic and environmental heterogeneity within the general construct of aggression, and is the first study to examine specific aggressive behaviors measured by the Aggression subscale of the Lifetime History of Aggression Questionnaire. Phenotypic and multivariate genetic modeling showed that genetic and environmental covariance across the five behaviors studied (temper tantrums, verbal aggression, indirect aggression, fighting and physical assault) could not be explained by a single underlying latent factor, but instead was accounted for by two etiological factors. Our results indicate that despite moderate phenotypic correlations between all individual aggressive behaviors (r = .26–.57), there are both common and distinct genetic and nonshared environmental factors underlying the covariance across behaviors. Furthermore, we found both similarities and differences across sex in the genetic and environmental architecture of the five behaviors.
The best fitting multivariate model for the LHA-AGG items was a 2-factor common pathway model in which two latent factors were delineated. Shared environmental influences, both common and behavior-specific, could be omitted from the model. Simplification of the full common pathway model involved dropping a nonsignificant loading from temper tantrums on one of the factors, as well as the behavior-specific genetic effects on temper tantrums and physical assault. The resulting solution yielded a General Aggression factor which drew strong loadings from temper tantrums, verbal aggression, and indirect aggression and significant but smaller loadings from physical assault and fighting, and a Physical Aggression factor with strong loadings from physical assault and fighting and weak loadings from verbal aggression and indirect aggression. Common genetic influences on the General Aggression factor were slightly greater than common nonshared environmental influences (54 vs. 46%). In contrast, the majority of the variance of the Physical Aggression factor could be attributed to common nonshared environmental influences (62%) with only 38% due to common genetic influences. Interestingly, the General Aggression factor was also significantly more heritable (.54) than the Physical Aggression factor (.38).
The structure of aggression
While the phenotypic factor analysis yielded a correlated 2-factor solution with physical and nonphysical aggression factors, the genetic factor analysis revealed a somewhat different pattern of results. The results from the genetic analyses indicate that there is an overall tendency to behave aggressively which encompasses behaviors that are both physical and nonphysical in nature, as indexed by the General Aggression factor. However, the significant and substantial loadings of fighting and physical assault on a second factor suggest that some physical aggression is, in part, etiologically distinguishable from this general tendency. The necessity of a two-factor structure found in the present study lends support to the current hypothesis that aggression is not easily described as a unitary phenomenon (Loeber and Hay 1997; Volavka and Nolan 2008). While intercorrelations amongst types of aggressive behavior have been well documented, variations in severity, correlates, and onset differentiate various subtypes at all ages (e.g., Berkowitz 1974; Björkqvist et al. 1992; Vaillancourt et al. 2003; Vitaro et al. 2006).
Univariate studies of aggression provide some evidence for the heritability of different aggressive behavior subtypes, but do not inform the question of whether subtypes such as physical and nonphysical aggression share underlying genetic and environmental influences, which requires examination in a multivariate context. In the current literature, multivariate endeavors such as the present study are few in number. Those that do exist in the extant literature for the most part provide evidence for shared genetic influences among aggressive behavior subtypes, but also some etiological distinctions (Coccaro et al. 1997b; Saudino and Hines 2007; Sluyter et al. 2003; Vernon et al. 1999). The emergence of a General Aggression factor from the present study lends support to this notion of shared etiology, but our finding of a second Physical Aggression factor also suggests that there is some significant independence of etiological influences. Although the total heritability was remarkably similar across different types of aggression, the patterning of genetic variance differed substantially across behaviors. For physical assault and fighting, most of the genetic variance came from the Physical Aggression factor whereas for temper tantrums, indirect aggression, and verbal aggression, most of the genetic variance came from the General Aggression factor. In addition, there were significant behavior-specific genetic influences on fighting, verbal aggression and indirect aggression but all of the genetic variance of temper tantrums and physical assault was shared with other behaviors.
Sex differences
Of the five aggressive behaviors included in the present study, we found mean level differences between males and females only for the more physical behaviors (physical aggression, fighting, and indirect aggression), where males scored higher than females. In contrast, mean sex differences were not found for temper tantrums and verbal aggression, consistent with several reviews indicating that sex differences for verbal aggression are generally much smaller than for physical aggression (e.g., Archer 2004; Eagly and Steffen 1986; Knight et al. 2002). For the most part, the heritabilities of the individual behaviors were similar across males and females, however our study showed significant sex differences in the patterns of genetic and environmental variance and covariance. Heritability of temper tantrums was significantly higher in females than in males (.48 vs. .33). Fighting and physical assault loaded more strongly on the General Aggression factor for females than for males, perhaps indicating that physically aggressive behaviors in males are less etiologically related to an overall tendency toward aggressive behavior. Conversely, a greater proportion of the genetic variance of indirect aggression in males was due to genetic influences shared with the other aggressive behaviors (from the General Aggression factor) than in females (69 vs. 44%), whereas a larger portion of the female heritability of indirect aggression was due to genetic influences specific to that behavior. Interestingly, there were no sex differences for verbal aggression, indicating that the pattern of genetic and nonshared environmental influences on verbal aggression are similar in males and females.
We note that the sex differences reported here are subtle and vary across behavior, and do not suggest a stark difference between males and females. Total heritabilities of individual behaviors were also similar for males and females. However, the fact that we did find subtle (but significant) sex differences in the patterning of genetic and environmental influences across behaviors may explain some of the discrepancies that have been found in earlier univariate studies of sex differences of aggression.
Limitations
There are a few limitations to this study that should be considered in its interpretation. Overall, magnitudes of variance component estimates from the present study were in line with previous behavioral genetic studies of aggressive behavior (Miles and Carey 1997; Rhee and Waldman 2002), with individual behavior heritabilities ranging from .23 to .48, and heritabilities of the latent aggression factors ranging from .38 to .54. However, the results of this study may not generalize to other aggression measures, in particular those which employ different modes of assessment (e.g., observational or projective methods, other-report or clinician diagnosis). This is a necessary aspect of the study design; to further our understanding of the etiology of aggression at large, we must evaluate measures individually, subsequently examining the results in aggregate to gain consensus and identify similarities across measures (and thus behaviors). Relatedly, not all subtypes of aggression were included in the LHA-AGG (e.g., proactive, reactive, and relational aggression) nor did our analyses include associated personality aspects such as hostility and negativity that have been included by others (e.g., Sluyter et al. 2000). This limits a direct comparison of our results with prior research. In addition, the aggressive behaviors included in the LHA-AGG were measured with single items, which may have attenuated estimates of heritability. Finally, the LHA is a primarily self-report, retrospective measure, and thus scores may be subject to recall bias and error.
A second limitation of this study is that it is likely that our analyses were overpowered. For example, we could not drop the factor loadings for verbal aggression and indirect aggression from the Physical Aggression factor, even though this factor accounted for less than 10% of the variance in these behaviors. Similarly, although the magnitudes of sex differences were relatively small, models equating all parameters for males and females consistently fit more poorly than models allowing for sex differences. Though specific sex differences in given parameters could be pinpointed, we note that the overall patterns of genetic and nonshared environmental influences appear more similar than different between males and females.
Finally, as with many twin studies that rely primarily on volunteer participants, some study limitations may have arisen due to sample characteristics. Firstly, our sample had a wide age range, which could have affected results given that differences in heritability of aggressive behaviors have been reported when comparing child- and adolescent-aged samples to adult samples (e.g., Miles and Carey 1997). However, this concern is mitigated by the fact that all of our participants were adults, and furthermore, a series of secondary analyses directly investigating possible effects of age on our genetic analyses indicated no such effects. Secondly, in large community samples such as ours, non-participation rates can introduce bias. While response rates for the Zygosity Questionnaire and the Behavioral Health Questionnaire were between 70 and 75%, only 41% of twins initially contacted actively consented to be part of the PennTwins Cohort. However, our study pool was recruited through systematic identification of twins (as opposed to general advertising strategies), and demographic and geocode data revealed few meaningful differences between responders and non-responders (Coccaro and Jacobson 2006). Moreover, our analyses investigating effects of cotwin participation in the present study found no significant differences in mean levels of aggression among twins in which both twins participated, and twins in which only one twin participated.
Summary
The present study makes a number of contributions to our understanding of the genetic and environmental underpinnings of aggressive behaviors over the lifespan. First, our results provide evidence for moderate but significant heritability of these behaviors, along with strong nonshared environmental influences. Second, though some significant sex differences were found, the etiological architecture of the aggressive behaviors studied was remarkably similar between males and females. Finally, these findings warn against blindly lumping aggressive behaviors together in research. Both phenotypically and etiologically, the five items of the Aggression subscale of the Lifetime History of Aggression Questionnaire clearly could not be explained by one factor, and required the specification of a two-factor structure. The distinction between physical and nonphysical aggressive behaviors is made on the level of etiological influences, which can have downstream effects on relationships with other variables of study. Thus, future research should systematically evaluate whether the factors that predict aggressive behavior are similar for various types of aggression.
Acknowledgments
This study was supported by the National Institute of Mental Health (NIMH) Grant R01 MH063262 to Emil F. Coccaro and an NIMH Mentored Scientist Career Development Award K01 MH068484 to Kristen C. Jacobson. We wish to thank Crystal Cole, Jing (Sam) Pan, Bing Chen, and the rest of the Clinical Neuroscience and Psychopharmacology Research Unit at the University of Chicago for their assistance in data collection and scoring. We are also grateful to the twins in the PennTwins Cohort for participating in this research.
Footnotes
Within the larger body of the aggression literature, indirect aggression usually refers to a subtype of aggression which includes behaviors of social manipulation, such as spreading malicious lies about someone, or excluding someone from a group. However, the indirect aggression item of the LHA asks whether the respondent has “deliberately struck or broken objects…in anger” (see Table 1).
We also tested 1- and 2-factor independent pathway (IP) models, which are unconstrained versions of the CP models. The CP models did not show a significant reduction in fit compared to the IP models, so only results from the CP models are presented here.
Allowing correlations between the factors in a full 2-factor CP model results in model nonidentification. Thus, correlations between the common A, C, and E factors are only added to factor models in which the factor structure has been simplified.
Though the items temper tantrums and indirect aggression index aggression that is carried out physically (i.e., slamming doors, throwing, striking, or breaking objects, see Table I), the ad hoc factor name “Nonphysical Aggression” is used to reflect the non-person directed nature of the physicality.
Additional analyses (not shown) tested whether the observed sex differences were due solely to differences in variances across sex. Even while allowing for scalar differences, the differences between males and females in the CP 2-factor models were still statistically significant. However, given the significant variance differences across sex, exploration of sex differences was done via constraints on the standardized parameters (results available upon request).
Contributor Information
Michelle T. Yeh, Department of Psychiatry and Behavioral Neuroscience, University of Chicago, CNPRU, Chicago, IL, USA
Emil F. Coccaro, Department of Psychiatry and Behavioral Neuroscience, University of Chicago, CNPRU, Chicago, IL, USA
Kristen C. Jacobson, Department of Psychiatry and Behavioral Neuroscience, University of Chicago, CNPRU, Chicago, IL, USA; Department of Psychiatry and Behavioral Neuroscience, L-466D, University of Chicago, CNPRU, 5841. S Maryland Ave., Chicago, IL 60613, USA
References
- Achenbach TM. Integrative guide to the 1991 CBCL/4–18, YSR, and TRF profiles. University of Vermont, Department of Psychology; Burlington, VT: 1991. [Google Scholar]
- Akaike H. Factor analysis and AIC. Psychometrika. 1987;52:317–332. [Google Scholar]
- Archer J. Sex differences in aggression in real-world settings: a meta-analytic review. Rev Gen Psychol. 2004;8(4):291–332. [Google Scholar]
- Asberg M. Monomamine neurotransmitters in human aggressiveness and violence: a selective review. Crim Behav Ment Health. 1994;4:303–327. [Google Scholar]
- Baker LA, Jacobson KC, Raine A, Lozano DI, Bezdjian S. Genetic and environmental bases of childhood antisocial behavior: a multi-informant twin study. J Abnorm Psychol. 2007;116(2):219–235. doi: 10.1037/0021-843X.116.2.219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker LA, Raine A, Liu J, Jacobson K. Differential genetic and environmental influences on reactive and proactive aggression in children. J Abnorm Child Psychol. 2008;36(8):1265–1278. doi: 10.1007/s10802-008-9249-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barratt ES, Stanford M, Dowdy L, Liebman MJ, Kent TA. Impulsive and premeditated aggression: a factor analysis of self-reported acts. Psychiatry Res. 1999;86:163–173. doi: 10.1016/s0165-1781(99)00024-4. [DOI] [PubMed] [Google Scholar]
- Berkowitz L. Some determinants of impulsive aggression: Role of mediated associations with reinforcements for aggression. Psychol Rev. 1974;81:165–176. doi: 10.1037/h0036094. [DOI] [PubMed] [Google Scholar]
- Björkqvist K, Lagerspetz K, Kaukiainen A. Do girls manipulate and boys fight? Developmental trends in regard to direct and indirect aggression. Aggress Behav. 1992;18:117–127. [Google Scholar]
- Björkqvist K, Österman K, Lagerspetz KMJ. Sex differences in covert aggression among adults. Aggress Behav. 1994;20:27–33. [Google Scholar]
- Boyle DJ, O’Leary KD, Rosenbaum A, Hassett-Walker C. Differentiating between generally and partner-only violent subgroups: Lifetime antisocial behavior, family of origin violence, and impulsivity. J Fam Violence. 2008;23(1):47–55. [Google Scholar]
- Brendgen M, Dionne G, Girard A, Boivin M, Vitaro F, Pérusse D. Examining genetic and environmental effects on social aggression: a study of 6-year-old twins. Child Dev. 2005;76:930–946. doi: 10.1111/j.1467-8624.2005.00887.x. [DOI] [PubMed] [Google Scholar]
- Brown GL, Goodwin FK, Ballenger JC, Goyer PF, Major LF. Aggression in humans correlates with cerebrospinal fluid amine metabolites. Psychiatry Res. 1979;1:131–139. doi: 10.1016/0165-1781(79)90053-2. [DOI] [PubMed] [Google Scholar]
- Buss AH, Durkee A. An inventory for assessing different kinds of hostility. J Consult Psychol. 1957;21:343–349. doi: 10.1037/h0046900. [DOI] [PubMed] [Google Scholar]
- Cadoret RJ. Psychopathology in adopted-away offspring of biologic parents with antisocial behavior. Arch Gen Psychiatry. 1978;35:176–184. doi: 10.1001/archpsyc.1978.01770260054005. [DOI] [PubMed] [Google Scholar]
- Cadoret RJ, Yates WR, Troughton E, Woodworth G. Genetic– environmental interaction in the genesis of aggressivity and conduct disorders. Arch Gen Psychiatry. 1995;52:916–924. doi: 10.1001/archpsyc.1995.03950230030006. [DOI] [PubMed] [Google Scholar]
- Caspi A, Moffitt TE, Newman DL, Silva PA. Behavioral observations at age 3 years predict adult psychiatric disorders: longitudinal evidence from a birth cohort. In: Herzig ME, Farber EA, editors. Annual progress in child psychiatry and child development. Brunner/Mazel Inc.; Bristol, PA: 1998. pp. 319–331. [DOI] [PubMed] [Google Scholar]
- Cates DS, Houston BK, Vavak CR, Crawford MH, Uttley M. Heritability of hostility-related emotions, attitudes, and behaviors. J Behav Med. 1993;16:237–256. doi: 10.1007/BF00844758. [DOI] [PubMed] [Google Scholar]
- Coccaro EF, Jacobson KJ. PennTwins: a population-based cohort for twin studies. Twin Res Hum Genet. 2006;9(6):998–1005. doi: 10.1375/183242706779462633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coccaro EF, Berman ME, Kavoussi RJ. Assessment of life history of aggression: development and psychometric characteristics. Psychiatry Res. 1997a;73:147–157. doi: 10.1016/s0165-1781(97)00119-4. [DOI] [PubMed] [Google Scholar]
- Coccaro EF, Bergerman C, Kavoussi RJ, Seroczynski A. Heritability of aggression and irritability: a twin study of the Buss—Durkee Aggression Scales in adult male subjects. Biol Psychiatry. 1997b;41(3):273–284. doi: 10.1016/s0006-3223(96)00257-0. [DOI] [PubMed] [Google Scholar]
- Coccaro EF, Lee R, McCloskey M. Norepinephrine function in personality disorder: plasma free MHPG correlates inversely with life history of aggression. CNS Spectr. 2003;8(10):731–736. doi: 10.1017/s1092852900019106. [DOI] [PubMed] [Google Scholar]
- Coie JD, Dodge KA. Aggression and antisocial behavior. In: Eisenberg N, Damon W, editors. Handbook of child psychology. vol 3: social, emotional, and personality development. Wiley; New York: 1998. pp. 779–862. [Google Scholar]
- Crick NR, Grotpeter JK. Relational Aggression, gender, and social-psychological adjustment. Child Dev. 1995;66(3):710–722. doi: 10.1111/j.1467-8624.1995.tb00900.x. [DOI] [PubMed] [Google Scholar]
- DiLalla LF. Behavior genetics of aggression in children: review and future directions. Dev Rev. 2002;22:593–622. [Google Scholar]
- Dodge KA. Behavioral antecedents of peer social status. Child Dev. 1983;54:1386–1399. [Google Scholar]
- Eagly AH, Steffen VJ. Gender and aggressive behavior: a meta-analytic review of the social psychological literature. Psychol Bull. 1986;100(3):309–330. [PubMed] [Google Scholar]
- Eley TC, Lichtenstein P, Stevenson J. Sex differences in the etiology of aggressive and nonaggressive antisocial behavior: results from two twin studies. Child Dev. 1999;70(1):155–168. doi: 10.1111/1467-8624.00012. [DOI] [PubMed] [Google Scholar]
- Finkel D, McGue M. Sex differences and nonadditivity in heritability of MPQ personality scales. J Pers Soc Psychol. 1997;72:929–938. doi: 10.1037//0022-3514.72.4.929. [DOI] [PubMed] [Google Scholar]
- Frick PJ. Family dysfunction and the disruptive behavior disorders: a review of recent empirical findings. In: Ollendick TH, Prinz RJ, editors. Advances in clinical child. vol 16. Plenum; New York: 1994. pp. 203–226. [Google Scholar]
- Friedman HS, Booth-Kewley S. The ‘disease-prone personality’. A meta-analytic view of the construct. Am Psychol. 1987;42:439–455. doi: 10.1037//0003-066x.42.6.539. [DOI] [PubMed] [Google Scholar]
- Frodi A, Macaulay J, Thorne PR. Are women always less aggressive than men? A review of the experimental literature. Psychol Bull. 1977;84:634–660. [PubMed] [Google Scholar]
- Goodman G, Gerstadt C, Pfeffer CR, Stroh M, Valdez A. ADHD and aggression as correlates of suicidal behavior in assaultive prepubertal psychiatric inpatients. Suicide Life Threat Behav. 2008;38(1):46–59. doi: 10.1521/suli.2008.38.1.46. [DOI] [PubMed] [Google Scholar]
- Jang KL, Livesley WJ, Vernon PA, Jackson DN. Heritability of personality disorder traits: a twin study. Acta Psychiatrica Scandinavia. 1996;94:438–444. doi: 10.1111/j.1600-0447.1996.tb09887.x. [DOI] [PubMed] [Google Scholar]
- Knight GP, Guthrie IK, Page MC, Fabes RA. Emotional arousal and gender differences in aggression: a meta-analysis. Aggress Behav. 2002;28(5):366–393. [Google Scholar]
- Ligthart L, Bartels M, Hoekstra R, Hudziak J, Boomsma D. Genetic contributions to subtypes of aggression. Twin Res Hum Genet. 2005;8:483–491. doi: 10.1375/183242705774310169. [DOI] [PubMed] [Google Scholar]
- Linnoila M, Virkkunen M, Scheinin M, Nuutila A, Rimon R, Goodwin FK. Low cerebrospinal fluid 5-hydroxy indoleacetic acid concentration differentiates impulsive from nonimpulsive violent behavior. Life Sci. 1983;33:2609–2614. doi: 10.1016/0024-3205(83)90344-2. [DOI] [PubMed] [Google Scholar]
- Loeber R, Hay DF. Key issues in the development of aggression and violence from childhood to early adulthood. Annu Rev Psychol. 1997;48:371–410. doi: 10.1146/annurev.psych.48.1.371. [DOI] [PubMed] [Google Scholar]
- Lykken DT. The diagnosis of zygosity in twins. Behav Genet. 1978;8:437–473. doi: 10.1007/BF01067939. [DOI] [PubMed] [Google Scholar]
- Mason DA, Frick PJ. The heritability of antisocial behavior: a meta-analysis of twin and adoption studies. J Psychopathol Behav Assess. 1994;16(4):301–323. [Google Scholar]
- Miles DR, Carey G. Genetic and environmental architecture of human aggression. J Pers Soc Psychol. 1997;72(1):207–217. doi: 10.1037//0022-3514.72.1.207. [DOI] [PubMed] [Google Scholar]
- Muthén LK, Muthén BO. Mplus user’s guide. 5th edn Muthén & Muthén; Los Angeles, CA: 1998–2007. [Google Scholar]
- Neale M, Boker SM, Xie G, Maes HH. Mx: statistical modeling. 6th edn. Department of Psychiatry; Richmond, VA: 2003. p. 23298. VCU Box 900126. [Google Scholar]
- Plomin R, Daniels D. Why are the children in the same family so different from one another? Behav Brain Sci. 1987;10:1–60. [Google Scholar]
- Plomin R, Foch TT, Rowe DC. Bobo clown aggression in childhood: environment, not genes. J Res Pers. 1981;15(3):331–342. [Google Scholar]
- Poulin F, Boivin M. Reactive and proactive aggression: evidence of a two-factor model. Psychol Assess. 2000;12(2):115–122. doi: 10.1037//1040-3590.12.2.115. [DOI] [PubMed] [Google Scholar]
- Pulkkinen L. The path to adulthood for aggressively inclined girls. In: Björkqvist K, Niemelä P, editors. Of mice and women: aspects of female aggression. Academic Press; New York: 1992. pp. 113–121. [Google Scholar]
- Rhee SH, Waldman ID. Genetic and environmental influences on antisocial behavior: a meta-analysis of twin and adoption studies. Psychol Bull. 2002;128:490–529. [PubMed] [Google Scholar]
- Riley WT, Treiber FA, Woods MG. Anger and hostility in depression. J Nerv Ment Dis. 1989;177:668–674. doi: 10.1097/00005053-198911000-00002. [DOI] [PubMed] [Google Scholar]
- Saudino KJ, Hines DA. Etiological similarities between psychological and physical aggression in intimate relationships: a behavioral genetic exploration. J Fam Violence. 2007;22:121–129. [Google Scholar]
- Schwartz D, Dodge KA, Coie JD, Hubbard JA, Cillessen AHN, Lemerise EA, Bateman H. Social-cognitive and behavioral correlates of aggression and victimization in boys’ play groups. J Abnorm Child Psychol. 1998;26:431–440. doi: 10.1023/a:1022695601088. [DOI] [PubMed] [Google Scholar]
- Schwarz G. Estimating the dimension of a model. Ann Stat. 1978;6:461–464. [Google Scholar]
- Silberg JL, Erickson MT, Meyer JM, Eaves LJ. The application of structural equation modeling to maternal ratings of twins’ behavioral and emotional problems. Special section: structural equation modeling in clinical research. J Consult Clin Psychol. 1994;62:510–521. [PubMed] [Google Scholar]
- Sluyter F, Keisjer JN, Boomsma DI, van Doornen LJP, van den Oord EJCG, Sneider H. Genetics of testosterone and the aggression-hostility-anger (AHA) syndrome: a study of middle-aged male twins. Twin Res. 2000;3:266–276. [PubMed] [Google Scholar]
- Stouthamer-Loeber M, Loeber R. The use of prediction data in understanding delinquency. In: Bond LA, Compas BE, editors. Primary prevention and promotion in the schools. Sage; Newbury Park, CA: 1989. pp. 179–202. [Google Scholar]
- Straus MA, Hamby SL, Boney-McCoy S, Sugarman DB. The revised Conflict Tactics Scales (CTS2): development and preliminary psychometric data. J Fam Issue. 1996;17(3):283–316. [Google Scholar]
- Suris A, Lind L, Emmett G, Borman PD, Kashner M, Barratt ES. Measures of aggressive behavior: overview of clinical and research instruments. Aggress Violent Behav. 2004;9:165–227. [Google Scholar]
- Swanson JW, Holzer CE, Ganju VK, Jono RT. Violence and psychiatric disorder in the community: evidence from the Epidemiologic Catchment Area surveys. Hosp Community Psychiatry. 1990;41:761–770. doi: 10.1176/ps.41.7.761. [DOI] [PubMed] [Google Scholar]
- Torgersen S. The determination of twin zygosity by means of a mailed questionnaire. Acta Genet Med Gemellol. 1979;28(3):225–236. doi: 10.1017/s0001566000009077. [DOI] [PubMed] [Google Scholar]
- Torgersen S. Behavioral genetics of personality. Curr Psychiatry Rep. 2005;7:51–56. doi: 10.1007/s11920-005-0025-4. [DOI] [PubMed] [Google Scholar]
- Vaillancourt T, Brendgen M, Boivin M, Tremblay RE. A longitudinal confirmatory factor analysis of indirect and physical aggression: evidence of two factors over time? Child Dev. 2003;74:1628–1638. doi: 10.1046/j.1467-8624.2003.00628.x. [DOI] [PubMed] [Google Scholar]
- Van den Oord EJCG, Boomsma DI, Verhulst FC. A study of problem behaviors in 10- to 15-year-old biologically related and unrelated international adoptees. Behav Genet. 1994;24:193–205. doi: 10.1007/BF01067187. [DOI] [PubMed] [Google Scholar]
- Vernon PA, McCarthy JM, Johnson AM, Jang KL, Harris JA. Individual differences in multiple dimensions of aggression: a univariate and multivariate genetic analysis. Twin Res. 1999;2:16–21. doi: 10.1375/136905299320566068. [DOI] [PubMed] [Google Scholar]
- Vitaro F, Gendreau PL, Tremblay RE, Oligny P. Reactive and proactive aggression differentially predict later conduct problems. J Child Psychol Psychiatry. 1998;39(3):377–385. [PubMed] [Google Scholar]
- Vitaro F, Brendgen M, Barker ED. Subtyptes of aggressive behaviors: a developmental perspective. Int J Behav Dev. 2006;30(1):1–19. [Google Scholar]
- Volavka J, Nolan KA. Methodological structure for aggression research. Psychiatr Q. 2008;79(4):293–300. doi: 10.1007/s11126-008-9074-2. [DOI] [PubMed] [Google Scholar]
- Xie H, Cairns RB, Cairns BD. The development of social aggression and physical aggression: A narrative analysis of interpersonal conflicts. Aggress Behav. 2002a;28:341–355. [Google Scholar]
- Xie H, Swift DJ, Cairns BD, Cairns RB. Aggressive behaviors in social interaction and developmental adaptation: a narrative analysis of interpersonal conflicts during early adolescence. Social Dev. 2002b;11:205–224. [Google Scholar]
- Zahn-Waxler C. Warriors and worriers: gender and psychopathology. Dev Psychopathol. 1993;5:79–89. [Google Scholar]


