Abstract
BACKGROUND
High-quality lung cancer care includes physician-patient communication about the disease and treatment, patient needs/preferences, and care goals. In this study, the authors evaluated communication with patients at all stages across multiple topics.
METHODS
A standardized questionnaire asked patients with lung cancer to rate (on 5-point, verbal descriptor scale) the extent of communication with physicians on symptoms, spiritual concerns, practical needs, proxy appointment, living will preparation, prognosis, care goals, potential complications of therapy, life support preferences, and hospice. Communication was defined as inadequate if the patient reported discussing ≥5 of 11 questionnaire topics “not at all” or “a little bit.” Multivariate logistic regression was used to evaluate the factors associated with inadequate communication.
RESULTS
In total, 276 of 348 (79%) eligible patients were enrolled (mean age [±standard deviation], 65 ± 10 years; 55% white, 21% black, and 19% Hispanic; all disease stages). For most topics, the majority of respondents reported that physicians communicated “not at all” or “a little bit.” Low ratings were frequent for discussion of emotional symptoms (56%; 95% confidence interval [CI], 49%–62%), practical needs (71%; 95% CI, 65%–76%), spiritual concerns (80%; 95% CI, 75%–85%), proxy appointment (63%; 95% CI, 57%–69%), living will preparation (90%; 95% CI, 85%–93%), life support preferences (80%; 95% CI, 75%–84%), and hospice (88%; 95% CI, 86%–94%). Communication was inadequate for patients of different ages, stages, and races, although Hispanics were less likely than non-Hispanic whites and blacks to report inadequate communication (odds ratio, 0.31; 95% CI, 0.15–0.65).
CONCLUSIONS
Across all stages, patients with lung cancer reported low rates of physician-patient communication on key topics, which may increase patient distress, impair decision-making, and compromise clinical outcomes and use patterns.
Keywords: lung neoplasms, communication, physician-patient relations, quality of health care, symptoms, palliative care
High-quality lung cancer care includes effective physician-patient communication about the disease and treatment, patient needs and preferences, and goals of care.1 To inform rational decision-making about therapy and adequate planning for expected outcomes, patients need and generally want to know about the extent of their disease and the chances that it will be cured.2 Discussion of symptoms and other concerns that may arise from the illness also helps to optimize the patient’s quality of life at any stage of lung cancer. In addition, whether the prognosis is favorable, poor, or uncertain, the physician should elicit each patient’s values and preferences to offer a concordant plan of care. Open, clear, and compassionate communication by clinicians with cancer patients and their families promotes favorable outcomes, including satisfaction with care, adherence to treatment, and physical and psychological well being.3–5 Despite physicians’ concerns that addressing issues related to prognosis and end-of-life care preferences may result in harm to patients or families, research continues to demonstrate the benefits of such discussions.6,7 Early introduction of palliative care for patients with lung cancer, including discussion of goals of care and assistance with decision-making, is associated with significant improvements in quality of life and mood and even with longer survival (evaluated as a secondary endpoint) despite less aggressive treatment at the end of life.8 Thus, communication should be optimized throughout the trajectory of lung cancer.9
Previous studies have documented deficiencies in communication about end-of-life care between physicians and patients with advanced lung cancer.10,11 For example, of over 1500 patients with stage IV lung cancer in the national Cancer Care Outcomes Research and Surveillance (CanCORS) study, barely 50% reported discussing hospice with a care provider, yet more than 70% of those patients died within 6 months of the study interview.12 A survey of more than 4000 physicians caring for Can-CORS patients indicated that, despite national guidelines recommending that they discuss prognosis, resuscitation status, hospice, and preferred site of death with patients expected to die within a year, the majority of physicians would not initiate such a discussion even if death were expected in ≤6 months. Instead, they would defer discussions until the patient became symptomatic or failed non-palliative treatment, and they might never have these discussions unless initiated by the patient or family.13 However, to our knowledge, no previous research has systematically evaluated communication by physicians on a broader range of important topics with patients at all stages of lung cancer. In the current study, we asked patients who had been diagnosed within the previous year with all stages of lung cancer about communication with their physicians on topics related to goals and preferences for treatment and to patients’ physical, emotional, practical, and spiritual needs. We sought the patients’ reports of the extent of communication by their lung cancer physicians while also eliciting information about characteristics of the patient, the illness, and its treatment that may influence this communication.
MATERIALS AND METHODS
Patient Eligibility and Recruitment
From January 2008 through November 2009, we prospectively recruited consecutive patients who received inpatient or outpatient lung cancer care at 4 medical centers in New York City: Mount Sinai Hospital, Montefiore Medical Center, Columbia Presbyterian Hospital, and Harlem Hospital. Mount Sinai, Montefiore, and Columbia are private, not-for-profit, university-affiliated hospitals, each with >700 beds; Harlem is a city hospital with approximately 300 beds. Eligible patients were aged ≥18 years, had biopsy-proven nonsmall cell lung cancer, were diagnosed within the past 12 months, spoke English or Spanish, and had a telephone contact number. Exclusion criteria were the diagnosis of another malignancy (except for nonmelanoma skin cancer) within the past 5 years and lack of capacity. To ensure completeness of the screening and recruitment process, we made systematic use of multiple patient-finding methods, including review of pathology logs and of administrative databases reflecting past or scheduled visits to oncology, radiation oncology, and chest clinics and admissions to the hospital. In addition, we directly contacted health care providers who participate in the care of lung cancer patients. All patients provided informed consent for participation in this research, which was approved by the institutional review board of each institution.
Questionnaire Development
Together with input from an interdisciplinary panel of experts in lung cancer, physician-patient communication, health services research, palliative care, and psychology, we performed a literature review to identify relevant domains and items for the study questionnaire. Our questionnaire focused on communication between physicians and patients related to decision-making about treatment and goals of care; patients’ physical, emotional, spiritual, and practical needs; and health-related quality of life. Lung cancer symptoms were assessed using items from the European Organization for Research and Treatment of Cancer Quality of Life-Questionnaire (EORTC-QLQ-LC13).14 The extent of spiritual distress among responders was ascertained using the Functional Assessment of Chronic Illness Therapy-Spiritual Well Being (FACIT-Sp) measure.15 We also adapted items from the caregiver burden instrument reported by Emanuel et al16 to evaluate the impact of lung cancer on the respondent and family.
We pilot-tested the questionnaire in a sample of 12 patients with lung cancer, obtaining specific feedback on clarity of items and response options and on respondent burden. After making appropriate revisions, we tested the clinical sensibility of the questionnaire with 5 senior clinical researchers who have expertise in physician communication with patients about cancer care, palliative care, racial and ethnic disparities in care, and communication during serious illness. These reviewers provided quantitative ratings in 6 sensibility domains (clarity, face validity, content validity, utility, redundancy, and discriminability)17 as well as qualitative feedback, which we used to finalize the questionnaire. Our final questionnaire was translated into Spanish and back-translated into English by native Spanish speakers, with comparison to confirm semantic and content equivalence.
Data Collection
Trained, bilingual research assistants administered the questionnaire (in English or Spanish) during in-person interviews. If the patient was too ill or otherwise unavailable to meet in person, then the questionnaire was administered by telephone. We collected information about sociodemographic (age, sex, race, ethnicity, income) and clinical (stage, comorbidities) characteristics of patients and about the course and treatment of their lung cancer (including surgery, radiotherapy, and chemotherapy). Clinical information about cancer stage and treatment was confirmed by an independent review of the medical record. The main outcomes of interest were extent of communication on the following 11 topics: physical symptoms, emotional symptoms, spiritual concerns, practical needs, health care proxy appointment, advance directive preparation, chances of cure, potential complications of lung cancer treatment, goals of care, life support preferences, and hospice care. Ratings of communication used a 5-point Likert scale: discussed “not at all,” “a little bit,” “somewhat,” “quite a bit,” or “a lot.”
Data Analysis
We used descriptive statistics to characterize the patients in the study sample and to summarize patients’ ratings of physician communication on each topic in the questionnaire. For this study, in the absence of a gold standard for evaluating the adequacy of communication about lung cancer from the perspective of its content or extent, we defined communication as inadequate if the patient reported that the physician discussed ≥5 of the 11 topics “not at all” or “a little bit.” We calculated the proportion of patients for whom communication was inadequate by this definition with 95% confidence intervals (CIs) based on the binomial distribution. We used multiple logistic regression analyses to identify factors that were associated independently with inadequate communication. All analyses were performed with SAS statistical software (SAS Inc., Cary, NC) using 2 sided P values.
RESULTS
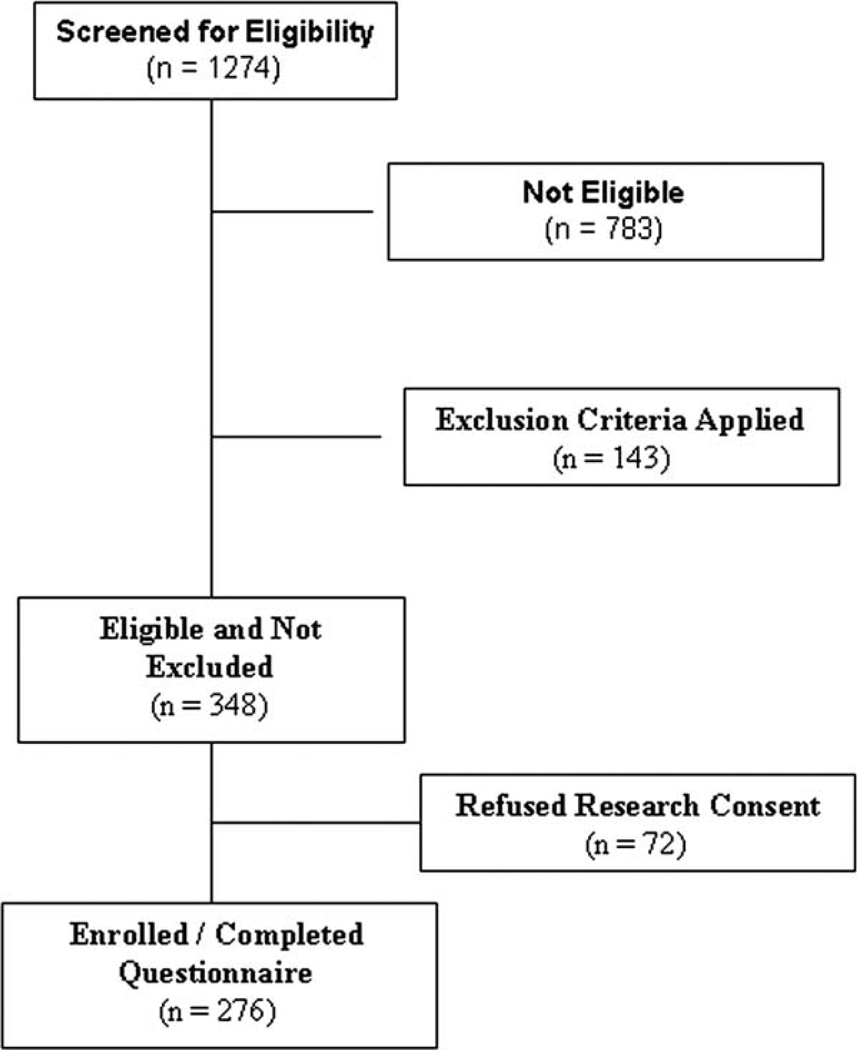
Between January 2008 and November 2009, we identified 348 patients who were eligible for the study and 276 of those patients (79.3%) were enrolled (Fig. 1). Characteristics of these patients are listed in Table 1. The mean (±standard deviation) age was 65.3 ± 10.3 years, and 44% of patients were men. Approximately 55% of patients were white, 21% were African American, and 19% were Hispanic. According to pathologic or clinical staging, 47%, 8%, 22%, and 22% of patients had stage I, II, III, and IV disease, respectively. The sex and race/ethnicity of patients who declined study participation did not differ significantly from those of study participants, although the mean age of nonparticipants (72.4 ± 11.0 years) differed significantly from that of participants (P = .0001). The median time from diagnosis of lung cancer to the study interview was 3.3 months (interquartile range, 1.6–5.7 months).
Figure 1.
The screening and recruitment of study participants are illustrated. This chart illustrates the process that led to the enrollment of 276 of 348 patients (79.3% enrollment rate) who were eligible for the current study.
Table 1.
Characteristics of the Study Patients (N=276)a
| Characteristic | No. of Patients (%) |
|---|---|
| Men | 121 (43.8) |
| Age: Mean±SD, y | 65.3±10.3 |
| Race | |
| White | 151 (54.7) |
| Black | 59 (21.3) |
| Hispanic | 53 (19.2) |
| Other | 13 (4.7) |
| Religious preference | |
| Protestant | 48 (17.4) |
| Catholic | 117 (42.4) |
| Jewish | 34 (12.3) |
| None | 31 (11.2) |
| Other | 46 (16.7) |
| Education | |
| Did not graduate high school | 63 (25.3) |
| High school graduate | 109 (43.8) |
| College graduate | 77 (30.9) |
| Household income during previous y | |
| ≤$15,000 | 59 (32.6) |
| $15,001–$50,000 | 64 (35.4) |
| >$50,000 | 58 (32) |
| Uninsured during previous y | 25 (9.1) |
| Lung cancer stage | |
| IA | 92 (33.3) |
| IB | 37 (13.4) |
| IIA | 6 (2.2) |
| IIB | 17 (6.2) |
| IIIA | 31 (11.3) |
| IIIB | 30 (10.9) |
| IV | 61 (22.1) |
| Time from diagnosis to study interview: Median [IQR], mo | 3.3 [1.6–5.7] |
Abbreviations: IQR, interquartile range; SD, standard deviation.
For several characteristics, totals are <276 because some patients did not answer every item in the questionnaire. Data on stage were unavailable for 2 patients (0.7%).
Patients across all stages of lung cancer frequently reported symptom distress, functional impairment, and other concerns. Greater than 20% of patients rated their pain within the prior week at the highest levels on the scale (“quite a bit” or “very much”). Approximately 25% reported that the illness left them “completely unable to work at a job or to do household jobs.” Patients also reported spiritual concerns at the highest levels, endorsing the statements that “my life lacks meaning and purpose” and “I have trouble feeling peace of mind” (17% and 19% of respondents, respectively). Patients described a range of practical needs (eg, obtaining transportation to medical appointments and nursing care at home), and about 10% reported that their illness had caused significant financial difficulty.
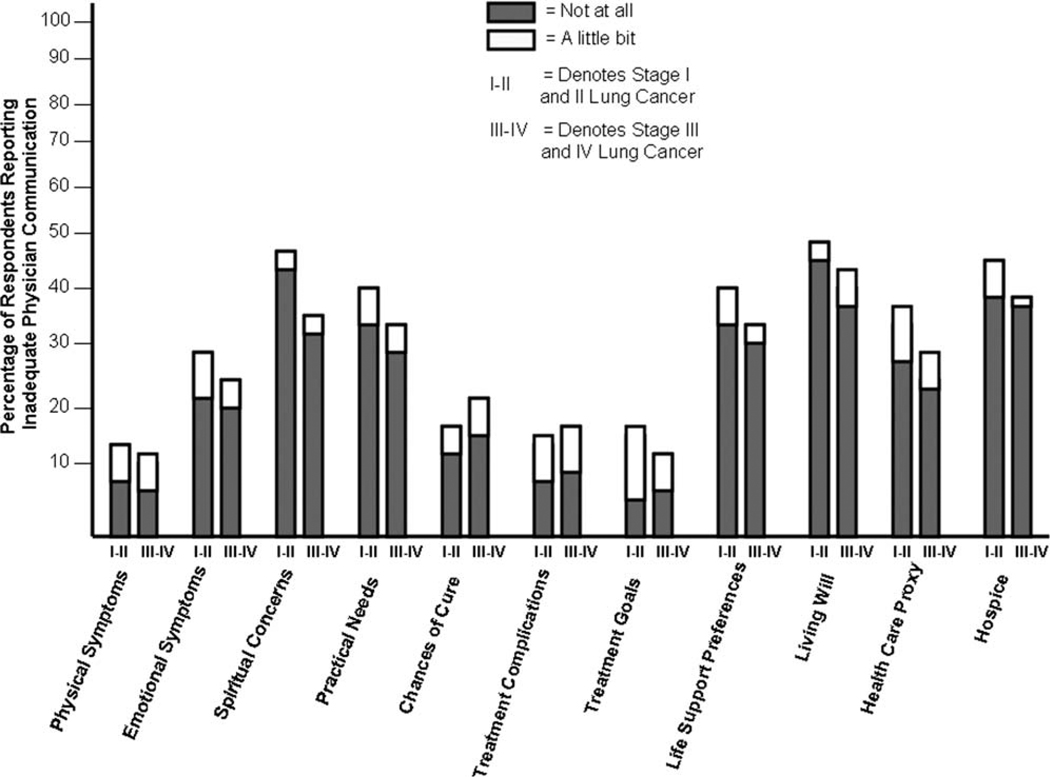
Patients reported low levels of physician communication about most of the topics that were assessed in the questionnaire (Fig. 2). Twenty percent of respondents (95% CI, 15%–25%) reported that their physicians communicated “not at all” or “a little bit” on all 11 topics. The percentages of patients who gave these low ratings for individual topics were 40% (95% CI, 34%–46%) for the chances of curing their lung cancer, 80% (95% CI, 75%–84%) for preferences for resuscitation/life-sustaining treatment, and 90% (95% CI, 85%–93%) for preparation of a written living will. However, 111 of 276 respondents (40%; 95% CI, 36%–48%) who had a preference with respect to cardiopulmonary resuscitation stated that they would not want cardiopulmonary resuscitation to be attempted in the event of an arrest, and 60% (95% CI, 54%–65%) would not want mechanical ventilation for respiratory failure. Almost 90% (95% CI, 86%–94%) of respondents with stage III and IV disease (N = 121) reported they received no or little information about hospice from their physicians, whereas misconceptions about hospice were prevalent: >33% (95% CI, 29%–41%) of these respondents believed that hospice care was restricted to patients in the last few days of life, and 66% (95% CI, 60%–71%) thought that such care could not be provided at home.
Figure 2.
This chart illustrates the extent of communication on key topics. Patients with lung cancer reported that their physicians communicated “not at all” or “a little bit.” Patients were asked to rate (on a 5-point, quantitative, verbal descriptor Likert scale) the extent of physician-patient communication on 11 topics, as indicated on the horizontal axis. The chart indicates the proportion of respondents (N = 276), stratified by early stage disease (stages I and II) and late stage disease (stages III and IV), who reported that their physicians discussed the topic “not at all” or “a little bit” (the 2 lowest ratings on the scale).
The extent of communication with physicians, as rated by their patients with lung cancer, did not differ according to stage of disease. Overall, 52% of patients reported that communication with physicians was inadequate (ie, communication occurred “not at all” or only “a little bit” on ≥5 key topics). Table 2 indicates that the rates of inadequate communication were equally high for patients with late stage disease (stage III or IV) and early stage disease (stages I and II). Multiple logistic regression analysis indicated that these rates also were similar among patients of different age, sex, income, and pathologic stage (P > .05 for all comparisons). Compared with whites, blacks reported similarly high rates (OR, 0.66; 95% CI, 0.32–1.4); however, Hispanics were less likely (OR, 0.31; 95% CI, 0.15–0.65) to report inadequate communication with their health care providers.
Table 2.
Rates of Communication About Palliative Care Needs According to Stage at Diagnosis
| No. of Patients (%)a | |||
|---|---|---|---|
| Topic | Discussed | Not Discussed | P |
| Physical symptoms | |||
| Stage I–II | 109 (75) | 37 (25) | .28 |
| Stage III–IV | 97 (80) | 24 (20) | |
| Emotional symptoms | |||
| Stage I–II | 68 (46) | 78 (54) | .62 |
| Stage III–IV | 60 (50) | 61 (50) | |
| Spiritual concerns | |||
| Stage I–II | 26 (18) | 120 (82) | .04 |
| Stage III–IV | 34 (28) | 87 (72) | |
| Practical needs | |||
| Stage I–II | 44 (30) | 102 (70) | .42 |
| Stage III–IV | 42 (35) | 79 (65) | |
| Chances of curing cancer | |||
| Stage I–II | 97 (66) | 49 (34) | .03 |
| Stage III–IV | 65 (54) | 56 (46) | |
| Goal of treatment | |||
| Stage I–II | 116 (81) | 27 (19) | .51 |
| Stage III–IV | 90 (78) | 26 (22) | |
| Healthcare proxy | |||
| Stage I–II | 55 (38) | 88 (62) | .88 |
| Stage III–IV | 46 (39) | 71 (61) | |
| Living will | |||
| Stage I–II | 18 (12) | 126 (88) | .29 |
| Stage III–IV | 10 (8) | 108 (92) | |
| Life-sustaining treatment | |||
| Stage I–II | 29 (22) | 108 (78) | .98 |
| Stage III–IV | 24 (21) | 90 (79) | |
| Potential complications | |||
| Stage I–II | 102 (70) | 44 (30) | .47 |
| Stage III–IV | 79 (65) | 42 (35) | |
| Hospiceb | |||
| Stage III–IV | 14 (12) | 107 (88) | |
The numbers for stage groups are not identical across items, because some patients did not answer every item in the study questionnaire.
The number for this item was 121, the number of patients with stage III and IV disease, for whom a discussion of hospice likely would be most relevant.
DISCUSSION
In this prospective, multicenter study of 276 patients with lung cancer who were recruited across all disease stages, patients’ reports revealed little or no communication with physicians about important topics, including symptoms, prognosis, potential benefits and complications of treatment, treatment preferences, surrogate decision-making, practical needs, spiritual concerns, and hospice care. Poor communication was a generalized problem for patients of different age, sex, and disease stage and likely resulted in unattended patient needs. Because effective communication on topics in our questionnaire is integral to high-quality cancer care, our findings suggest that communication is an important target for improvement efforts.
Our study adds to existing evidence of deficiencies in communication with lung cancer patients. More than a decade ago, the Study to Understand Prognoses and Preferences for Outcomes and Risks of Treatments (SUPPORT) study demonstrated that patients with advanced (stage III or IV) nonsmall cell lung cancer significantly overestimated their chances of surviving for 6 months compared with their physicians’ estimates. Patients who received sufficient information to understand that there was even a small chance (as low as 10%) of dying within 6 months were more likely to choose comfort as a primary goal of care.18 Two subsequent studies of patients with newly diagnosed lung cancer indicated that, although patients knew their diagnosis after discussion with the physician, many were unclear about the extent of their disease and about whether the intent of treatment they were receiving was to cure the cancer or to improve symptoms and prolong life.19,20 Similarly, a qualitative analysis of audiotaped discussions between patients with lung cancer and their thoracic surgeons or oncologists revealed that physicians rarely responded empathically to patients’ stated concerns about the impact of the cancer on their lives.21
The current study contributes new evidence about communication across a broad range of topics with lung cancer patients at all stages of the disease. Our questionnaire addressed not only prognosis and end-of-life care but also topics related to decision-making and patient needs throughout the trajectory of lung cancer. We elicited ratings of communication from patients who presented at early or late stages and from those who pursued disease-modifying treatments or received care focused primarily or exclusively on comfort. In addition, we investigated factors that may influence the extent of communication between lung cancer patients and their physicians. Our results indicate that important opportunities exist to improve communication across topics that are relevant for patients with earlier stages of lung cancer and for those with more advanced disease.
A variety of barriers to communication with lung cancer patients have been identified. Many physicians lack the training and supervised experiences needed for strengthening skills in communication.22,23 For most patients with lung cancer, the physician not only must disclose distressing information but also must address the attendant emotions, 2 tasks that can be especially challenging without advanced skills.21,24,25 Our finding that, as reported by patients, physicians were more likely to discuss prognosis (“chances of curing the cancer”) with patients who presented at stages I and II than those who presented at stages III and IV suggests that that this topic is especially challenging in the context of advanced disease. Even experienced clinicians can benefit from focused training for such difficult discussions. Comprehensive conversations that include discussion of treatment preferences and goals as well as physical, emotional, and practical concerns often are time-consuming, yet physicians may not receive appropriate recognition or compensation for this effort. Patients, in turn, may not be explicit about their concerns.26 Issues related to low health literacy and discordance between physicians and patients with respect to language, race, ethnicity, or culture may further complicate communication about lung cancer.27
The established benefits of effective communication between physicians and patients with cancer highlight the importance of overcoming these barriers. Communication that meets patient needs enhances patient satisfaction and psychological well being.7,20 For patients with advanced cancer (including lung cancer), Wright et al reported that discussion with the physician about care the patient would want to receive if they were dying was associated not with depression or anxiety but, rather, with greater acceptance of the gravity of the illness, preference for stage-appropriate goals of care, completion of an order not to attempt resuscitation, earlier enrollment in hospice, and better quality of life near death. Better outcomes also were observed during the bereavement period for the patients’ informal caregivers.6 In addition, patients who reported these discussions were less likely to receive mechanical ventilation in the last week of life, which translated to substantial cost savings for this preterminal period during which such treatment generally is more burdensome than beneficial.28,29 Wagner et al reported that, among patients with terminal illness, those with a poorer understanding of prognosis were less likely to discuss care preferences with their surrogate decision-makers, suggesting that better provider communication about prognosis may translate into greater concordance between care planning and patient preferences.30 Ambulatory patients with metastatic lung cancer who received early integration of palliative care with standard cancer care, including timely communication about treatment goals and preferences, had better quality of life and mood as well as longer survival.8
Although disparities in lung cancer care are well documented,31 Hispanic patients in our cohort were less likely to report inadequate communication by their physicians. Because we measured the adequacy of communication from patients’ reports rather than from direct observation, there are multiple possible explanations for this finding. It is possible that Hispanic patients began with lower expectations for communication with their physicians and, thus, were less likely to rate the communication they received as inadequate. Another possibility is that Hispanic patients hesitated to report negatively on the communication because that may have suggested a lack of appreciation or respect for the physician responsible for their lung cancer care. In a recent report by Beach et al,32 Hispanic patients with human immunodeficiency virus/acquired immunodeficiency syndrome rated communication with physicians more positively than white patients, although this communication was lacking by direct observation. Wallace et al, in a recent cross-sectional analysis of a nationally representative survey on medical care, reported that, compared with non-Hispanic whites, Hispanic whites were more likely to report positively on communication with their health care providers.33 Alternatively, the results from our current analysis may reflect a preference on the part of some Hispanics to avoid discussing some of the topics that were covered by our questionnaire, such as prognosis, the appointment of a surrogate decision-maker, or other advance care planning,34,35 or to avoid involving their families to a greater extent than themselves in communication with their physicians.36 Finally, the possibility exists that prior evidence of health care disparities or other factors led physicians in our study to make special efforts to ensure adequate communication with Hispanic lung cancer patients.
Many resources are available to assist physicians in their efforts to optimize communication with lung cancer patients. The National Cancer Institute sponsored the “Oncotalk” program specifically to provide training in communication skills to physicians who care for cancer patients.23 Randomized, controlled research has indicated that appropriate training improves the communication skills of cancer physicians.37 In addition, research illuminates the concerns that many cancer patients wish to discuss with their physicians and the communication approaches they prefer. The majority of patients want physicians to provide realistic prognostic information in a straightforward way that allows the patient to maintain hope and a sense of control.7,38 Effective strategies for identification of empathic opportunities and for explicit verbalization of empathy have been described.21,39
In the majority of US hospitals, palliative care consultants currently are available to facilitate communication with patients and families. They typically provide multidisciplinary expertise, including physicians, nurses, social workers, and chaplains who have been trained and certified in skills for communicating about serious illness.40 Some institutions have established a program of proactive palliative care consultation after a diagnosis of lung cancer, even for patients with early stage disease.41 Brief, practical tools are available for assessment of a range of needs (symptom control, practical support, spiritual concerns) that are common among patients with lung cancer and their families.14,42
This study has limitations. First, we relied on patients’ reports rather than direct observation of physician-patient encounters. What physicians actually said to the patients is not necessarily the same as what the patients heard at the time or remembered or reported in the study interview. The information that patients understand and retain, however, is an important indicator of the adequacy of communication.3,43 Second, our study investigated communication on a defined set of topics for all patients, whereas prior literature suggests that patients’ informational needs are variable in some respects and that optimal communication is sensitive to the specific preferences of individual patients.11,44 Some patients may not have appreciated a need for communication on all topics in our questionnaire, whereas others may have wished for further discussion of topics we did not include. We focused on topics of broad importance while also attempting to minimize the burden on patients participating in the study and to optimize the rate of response. We acknowledge that prior research has not clearly established a gold standard for adequacy of physician communication in terms of content or extent. Third, the questionnaire in this study asked about communication in quantitative descriptor terms (scale ranging from “not at all” to “a lot”), whereas qualitative ratings may have provided a different perspective on the adequacy of communication. Fourth, most of the study sites were large medical centers in New York City and, although the patients were a diverse cohort evaluated by a large number of physicians across 4 different hospitals, our observations may not be representative of practice in other settings. However, our population included a significant percentage of minorities, who are at higher risk of poor lung cancer outcomes. Fifth, men represented 44% of our study cohort, whereas the proportion of men with lung cancer nationally is slightly greater than 50%. Sixth, because of referral patterns in some of the participating centers, stage I disease was over represented in our study sample compared with the proportion of such patients in national statistics on lung cancer stages. In addition, the median time between diagnosis and questionnaire administration was 3.3 months. However, communication on topics in our study questionnaire is relevant across the trajectory of lung cancer, and we did not observe differences in reports of communication between patients with early stage disease and those with advanced lung cancer. Rates of death from disease progression are substantial even for patients with earlier stages of lung cancer, and these patients face mortality and morbidity associated with treatments aimed at cure as well as with their underlying illness. For these patients, as for patients with more advanced disease, communication about treatment preferences, symptoms, and other topics in our questionnaire (apart from hospice, which we analyzed separately) is important for informed decision-making, symptom control, treatment adherence, and coping. Our questionnaire focused on communication with “lung cancer physicians” and did not ask patients whether they had discussed these topics with a palliative care clinician or with another professional caregiver, such as a nurse or a chaplain; this is also a limitation. Finally, it is possible that patients who declined to participate in this study would have responded differently than those who participated. However, we were able to capture a high proportion of eligible lung cancer patients across disease stages at the study sites, and the participants did not differ significantly in sex or race/ethnicity from the patients who declined enrollment.
In conclusion, current clinical practice guidelines recommend early integration of palliative care in lung cancer management, even for patients who pursue curative or life-prolonging therapies.1,45–47 Our current study documents opportunities to improve physician-patient communication across a range of topics that are relevant at every stage of lung cancer. Evidence-based strategies for effective communication about cancer, communication skills training for physicians who provide cancer care, and interdisciplinary teams of palliative care specialists all are available to support primary physicians in the communication process. Throughout the trajectory of lung cancer, clinicians should make use of available resources to ensure that patients receive opportunities to discuss goals of treatment in relation to prognosis and preferences and to report distressing symptoms and other concerns arising from the illness.
Acknowledgments
We are grateful to Amy Walker, MA, Adjoa Boateng, BA, Andrea Maldonado, BA, Kivil Stacik, BA, and Liliana Serrano, BA, for assistance with the data collection and to Linda Lurslurchachai, MPH, for overall project management. We also gratefully acknowledge the cooperation of Stuart Packer, MD, and other lung cancer clinicians at our institutions with recruitment and other aspects of this research and the willingness of the patients to participate in study interviews. Jean S. Kutner, MD, MSPH, and Leslie J. Blackhall, MD, MTS, generously contributed their expertise to the refinement of the questionnaire for individual interviews. Holly Prigerson, PhD, also provided valuable insights for the questionnaire.
FUNDING SOURCES
This work was supported by research grants from the American Cancer Society (PEP2-114269 and RSGT-07-162-01-CPHPS to Dr. Nelson and Dr. Wisnivesky, respectively). The sponsor had no role in the design or conduct of the study; collection, management, analysis, or interpretation of the data; or preparation, review, or approval of the article.
CONFLICT OF INTEREST DISCLOSURES
Dr. Wisnivesky is a member of the research board of EHE International, has received a research grant from GlaxoSmithKline, and has received lecture fees from Novartis Pharmaceutical. Dr. Powell has received a research grant from Philips, NA.
REFERENCES
- 1.Griffin JP, Koch KA, Nelson JE, Cooley ME. Palliative care consultation, quality-of-life measurements, and bereavement for end-of-life care in patients with lung cancer: ACCP evidence-based clinical practice guidelines (2nd edition) Chest. 2007;132(3 suppl):404S–422S. doi: 10.1378/chest.07-1392. [DOI] [PubMed] [Google Scholar]
- 2.Hagerty RG, Butow PN, Ellis PM, et al. Communicating with realism and hope: incurable cancer patients’ views on the disclosure of prognosis. J Clin Oncol. 2005;23:1278–1288. doi: 10.1200/JCO.2005.11.138. [DOI] [PubMed] [Google Scholar]
- 3.Heyland DK, Allan DE, Rocker G, Dodek P, Pichora D, Gafni A. Discussing prognosis with patients and their families near the end of life: impact on satisfaction with end-of-life care. Open Med. 2009;3:e101–e110. [PMC free article] [PubMed] [Google Scholar]
- 4.Zolnierek KB, Dimatteo MR. Physician communication and patient adherence to treatment: a meta-analysis. Med Care. 2009;47:826–834. doi: 10.1097/MLR.0b013e31819a5acc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Stewart MA. Effective physician-patient communication and health outcomes: a review. CMAJ. 1995;152:1423–1433. [PMC free article] [PubMed] [Google Scholar]
- 6.Wright AA, Zhang B, Ray A, et al. Associations between end-of-life discussions, patient mental health, medical care near death, and caregiver bereavement adjustment. JAMA. 2008;300:1665–1673. doi: 10.1001/jama.300.14.1665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Trice ED, Prigerson HG. Communication in end-stage cancer: review of the literature and future research. J Health Commun. 2009;14 suppl 1:95–108. doi: 10.1080/10810730902806786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Temel JS, Greer JA, Muzinkansky A, et al. Early palliative care for patients with metastatic non-small cell lung cancer. N Engl J Med. 2010;363:733–742. doi: 10.1056/NEJMoa1000678. [DOI] [PubMed] [Google Scholar]
- 9.Foley K, Gelband H, editors. Improving Palliative Care for Cancer. Washington, DC: National Academy Press; 2001. [PubMed] [Google Scholar]
- 10.Haidet P, Hamel MB, Davis RB, et al. Outcomes, preferences for resuscitation, and physician-patient communication among patients with metastatic colorectal cancer. SUPPORT Investigators. Study to Understand Prognoses and Preferences for Outcomes and Risks of Treatments. Am J Med. 1998;105:222–229. doi: 10.1016/s0002-9343(98)00242-3. [DOI] [PubMed] [Google Scholar]
- 11.Hofmann JC, Wenger NS, Davis RB, et al. Patient preferences for communication with physicians about end-of-life decisions. SUPPORT Investigators. Study to Understand Prognoses and Preference for Outcomes and Risks of Treatment. Ann Intern Med. 1997;127:1–12. doi: 10.7326/0003-4819-127-1-199707010-00001. [DOI] [PubMed] [Google Scholar]
- 12.Huskamp HA, Keating NL, Malin JL, et al. Discussions with physicians about hospice among patients with metastatic lung cancer. Arch Intern Med. 2009;169:954–962. doi: 10.1001/archinternmed.2009.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Keating NL, Landrum MB, Rogers SO, Jr, et al. Physician factors associated with discussions about end-of-life care. Cancer. 2010;116:998–1006. doi: 10.1002/cncr.24761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bergman B, Aaronson NK, Ahmedzai S, Kaasa S, Sullivan M. The EORTC QLQ-LC13: a modular supplement to the EORTC Core Quality of Life Questionnaire (QLQ-C30) for use in lung cancer clinical trials. EORTC Study Group on Quality of Life. Eur J Cancer. 1994;30A:635–642. doi: 10.1016/0959-8049(94)90535-5. [DOI] [PubMed] [Google Scholar]
- 15.Peterman AH, Fitchett G, Brady MJ, Hernandez L, Cella D. Measuring spiritual well-being in people with cancer: the functional assessment of chronic illness therapy—Spiritual Well-Being Scale (FACIT-Sp) Ann Behav Med. 2002;24:49–58. doi: 10.1207/S15324796ABM2401_06. [DOI] [PubMed] [Google Scholar]
- 16.Emanuel EJ, Fairclough DL, Slutsman J, Emanuel LL. Understanding economic and other burdens of terminal illness: the experience of patients and their caregivers. Ann Intern Med. 2000;132:451–459. doi: 10.7326/0003-4819-132-6-200003210-00005. [DOI] [PubMed] [Google Scholar]
- 17.Feinstein AR. Clinical sensibility. In: Feinstein AR, editor. Clinimetrics. New Haven, CT: Yale University Press; 1987. pp. 141–166. [Google Scholar]
- 18.Hakim RB, Teno JM, Harrell FE, Jr, Knaus WA, Wenger N, Phillips RS. Factors associated with do-not-resuscitate orders: patients’ preferences, prognoses, and physicians’ judgments. SUPPORT Investigators. Study to Understand Prognoses and Preferences for Outcomes and Risks of Treatment. Ann Intern Med. 1996;125:284–293. doi: 10.7326/0003-4819-125-4-199608150-00005. [DOI] [PubMed] [Google Scholar]
- 19.Quirt CF, Mackillop WJ, Ginsburg AD, et al. Do doctors know when their patients don’t? A survey of doctor-patient communication in lung cancer. Lung Cancer. 1997;18:1–20. doi: 10.1016/s0169-5002(97)00048-2. [DOI] [PubMed] [Google Scholar]
- 20.Gabrijel S, Grize L, Helfenstein E, et al. Receiving the diagnosis of lung cancer: patient recall of information and satisfaction with physician communication. J Clin Oncol. 2008;26:297–302. doi: 10.1200/JCO.2007.13.0609. [DOI] [PubMed] [Google Scholar]
- 21.Morse DS, Edwardsen EA, Gordon HS. Missed opportunities for interval empathy in lung cancer communication. Arch Intern Med. 2008;168:1853–1858. doi: 10.1001/archinte.168.17.1853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Back AL, Arnold RM, Baile WF, et al. Faculty development to change the paradigm of communication skills teaching in oncology. J Clin Oncol. 2009;27:1137–1141. doi: 10.1200/JCO.2008.20.2408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Back AL, Arnold RM, Baile WF, et al. Efficacy of communication skills training for giving bad news and discussing transitions to palliative care. Arch Intern Med. 2007;167:453–460. doi: 10.1001/archinte.167.5.453. [DOI] [PubMed] [Google Scholar]
- 24.Baile WF, Aaron J. Patient-physician communication in oncology: past, present, and future. Curr Opin Oncol. 2005;17:331–335. doi: 10.1097/01.cco.0000167738.49325.2c. [DOI] [PubMed] [Google Scholar]
- 25.Schapira L, Butow P, Brown R, Boyle F. Pessimism is no poison. J Clin Oncol. 2010;28:705–707. doi: 10.1200/JCO.2009.25.0027. [DOI] [PubMed] [Google Scholar]
- 26.Anderson WG, Alexander SC, Rodriguez KL, et al. “What concerns me is…” Expression of emotion by advanced cancer patients during outpatient visits. Support Care Cancer. 2008;16:803–811. doi: 10.1007/s00520-007-0350-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Amalraj S, Starkweather C, Nguyen C, Naeim A. Health literacy, communication, and treatment decision-making in older cancer patients. Oncology (Williston Park) 2009;23:369–375. [PubMed] [Google Scholar]
- 28.Reichner CA, Thompson JA, O’Brien S, Kuru T, Anderson ED. Outcome and code status of lung cancer patients admitted to the medical ICU. Chest. 2006;130:719–723. doi: 10.1378/chest.130.3.719. [DOI] [PubMed] [Google Scholar]
- 29.Zhang B, Wright AA, Huskamp HA, et al. Health care costs in the last week of life: associations with end-of-life conversations. Arch Intern Med. 2009;169:480–488. doi: 10.1001/archinternmed.2008.587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wagner GJ, Riopelle D, Steckart J, Lorenz KA, Rosenfeld KE. Provider communication and patient understanding of life-limiting illness and their relationship to patient communication of treatment preferences. J Pain Symptom Manage. 2010;39:527–534. doi: 10.1016/j.jpainsymman.2009.07.012. [DOI] [PubMed] [Google Scholar]
- 31.Wisnivesky JP, McGinn T, Henschke C, Hebert P, Iannuzzi MC, Halm EA. Ethnic disparities in the treatment of stage I non-small cell lung cancer. Am J Respir Crit Care Med. 2005;171:1158–1163. doi: 10.1164/rccm.200411-1475OC. [DOI] [PubMed] [Google Scholar]
- 32.Beach MC, Saha S, Korthuis PT, et al. Differences in patient-provider communication for Hispanic compared with non-Hispanic white patients in HIV care. J Gen Intern Med. 2010;25:682–687. doi: 10.1007/s11606-010-1310-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wallace LS, DeVoe JE, Rogers ES, Malagon-Rogers M, Fryer GE., Jr The medical dialogue: disentangling differences between Hispanic and non-Hispanic whites. J Gen Intern Med. 2007;22:1538–1543. doi: 10.1007/s11606-007-0368-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Smith AK, Sudore RL, Perez-Stable EJ. Palliative care for Latino patients and their families: whenever we prayed, she wept. JAMA. 2009;301:1047–1057. E1. doi: 10.1001/jama.2009.308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Smith AK, McCarthy EP, Paulk E, et al. Racial and ethnic differences in advance care planning among patients with cancer: impact of terminal illness acknowledgment, religiousness, and treatment preferences. J Clin Oncol. 2008;26:4131–4137. doi: 10.1200/JCO.2007.14.8452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Morrison RS, Meier DE. High rates of advance care planning in New York City’s elderly population. Arch Intern Med. 2004;164:2421–2426. doi: 10.1001/archinte.164.22.2421. [DOI] [PubMed] [Google Scholar]
- 37.Fallowfield L, Jenkins V, Farewell V, Saul J, Duffy A, Eves R. Efficacy of a Cancer Research UK communication skills training model for oncologists: a randomised controlled trial. Lancet. 2002;359:650–656. doi: 10.1016/S0140-6736(02)07810-8. [DOI] [PubMed] [Google Scholar]
- 38.Butow PN, Dowsett S, Hagerty R, Tattersall MH. Communicating prognosis to patients with metastatic disease: what do they really want to know? Support Care Cancer. 2002;10:161–168. doi: 10.1007/s005200100290. [DOI] [PubMed] [Google Scholar]
- 39.Hack TF, Degner LF, Parker PA. The communication goals and needs of cancer patients: a review. Psychooncology. 2005;14:831–845. doi: 10.1002/pon.949. discussion 846–847. [DOI] [PubMed] [Google Scholar]
- 40.Goldsmith B, Dietrich J, Du Q, Morrison RS. Variability in access to hospital palliative care in the United States. J Palliat Med. 2008;11:1094–1102. doi: 10.1089/jpm.2008.0053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Borneman T, Koczywas M, Cristea M, Reckamp K, Sun V, Ferrell B. An interdisciplinary care approach for integration of palliative care in lung cancer. Clin Lung Cancer. 2008;9:352–360. doi: 10.3816/CLC.2008.n.051. [DOI] [PubMed] [Google Scholar]
- 42.Steinhauser KE, Voils CI, Clipp EC, Bosworth HB, Christakis NA, Tulsky JA. “Are you at peace?”: one item to probe spiritual concerns at the end of life. Arch Intern Med. 2006;166:101–105. doi: 10.1001/archinte.166.1.101. [DOI] [PubMed] [Google Scholar]
- 43.Beckett MK, Elliott MN, Richardson A, Mangione-Smith R. Outpatient satisfaction: the role of nominal versus perceived communication. Health Serv Res. 2009;44(5 pt 1):1735–1749. doi: 10.1111/j.1475-6773.2009.01001.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Fried TR, Bradley EH, O’Leary J. Prognosis communication in serious illness: perceptions of older patients, caregivers, and clinicians. J Am Geriatr Soc. 2003;51:1398–1403. doi: 10.1046/j.1532-5415.2003.51457.x. [DOI] [PubMed] [Google Scholar]
- 45.National Consensus Project for Quality Palliative Care. Clinical Practice Guidelines for Quality Palliative Care. 2nd ed. Pittsburgh, PA: National Consensus Project for Quality Palliative Care; 2009. [Google Scholar]
- 46.Ettinger DS, Bepler G, Bueno R, et al. Non-small cell lung cancer clinical practice guidelines in oncology. J Natl Compr Canc Netw. 2006;4:548–582. doi: 10.6004/jnccn.2006.0046. [DOI] [PubMed] [Google Scholar]
- 47.Silvestri GA, Sherman C, Williams T, Leong SS, Flume P, Turrisi A. Caring for the dying patient with lung cancer. Chest. 2002;122:1028–1036. doi: 10.1378/chest.122.3.1028. [DOI] [PubMed] [Google Scholar]