Abstract
Focal adhesion kinase (FAK) is a non-receptor tyrosine kinase that resides at the sites of at focal adhesions. The 125 kDa FAK protein is encoded by the FAK gene located on human chromosome 8q24. Structurally, FAK consists of an amino-terminal regulatory FERM domain, a central catalytic kinase domain, and a carboxy-terminal focal adhesion targeting domain. FAK has been shown to be an important mediator of cell adhesion, growth, proliferation, survival, angiogenesis and migration, all of which are often disrupted in cancer cells. Normal tissues have low expression of FAK, while primary and metastatic tumors significantly overexpress this protein. This review summarizes expression of FAK by immunohistochemical staining in different tumor types and presents several FAK inhibition therapy approaches.
Keywords: Adhesion, cancer, Focal Adhesion Kinase, inhibitor, metastasis, tumor
BREAST CANCER
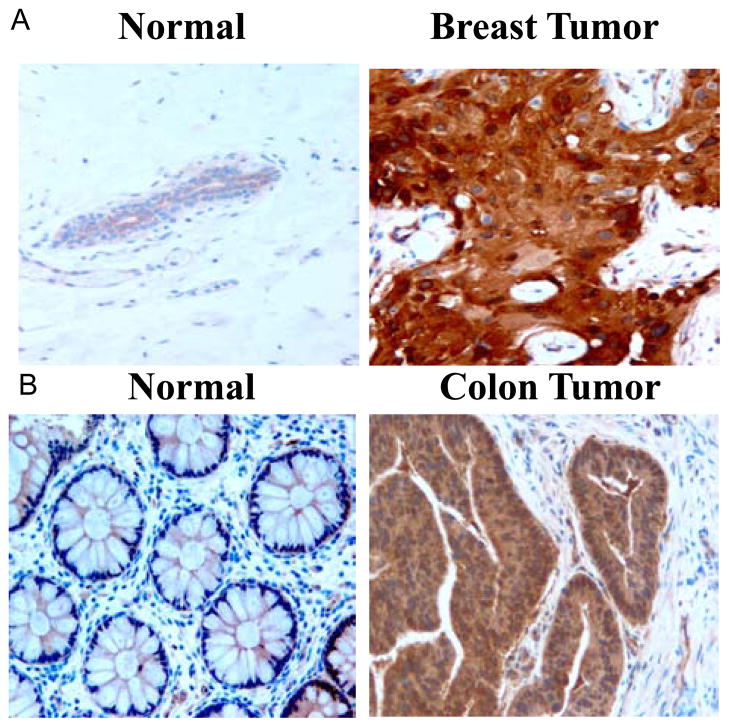
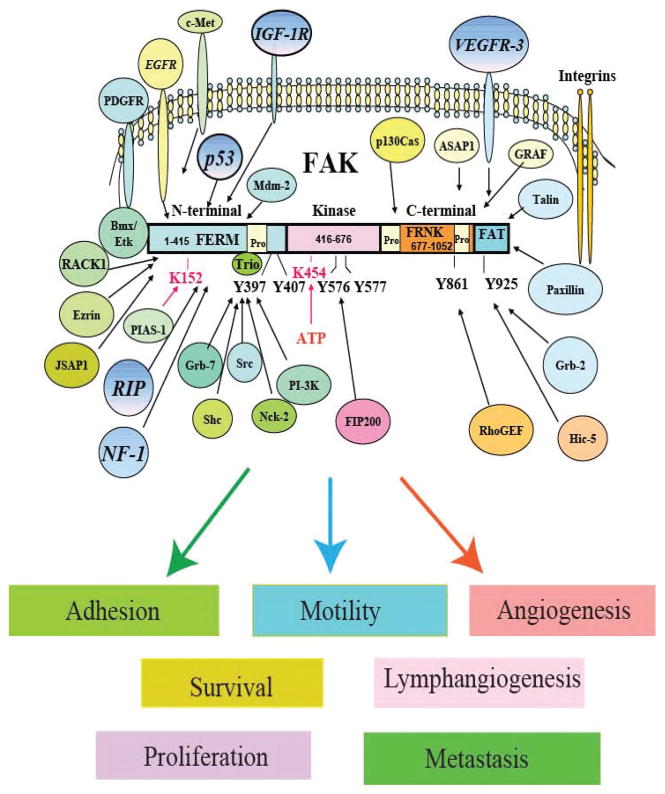
Focal Adhesion Kinase was significantly elevated in invasive and metastatic breast tumors (Fig. 1), suggesting that FAK can be a marker of invasive tumors [1]. The structure of Focal Adhesion Kinase and proteins interacting with Focal Adhesion Kinase are shown on Fig. (2). FAK has three domains (N-terminal, Kinase domain and C-terminal domain), interacts with many proteins and plays important role in adhesion, survival, proliferation, angiogenesis, lympangiogenesis, motility, invasion and metastasis (Fig. 2). Dual inhibition of FAK and EGFR (which are both overexpressed in breast tumors) signaling pathways cooperatively enhanced apoptosis in breast cancers [2]. Also dual inhibition of activated form of the Src tyrosine kinase and FAK in breast cancer cells (BT474 and MCF-7) increased cancer cell detachment and apoptosis, indicating cooperative FAK and Src signaling in breast tumorigenesis [3–5]. FAK expression has been shown to be up-regulated in DCIS breast tumors suggesting as an early event in breast tumorigenesis [6]. High FAK expression was associated with poor prognostic indicators, including high mitotic index, nuclear grade 3, architectural grade 3, estrogen and progesterone receptor negative, and overexpression of HER-2/neu in 629 breast cancer tumors[7]. Recently, disruption of FAK blocked mammary tumor progression in a transgenic mouse Cre/loxP breast cancer model [8]. Recently mutations in p53 has bee shown to increase FAK mRNA and protein expression in breast cancer cells [9]. High positive correlation between FAK overexpression and p53 mutations has been shown in 596 breast cancer tumors, demonstrating that p53 regulates FAK expression during breast tumorigenesis [10].
Fig. 1.
Overexpression of Focal Ahhesion Kinase (FAK) in breast cancer tumors. Immunohistochemistry (IHC) staining was performed on breast (A) and colon (B) tumor (right) and matched normal (left) tissue samples. FAK 4.47 antibody (Upstate) was used for IHC.
Fig. 2.
The structure of Focal Adhesion Kinase and its interacting proteins with intracellular signaling. FAK structure has N-terminal (including FERM domain); Central Kinase and C-terminal domains. The N-terminal domain has Y397 site, the main autophosphorylation site. The Kinase domain has Y576/Y577 tyrosines, critical for kinase activity of FAK. The C-terminal domain has main Y861 and Y925 tyrosines. FAK has many interacting binding partners and plays role in motility, invasion, metastasis, proliferation, adhesion, angiogenesis, lymphangiogenesis and survival.
FAK has been proposed a potential target in cancer therapy [11]. The FAK kinase inhibitor TAE226 (Novartis) induced apoptosis in breast cancer cells [12]. Another group used the Src kinase inhibitor SKI-606 (bosutinib) to block breast carcinogenesis and found that it blocked FAK phosporylation and suppressed human breast cancer cell migration and invasion [13]. Another inhibitor PF-271 (Pfizer) targeting ATP-binding site blocked FAK phosphorylation and breast cancer tumorigenesis [14]. The novel inhibitor of FAK, called Y15 or compound 14 targeting Y397 site of FAK also blocked breast tumorigenesis [15]. Recently, novel FAK kinase inhibitor, PND1186 blocked breast tumor progression and spontaneous breast to lung metastases [16, 17].
NEUROBLASTOMA
Treatment of neuroblastoma cells with okadaic acid (OA), a serine phosphatase inhibitor, increasing serine/threonine phosphorylation and inhibiting tyrosine phosphorylation, induced focal adhesion loss, actin cytoskeleton disorganization, and cellular detachment, which corresponded to a loss of FAK Tyr397 [18]. The authors suggested that inhibitors, causing FAK dephosphorylation may be potentially therapeutic drugs in neuroblastoma cells. We have demonstrated that N-Myc can regulate FAK expression in neuroblastoma through binding to the FAK promoter (Beirlie et al., 2007). Recently, FAK expression was analyzed on 70 formalin-fixed, paraffin-embedded human neuroblastoma specimens [19]. The authors demonstrated that FAK was expressed in 73% of neuroblastoma tumor samples [19]. FAK staining was significantly increased in stage IV tumors with the amplification of the N-MYC oncogene, providing basis for targeting FAK in neuroblastoma treatment [19].
Most recently the novel FAK molecule inhibitor TAE226 has been used on neuroblastoma cells, and the drug decreased cell viability and increased apoptosis suggesting that FAK is a potential therapy target of neurobalstoma [20]. Simultaneous inhibition of FAK with dominant-negative inhibitor, C-terminal domain of FAK and Src with PP2 inhibitor resulted in increased apoptosis in neuroblastoma cells [20]. Novel autophosphorylation inhibitor of FAK also effectively blocked neuroblastoma tumorigenesis especially in the case of N-myc-positive cells [21].
PANCREATIC CANCER
Inhibition of FAK with FAKsiRNA potentiated gemcitabine-induced cytotoxicity in pancreatic cells [22]. FAK siRNA treatment down-regulated AKT activity [22]. In addition, down-regulation of FAK with siRNA caused cell increased anoikis in pancreatic adenocarcinoma PANC-1, BxPC3 and MiaPaCa-2 cell lines [23]. FAKsiRNA also inhibited metastasis in a nude mouse model [23]. Furthermore, down-regulating FAK with siRNA decreased cell motility and invasiveness in pancreatic cells [24]. Analysis of FAK expression in 50 patients with pancreatic invasive ductal carcinoma by immunohistochemistry showed its detection in 24 samples (48%) [25]. There was a statistically significant correlation between FAK expression and tumor size (P=0.004), although FAK expression did not significantly correlate with other factors such as tumor histological grade, lymph node metastasis, distant metastasis, histological stage, and overall survival. The authors conclude that focal adhesion kinase expression may not be a prognostic marker for pancreatic cancer patients [25]. The more extended study of pancreatic cancer needed to be analyzed for FAK expression, as it correlated with tumor size. Recently, FAK was shown to promote survival in pancreatic adenocarcinoma by interacting with IGF-IR and activating pathways that lead to cell proliferation and survival [26]. FAK autophosphorylation inhibitor, Y15 blocked pancreatic tumorgenesis [27].
OVARIAN CANCER AND CERVICAL CANCERS
We have shown that FAK expression was increased in ovarian cancers (26 cancer samples from patients with Stage I–IV ovarian carcinoma and two ovarian carcinoma cell lines) compared to normal samples [28]. The authors suggested that FAK may be a potential target for therapeutic disruption of ovarian carcinoma progression [28]. Recently FAK overexpression in ovarian cancer and absence in normal ovarian epithelial cells was also demonstrated by several other groups [29–31]. It has also been suggested that FAK activated the PI3-Akt signaling pathway and induced expression of KFL8 (Kruppel-like factor 8), a transcription factor important in cell cycle regulation, oncogenic transformation, and epithelial to mesenchymal transition in human ovarian epithelial and cancer cells [32].
Ovarian cancer cells mobility has been inhibited recently by inhibiting FAK phosphorylation and focal adhesion assembly with the potential anti-cancer agent TGZ (Troglitazone) [33]. TAE226 (Novartis) has also been used in combination with docetaxel to significantly inhibit ovarian cancer cell growth [34]. The authors suggested that TAE226 can be an effective therapeutic approach in ovarian carcinoma [34]. FAK has been shown to be activated in human cervical cancer [35].
FAK overexpression has been suggested to be a marker for malignant transformation of cervical cancer [36]. The authors found minimal FAK expression in benign cervical epithelium and in low-grade squamous dysplasia (CIN I and CIN I–II) of the cervix, while most of the invasive SCCs of the cervix (13 of 16 cases) were positive for FAK [36]. In another studies, FAK phosphorylation was linked to the cervical cancer invasion [37], and a poor prognosis [29].
OSTEOSARCOMA
Expression analysis of 16 osteosarcoma tumors, 5 osteosarcoma cell lines and 6 normal tissues demonstrated FAK overexpression in high grade osteosarcomas [38]. FAK expression analysis performed in our group in 13 high grade sarcomas showed high levels in all tumor samples compared to benign, noninvasive mesenchymal specimens [1]. This analysis shows that FAK can be a potential target in these tumors, although more detailed study with correlation analysis of other tumor markers will be critical.
KIDNEY CANCER
Comparative analysis of FAK expression in metastatic and non-metastatic renal carcinoma cells revealed that FAK and paxillin mRNA expression were up-regulated 2.0–2.5 fold in the metastatic Caki-1 cells over normal renal cortex epithelial cells (RCEC), suggesting its potential role as a marker of metastasis [39].
Expression of FAK and HGF (hepatocyte growth factor) synergistically increased transformation of Madin-Darby canine kidney epithelial cells [40].
LUNG CANCER
In lung surgical specimens, phosphorylated FAK has been shown to be the major component among 100–130 kDa phosphorylated proteins correlating with poor patient prognosis [41]. In addition, increased phosphorylation of FAK has been demonstrated in lung cancer samples and its absence in normal tissues [42]. The increased phosphorylation of FAK was closely correlated with the nodal involvement of cancer and disease-free survival time [42]. Expression of laminin 5, regulating cell adhesion and anoikis, and increased phosphorylation of FAK in lung adenocarcinoma cells suggested the importance of the laminin-integrin-FAK pathway in tumorigenesis [43]. Stimulation of small cell lung cancer cells with HGF (hepatocyte growth factor) activated c-Met and increased phosphorylation of Y397 FAK, suggesting this pathway as a therapeutic target in lung tumorigenesis [44]. FAK signaling has been demonstrated to be important in the early stages of mammary adenocarcinoma lung metastasis [45]. In an experimental model of mammary metastasis, lung metastasis formation was prevented when the dominant-negative FAK inhibitor, FRNK, was expressed one day before tumor cell injection, whereas at 11 days after injection expression of FRNK did not affect lung metastasis formation [45]. Immunohistochemical staining of FAK in 60 formalin-fixed and paraffin-embedded non-small cell lung cancer (NSCLC) tumors demonstrated increased FAK levels compared with non-neoplastic tissues [46]. Moreover, Western blotting and real-time RT-PCR showed a statistically significant correlation between FAK up-regulation and higher disease stages (I+II versus III+IV, p=0.019 and 0.028, respectively), indicating FAK involvement in tumorigenesis [46].
HEAD AND NECK CANCER
In a recent study, hepatocyte growth factor/scatter factor HFG/SF induced phosphorylation of FAK and Erk in cultured squamous cell carcinoma of the head and neck, indicating its role in tumorigenesis [47]. Inhibition of Focal Adhesion Kinase with a dominant-negative C-terminal FAK, Ad-FRNK caused increased apoptosis and decrease of cell motility in epithelial cells from head and neck carcinomas (SCCHN cells) [48]. FAK expression analysis by immunohistochemistry in 211 head and neck squamous cell carcinoma (HNSCC) tissue samples, including 147 primary tumors, 56 lymph node metastases, 3 benign hyperplasias, and 5 dysplasias demonstrated elevated FAK expression in 62% of tumor samples [49]. Positive immunostaining was also detected in benign hyperplasias and preinvasive dysplastic lesions, indicating that FAK overexpression is an early event during tumorigenesis. FAK DNA copy number ratios were higher in 39% of the tumors compared with normal matched samples. However, not all cases of FAK overexpression FAK contained gene amplification indicating additional mechanisms of FAK expression regulation [49]. Inhibiting of FAK by adenoviral Ad-FRNK and introduction of exogenous p53 with Ad-p53 cause increased apoptosis of the carcinoma cells and improved cytotoxic effect of chemotherapy drugs [50]. Photodynamic therapy, effective in treatment of head and neck invasive cancers, decreased cancer cell motility and FAK-phosphorylation and downstream survival signaling [51]. Recently, FAK was shown to be important in cellular invasion of head and neck cancer [52]. Disruption of FAK caused decreased cell attachment, motility and invasion while FAK overexpression increased cell invasion.
ORAL CANCER
FAK was found to be overexpressed in oral cancers [53]. Overexpression of FAK in low-invading cells resulted in a 4.5-fold increase in the rate of invasion compared with control cell lines, suggesting that enhanced expression of FAK in oral carcinoma cells may lead to a selective growth advantage and increased invasive potential of the primary oral tumor [54]. In another study, authors suggested that activation of FAK by phosphorylation of c-Met could mediate the HGF/SF-induced motility of human oral squamous cell carcinoma cells, and that Rho protein could regulate the tyrosine phosphorylation of FAK through translocation from the nucleus to the membrane [55]. Increased FAK phosphorylation and expression was observed in oral carcinoma of the larynx [56].
HEPATOCELLULAR CARCINOMA
FAK expression analysis in 60 hepatocellular carcinoma demonstrated overexpression of FAK mRNA and protein in tumor samples compared to matched non-tumor liver samples [57]. The fact that FAK overexpression correlated significantly with the tumor size (P=0.034) and serum AFP level (P=0.030) led to the suggestion that FAK can be a prognostic therapeutic factor in these tumors [57]. Down-regulation of FAK with the dominant-negative FRNK caused metastatic adhesion of carcinoma cells within liver sinusoids [58]. The hepatitis B virus (HBV) involved in hepatic cell transformation activated FAK, suggesting importance of FAK signaling in HBV-associated hepatocellular carcinogenesis [59].
COLON CANCER
We have shown that FAK was overexpressed in invasive and metastatic colon tumors on both protein and mRNA levels [60, 61]. FAK protein overexpression was seen in 32 out of 80 cases of colon adenocarcinoma [62]. An increase of tyrosine phosphorylation of FAK (Y-397) was detected in colorectal cancer cells [63]. Phospho-FAK correlated with invasiveness and lymph node metastasis in colon cancers [64]. We have demonstrated that simultaneous inhibition of FAK and Src pathways caused increased apoptosis in colon cancer cell lines, suggesting that multiple signaling is involved in tumorigenesis [65]. Recently, FAK has been suggested to mediate pressure-induced colon cancer cell adhesion [66].
PROSTATE CANCER
Increased FAK protein and mRNA levels and phosphorylation was observed in metastatic prostate cancer cells that correlated with the progression and metastasis of tumors [67]. FAK signaling has been suggested to play a critical role in invasiveness and motility of prostate cancers [68, 69]. Recently, FAK has been shown to be important player in prostate cancer progression and essential for androgen-independent formation of neuroendocrine carcinoma in the transgenic adenocarcinoma of mouse prostate (TRAMP) model with decreased FAK expression by either Cre-mediated recombination or with inhibited FAK activity with the small-molecule inhibitor, PF-562,271, suggesting that inhibition of FAK can be potential approach for castrate-resistant prostate tumors [70].
BRAIN CANCER
Anaplastic astrocytoma brain tumors expressed higher levels of FAK and autophosphorylation compared to non-neoplastic adult normal brain tissues [71]. Overexpression of FAK promoted Ras activity through the formation of FAK/p120RasGAP complex in malignant astrocytoma cells cultured in aggregate suspension or as monolayers adherent to vitronectin [72]. Overexpression of FAK in serum-starved glioblastoma cells plated on recombinant (rec)-osteopontin resulted in enhancement of basal migration and in a more significant increase of PDGF-BB-stimulated migration. Both expression of mutant FAK(397F) and FAK down-regulation with small interfering siRNA inhibited basal and PDGF-stimulated migration [73]. Recently, a novel kinase inhibitor of FAK (TAE226) has been shown to increase apoptosis in brain tumors [74].
MELANOMA
Increased FAK constitutive phosphorylation was observed in human metastatic melanoma cells [75]. Focal adhesion increased expression correlated with motility of human melanoma cells [76]. FAK expression appeared to be important for tumor cell adhesion in melanoma [77]. Constitutive activation of FAK was regulated by the cytoskeleton in melanoma cells important for aggressive tumor growth [78]. We treated melanoma cell lines with antisense FAK oligonucleotides, inhibiting FAK expression, and demonstrated increased cell apoptosis and sensitivity to the chemotherapy drug 5-fluorouracil [79]. Treatment of melanoma cells with dominant-negative FAK-related FRNK increased the aggressive phenotype of the cells, demonstrated by decreased invasion and motility and inhibiting Erk1/2 mediated signaling pathway [80]. Recently FAK has been linked with the enhanced malignant properties of ganglioside GD-3- expressing melanoma cells [81].
The compound geraniin, a form of tannin separated from geranium, has been shown to induce apoptosis in human melanoma cells by promoting caspace-3 mediated cleavage of FAK [82]. A synthetic agent, Manbeta(1–4)[Fucalpha(1–3)]Glcbeta1-Cer, (gly-cosphingolipid 7), which was identified in the millipede Parafontaria laminata armigera, had an inhibitory effect on proliferation of the melanoma cells [83]. Glycosphingolipid 7 suppressed the activation of the FAK-Akt and Erk1/2 pathways, which caused decrease in the expression of cyclin D1 and CDK4 [83]. The authors suggest that glycosphingolipid 7 might be a candidate for an inhibitor of cell proliferation in melanomas [83].
THYROID CANCER
We analyzed p125FAK expression in 30 human thyroid tissue samples that included paired normal and malignant specimens [84]. The highest levels of p125FAK were seen in follicular carcinomas and tumors associated with distant metastatic foci [84]. FAK was not expressed in normal tissue and nodular hyperplasia but was expressed in all of the follicular, papillary, medullary, and anaplastic thyroid carcinomas [85]. This result indicates that the up-regulation of FAK may play a role in the development of thyroid carcinogenesis.
ACUTE MYELOID LEUKEMIA
FAK expression was analyzed in 60 cases of acute myeloid leukemia (AML) and both the FAK protein and mRNA were detected in about 42% of the cases. Aberrant expression of FAK was significantly correlated with high blast cell mount, early death and shorter survival rate. FAK expression in enhanced blast cell migration and celularity, and equated to a poor prognosis [86]. Understanding the role of FAK in this cancer may be critical in the development of an effective treatment.
CONCLUSION
This review demonstrates overexpression of FAK in many types of tumors that is important for motility, angiogenesis and metastasis. The novel inhibitors targeting FAK signaling by blocking FAK autophosphorylation site, ATP-binding site or protein interactions can be future therapy approach.
Acknowledgments
The work is supported by Susan G. Komen Grant to Vita Golubovskaya.
References
- 1.Owens LV, Xu L, Craven RJ, Dent GA, Weiner TM, Kornberg L, Liu ET, Cance WG. Overexpression of the focal adhesion kinase (p125FAK) in invasive human tumors. Cancer Res. 1995;55(13):2752–2755. [PubMed] [Google Scholar]
- 2.Golubovskaya V, Beviglia L, Xu LH, Earp HS, 3rd, Craven R, Cance W. Dual inhibition of focal adhesion kinase and epidermal growth factor receptor pathways cooperatively induces death receptor-mediated apoptosis in human breast cancer cells. J Biol Chem. 2002;277(41):38978–38987. doi: 10.1074/jbc.M205002200. [DOI] [PubMed] [Google Scholar]
- 3.Park HB, Golubovskaya V, Xu L, Yang X, Lee JW, Scully S, 2nd, Craven RJ, Cance WG. Activated Src increases adhesion, survival and alpha2-integrin expression in human breast cancer cells. Biochem J. 2004;378(2):559–567. doi: 10.1042/BJ20031392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Madan R, Smolkin MB, Cocker R, Fayyad R, Oktay MH. Focal adhesion proteins as markers of malignant transformation and prognostic indicators in breast carcinoma. Hum Pathol. 2006;37(1):9–15. doi: 10.1016/j.humpath.2005.09.024. [DOI] [PubMed] [Google Scholar]
- 5.Mitra SK, Schlaepfer DD. Integrin-regulated FAK-Src signaling in normal and cancer cells. Curr Opin Cell Biol. 2006;18(5):516–523. doi: 10.1016/j.ceb.2006.08.011. [DOI] [PubMed] [Google Scholar]
- 6.Lightfoot HM, Jr, Lark A, Livasy CA, Moore DT, Cowan D, Dressler L, Craven RJ, Cance WG. Upregulation of focal adhesion kinase (FAK) expression in ductal carcinoma in situ (DCIS) is an early event in breast tumorigenesis. Breast Cancer Res Treat. 2004;88(2):109–116. doi: 10.1007/s10549-004-1022-8. [DOI] [PubMed] [Google Scholar]
- 7.Lark AL, Livasy CA, Dressler L, Moore DT, Millikan RC, Geradts J, Iacocca M, Cowan D, Little D, Craven RJ, Cance W. High focal adhesion kinase expression in invasive breast carcinomas is associated with an aggressive phenotype. Mod Pathol. 2005;18(10):1289–1294. doi: 10.1038/modpathol.3800424. [DOI] [PubMed] [Google Scholar]
- 8.Lahlou H, Sanguin-Gendreau V, Zuo D, Cardiff RD, McLean GW, Frame MC, Muller WJ. Mammary epithelial-specific disruption of the focal adhesion kinase blocks mammary tumor progression. Proc Natl Acad Sci USA. 2007;104(51):20302–20307. doi: 10.1073/pnas.0710091104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Golubovskaya VM, Finch R, Kweh F, Massoll NA, Campbell-Thompson M, Wallace MR, Cance WG. 53 regulates FAK expression in human tumor cells. Mol Carcinog. 2008;47(5):373–382. doi: 10.1002/mc.20395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cance WG, Golubovskaya VM. Focal adhesion kinase versus p53: apoptosis or survival? Sci Signal. 2008;1(20):pe22. doi: 10.1126/stke.120pe22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.van Nimwegen MJ, van de Water B. Focal adhesion kinase: A potential target in cancer therapy. Biochem Pharmacol. 2007;73(5):597–609. doi: 10.1016/j.bcp.2006.08.011. [DOI] [PubMed] [Google Scholar]
- 12.Golubovskaya VM, Virnig C, Cance WG. TAE226-induced apoptosis in breast cancer cells with overexpressed Src or EGFR. Mol Carcinog. 2008;47(3):222–234. doi: 10.1002/mc.20380. [DOI] [PubMed] [Google Scholar]
- 13.Vultur A, Buettner R, Kowolik C, Liang W, Smith D, Boschelli F, Jove R. SKI-606 (bosutinib), a novel Src kinase inhibitor, suppresses migration and invasion of human breast cancer cells. Mol Cancer Ther. 2008;7(5):1185–1194. doi: 10.1158/1535-7163.MCT-08-0126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Roberts WG, Ung E, Whalen P, Cooper B, Hulford C, Autry C, Richter D, Emerson E, Lin J, Kath J, Coleman K, Yao L, Martinez-Alsina L, Lorenzen M, Berliner M, Luzzio M, Patel N, Schmitt E, LaGreca S, Jani J, Wessel M, Marr E, Griffor M, Vajdos F. Antitumor activity and pharmacology of a selective focal adhesion kinase inhibitor, PF-562,271. Cancer Res. 2008;68(6):1935–1944. doi: 10.1158/0008-5472.CAN-07-5155. [DOI] [PubMed] [Google Scholar]
- 15.Golubovskaya VM, Nyberg C, Zheng M, Kweh F, Magis A, Ostrov D, Cance WG. A small molecule inhibitor, 1,2,4,5-benzenetetraamine tetrahydrochloride, targeting the Y397 site of focal adhesion kinase decreases tumor growth. J Med Chem. 2008;51(23):7405–7416. doi: 10.1021/jm800483v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Walsh C, Tanjoni I, Uryu S, Tomar A, Nam JO, Luo H, Phillips A, Patel N, Kwok C, McMahon G, Stupack DG, Schlaepfer DD. Oral delivery of PND-1186 FAK inhibitor decreases tumor growth and spontaneous breast to lung metastasis in pre-clinical models. Cancer Biol Ther. 2010;9(10):778–790. doi: 10.4161/cbt.9.10.11433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tanjoni I, Walsh C, Uryu S, Tomar A, Nam JO, Mielgo A, Lim ST, Liang C, Koenig M, Sun C, Patel N, Kwok C, McMahon G, Stupack DG, Schlaepfer DD. PND-1186 FAK inhibitor selectively promotes tumor cell apoptosis in three-dimensional environments. Cancer Biol Ther. 2010;9(10):764–777. doi: 10.4161/cbt.9.10.11434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kim B, van Golen CM, Feldman EL. Degradation and dephosphorylation of focal adhesion kinase during okadaic acid-induced apoptosis in human neuroblastoma cells. Neoplasia. 2003;5(5):405–416. doi: 10.1016/s1476-5586(03)80043-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Beierle EA, Massoll NA, Hartwich J, Kurenova EV, Golubovskaya VM, Cance WG, McGrady P, London WB. Focal adhesion kinase expression in human neuroblastoma: immunohistochemical and real-time PCR analyses. Clin Cancer Res. 2008;14(11):3299–3305. doi: 10.1158/1078-0432.CCR-07-1511. [DOI] [PubMed] [Google Scholar]
- 20.Beierle EA, Trujillo A, Nagaram A, Golubovskaya VM, Cance WG, Kurenova EV. TAE226 inhibits human neuroblastoma cell survival. Cancer Invest. 2008;26(2):145–151. doi: 10.1080/07357900701577475. [DOI] [PubMed] [Google Scholar]
- 21.Beierle EA, Ma X, Stewart J, Nyberg C, Trujillo A, Cance WG, Golubovskaya VM. Inhibition of focal adhesion kinase decreases tumor growth in human neuroblastoma. Cell Cycle. 2010;9(5):1005–1015. doi: 10.4161/cc.9.5.10936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Duxbury MS, Ito H, Benoit E, Zinner MJ, Ashley SW, Whang EE. RNA interference targeting focal adhesion kinase enhances pancreatic adenocarcinoma gemcitabine chemosensitivity. Biochem Biophys Res Commun. 2003;311(3):786–792. doi: 10.1016/j.bbrc.2003.10.060. [DOI] [PubMed] [Google Scholar]
- 23.Duxbury MS, Ito H, Zinner MJ, Ashley SW, Whang EE. Focal adhesion kinase gene silencing promotes anoikis and suppresses metastasis of human pancreatic adenocarcinoma cells. Surgery. 2004;135(5):555–562. doi: 10.1016/j.surg.2003.10.017. [DOI] [PubMed] [Google Scholar]
- 24.Huang YT, Lee LT, Lee PP, Lin YS, Lee MT. Targeting of focal adhesion kinase by flavonoids and small-interfering RNAs reduces tumor cell migration ability. Anticancer Res. 2005;25(3B):2017–2025. [PubMed] [Google Scholar]
- 25.Furuyama K, Doi R, Mori T, Toyoda E, Ito D, Kami K, Koizumi M, Kida A, Kawaguchi Y, Fujimoto K. Clinical significance of focal adhesion kinase in resectable pancreatic cancer. World J Surg. 2006;30(2):219–226. doi: 10.1007/s00268-005-0165-z. [DOI] [PubMed] [Google Scholar]
- 26.Liu W, Bloom DA, Cance WG, Kurenova EV, Golubovskaya VM, Hochwald SN. Fak And Igf-Ir Interact To Provide Survival Signals In Human Pancreatic Adenocarcinoma Cells. Carcinogenesis. 2008;29(6):1096–1107. doi: 10.1093/carcin/bgn026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hochwald SN, Nyberg C, Zheng M, Zheng D, Wood C, Massoll NA, Magis A, Ostrov D, Cance WG, Golubovskaya VM. A novel small molecule inhibitor of FAK decreases growth of human pancreatic cancer. Cell Cycle. 2009;8(15):2435–2443. doi: 10.4161/cc.8.15.9145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Judson PL, He X, Cance WG, Van Le L. Overexpression of focal adhesion kinase, a protein tyrosine kinase, in ovarian carcinoma. Cancer. 1999;86(8):1551–1556. doi: 10.1002/(sici)1097-0142(19991015)86:6<1551::aid-cncr23>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- 29.Gabriel B, Mildenberger S, Weisser CW, Metzger E, Gitsch G, Schule R, Muller JM. Focal adhesion kinase interacts with the transcriptional coactivator FHL2 and both are overexpressed in epithelial ovarian cancer. Anticancer Res. 2004;24(2):921–927. [PubMed] [Google Scholar]
- 30.Grisaru-Granovsky S, Salah Z, Maoz M, Pruss D, Beller U, Bar-Shavit R. Differential expression of protease activated receptor 1 (Par1) and pY397FAK in benign and malignant human ovarian tissue samples. Int J Cancer. 2005;113(3):372–378. doi: 10.1002/ijc.20607. [DOI] [PubMed] [Google Scholar]
- 31.Sood AK, Coffin JE, Schneider GB, Fletcher MS, DeYoung BR, Gruman LM, Gershenson DM, Schaller MD, Hendrix MJ. Biological significance of focal adhesion kinase in ovarian cancer: role in migration and invasion. Am J Pathol. 2004;165(4):1087–1095. doi: 10.1016/S0002-9440(10)63370-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wang X, Urvalek AM, Liu J, Zhao J. Activation of KLF8 transcription by focal adhesion kinase in human ovarian epithelial and cancer cells. J Biol Chem. 2008;283(20):13934–13942. doi: 10.1074/jbc.M709300200. [DOI] [PubMed] [Google Scholar]
- 33.Yang YC, Ho TC, Chen SL, Lai HY, Wu JY, Tsao YP. Inhibition of cell motility by troglitazone in human ovarian carcinoma cell line. BMC Cancer. 2007;7:216–218. doi: 10.1186/1471-2407-7-216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Halder J, Lin YG, Merritt WM, Spannuth WA, Nick AM, Honda T, Kamat AA, Han LY, Kim TJ, Lu C, Tari AM, Bornmann W, Fernandez A, Lopez-Berestein G, Sood AK. Therapeutic efficacy of a novel focal adhesion kinase inhibitor TAE226 in ovarian carcinoma. Cancer Res. 2007;67(22):10976–10983. doi: 10.1158/0008-5472.CAN-07-2667. [DOI] [PubMed] [Google Scholar]
- 35.McCormack SJ, Brazinski SE, Moore JL, Jr, Werness BA, Goldstein DJ. Activation of the focal adhesion kinase signal transduction pathway in cervical carcinoma cell lines human genital epithelial cells immortalized with human papillomavirus type 18. Oncogene. 1997;15(3):265–274. doi: 10.1038/sj.onc.1201186. [DOI] [PubMed] [Google Scholar]
- 36.Oktay MH, Oktay K, Hamele-Bena D, Buyuk A, Koss LG. Focal adhesion kinase as a marker of malignant phenotype in breast and cervical carcinomas. Hum Pathol. 2003;34(3):240–245. doi: 10.1053/hupa.2003.40. [DOI] [PubMed] [Google Scholar]
- 37.Moon HS, Park WI, Choi EA, Chung HW, Kim SC. The expression and tyrosine phosphorylation of E-cadherin/catenin adhesion complex, and focal adhesion kinase in invasive cervical carcinomas. Int J Gynecol Cancer. 2003;13(5):640–646. doi: 10.1046/j.1525-1438.2003.13396.x. [DOI] [PubMed] [Google Scholar]
- 38.Schroder A, Delling G, Kaiser EA. Expression analysis of protein tyrosine kinases of the FAK (focal adhesion kinase) family in osteosarcoma. Pathologe. 2002;23(5):361–366. doi: 10.1007/s00292-002-0557-x. [DOI] [PubMed] [Google Scholar]
- 39.Jenq W, Cooper DR, Ramirez G. Integrin expression on cell adhesion function and up-regulation of P125FAK and paxillin in metastatic renal carcinoma cells. Connect Tissue Res. 1996;34(3):161–174. doi: 10.3109/03008209609000696. [DOI] [PubMed] [Google Scholar]
- 40.Chan PC, Liang CC, Yu KC, Chang MC, Ho WL, Chen BH, Chen HC. Synergistic effect of focal adhesion kinase over-expression and hepatocyte growth factor stimulation on cell transformation. J Biol Chem. 2002;277(52):50373–50379. doi: 10.1074/jbc.M204691200. [DOI] [PubMed] [Google Scholar]
- 41.Nishimura M, Machida K, Imaizumi M, Abe T, Umeda T, Takeshima E, Watanabe T, Ohnishi Y, Takagi K, Hamaguchi M. Tyrosine phosphorylation of 100–130 kDa proteins in lung cancer correlates with poor prognosis. Br J Cancer. 1996;74(5):780–787. doi: 10.1038/bjc.1996.436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Imaizumi M, Nishimura M, Takeuchi S, Murase M, Hamaguchi M. Role of tyrosine specific phosphorylation of cellular proteins, especially EGF receptor and p125FAK in human lung cancer cells. Lung Cancer. 1997;17(1):69–84. doi: 10.1016/s0169-5002(97)00650-8. [DOI] [PubMed] [Google Scholar]
- 43.Kodama K, Ishii G, Miyamoto S, Goya M, Zhang SC, Sangai T, Yoshikawa T, Hasebe T, Hitomi Y, Izumi K, Ochiai A. Laminin 5 expression protects against anoikis at aerogenous spread and lepidic growth of human lung adenocarcinoma. Int J Cancer. 2005;116(6):876–884. doi: 10.1002/ijc.21136. [DOI] [PubMed] [Google Scholar]
- 44.Maulik G, Kijima T, Ma PC, Ghosh SK, Lin J, Shapiro GI, Schaefer E, Tibaldi E, Johnson BE, Salgia R. Modulation of the c-Met/hepatocyte growth factor pathway in small cell lung cancer. Clin Cancer Res. 2002;8(2):620–627. [PubMed] [Google Scholar]
- 45.van Nimwegen MJ, Verkoeijen S, van Buren L, Burg D, van de Water B. Requirement for focal adhesion kinase in the early phase of mammary adenocarcinoma lung metastasis formation. Cancer Res. 2005;65(11):4698–4706. doi: 10.1158/0008-5472.CAN-04-4126. [DOI] [PubMed] [Google Scholar]
- 46.Carelli S, Zadra G, Vaira V, Falleni M, Bottiglieri L, Nosotti M, Di Giulio AM, Gorio A, Bosari S. Up-regulation of focal adhesion kinase in non-small cell lung cancer. Lung Cancer. 2006;53(3):263–271. doi: 10.1016/j.lungcan.2006.06.001. [DOI] [PubMed] [Google Scholar]
- 47.Fleigel J, Sedwick J, Kornberg LJ. Hepatocyte growth factor/scatter factor stimulates mitogenesis and migration of a head and neck squamous cell carcinoma cell line. Otolaryngol Head Neck Surg. 2002;127(4):271–278. doi: 10.1067/mhn.2002.127414. [DOI] [PubMed] [Google Scholar]
- 48.Kornberg LJ. Adenovirus-mediated transfer of FRNK augments drug-induced cytotoxicity in cultured SCCHN cells. Anticancer Res. 2005;25(6B):4349–4356. [PubMed] [Google Scholar]
- 49.Canel M, Secades P, Rodrigo JP, Cabanillas R, Herrero A, Suarez C, Chiara MD. Overexpression of focal adhesion kinase in head and neck squamous cell carcinoma is independent of fak gene copy number. Clin Cancer Res. 2006;12(11):3272–3279. doi: 10.1158/1078-0432.CCR-05-1583. [DOI] [PubMed] [Google Scholar]
- 50.Kornberg L. Ad-fRNK and Ad-p53 cooperate to augment drug-induced death of a transformed cell line. Anticancer Res. 2006;26(4B):3025–3031. [PubMed] [Google Scholar]
- 51.Yang TH, Chen CT, Wang CP, Lou PJ. Photodynamic therapy suppresses the migration and invasion of head and neck cancer cells in vitro. Oral Oncol. 2007;43(4):358–365. doi: 10.1016/j.oraloncology.2006.04.007. [DOI] [PubMed] [Google Scholar]
- 52.Canel M, Secades P, Garzon-Arango M, Allonca E, Suarez C, Serrels A, Frame M, Brunton V, Chiara MD. Involvement of focal adhesion kinase in cellular invasion of head and neck squamous cell carcinomas via regulation of MMP-2 expression. Br J Cancer. 2008;98(7):1274–1284. doi: 10.1038/sj.bjc.6604286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kornberg LJ. Focal adhesion kinase expression in oral cancers. Head Neck. 1998;20(7):634–639. doi: 10.1002/(sici)1097-0347(199810)20:7<634::aid-hed10>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- 54.Schneider GB, Kurago Z, Zaharias R, Gruman LM, Schaller MD, Hendrix MJ. Elevated focal adhesion kinase expression facilitates oral tumor cell invasion. Cancer. 2002;95(12):2508–2515. doi: 10.1002/cncr.10992. [DOI] [PubMed] [Google Scholar]
- 55.Kitajo H, Shibata T, Nagayasu H, Kawano T, Hamada J, Yamashita T, Arisue M. Rho regulates the hepatocyte growth factor/scatter factor-stimulated cell motility of human oral squamous cell carcinoma cells. Oncol Rep. 2003;10(5):1351–1356. [PubMed] [Google Scholar]
- 56.Aronsohn MS, Brown HM, Hauptman G, Kornberg LJ. Expression of focal adhesion kinase and phosphorylated focal adhesion kinase in squamous cell carcinoma of the larynx. Laryngoscope. 2003;113(11):1944–1948. doi: 10.1097/00005537-200311000-00017. [DOI] [PubMed] [Google Scholar]
- 57.Fujii T, Koshikawa K, Nomoto S, Okochi O, Kaneko T, Inoue S, Yatabe Y, Takeda S, Nakao A. Focal adhesion kinase is overexpressed in hepatocellular carcinoma and can be served as an independent prognostic factor. J Hepatol. 2004;41(1):104–111. doi: 10.1016/j.jhep.2004.03.029. [DOI] [PubMed] [Google Scholar]
- 58.von Sengbusch A, Gassmann P, Fisch KM, Enns A, Nicolson GL, Haier J. Focal adhesion kinase regulates metastatic adhesion of carcinoma cells within liver sinusoids. Am J Pathol. 2005;166(2):585–596. doi: 10.1016/S0002-9440(10)62280-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Bouchard MJ, Wang L, Schneider RJ. Activation of focal adhesion kinase by hepatitis B virus HBx protein: multiple functions in viral replication. J Virol. 2006;80(9):4406–4414. doi: 10.1128/JVI.80.9.4406-4414.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Weiner TM, Liu ET, Craven RJ, Cance WG. Expression of focal adhesion kinase gene and invasive cancer. Lancet. 1993;342(8878):1024–1025. doi: 10.1016/0140-6736(93)92881-s. [DOI] [PubMed] [Google Scholar]
- 61.Lark AL, Livasy CA, Calvo B, Caskey L, Moore DT, Yang X, Cance WG. Overexpression of focal adhesion kinase in primary colorectal carcinomas and colorectal liver metastases: immunohistochemistry and real-time PCR analyses. Clin Cancer Res. 2003;9(1):215–222. [PubMed] [Google Scholar]
- 62.Theocharis SE, Kouraklis GP, Kakisis JD, Kanelli HG, Apostolakou FE, Karatzas GM, Koutselinis AS. Focal adhesion kinase expression is not a prognostic predictor in colon adenocarcinoma patients. Eur J Surg Oncol. 2003;29(7):571–574. doi: 10.1016/s0748-7983(03)00120-3. [DOI] [PubMed] [Google Scholar]
- 63.Yu HG, Schrader H, Otte JM, Schmidt WE, Schmitz F. Rapid tyrosine phosphorylation of focal adhesion kinase, paxillin, and p130Cas by gastrin in human colon cancer cells. Biochem Pharmacol. 2004;67(1):135–146. doi: 10.1016/j.bcp.2003.08.015. [DOI] [PubMed] [Google Scholar]
- 64.Yu HG, Tong SL, Ding YM, Ding J, Fang XM, Zhang XF, Liu ZJ, Zhou YH, Liu QS, Luo HS, Yu JP. Enhanced expression of cholecystokinin-2 receptor promotes the progression of colon cancer through activation of focal adhesion kinase. Int J Cancer. 2006;119(12):2724–2732. doi: 10.1002/ijc.22207. [DOI] [PubMed] [Google Scholar]
- 65.Golubovskaya VM, Gross S, Kaur AS, Wilson RI, Xu LH, Yang XH, Cance WG. Simultaneous inhibition of focal adhesion kinase and SRC enhances detachment and apoptosis in colon cancer cell lines. Mol Cancer Res. 2003;1(10):755–764. [PubMed] [Google Scholar]
- 66.Thamilselvan V, Craig DH, Basson MD. FAK association with multiple signal proteins mediates pressure-induced colon cancer cell adhesion via a Src-dependent PI3K/Akt pathway. Faseb J. 2007;21(8):1730–1741. doi: 10.1096/fj.06-6545com. [DOI] [PubMed] [Google Scholar]
- 67.Tremblay L, Hauck W, Aprikian AG, Begin LR, Chapdelaine A, Chevalier S. Focal adhesion kinase (pp125FAK) expression, activation and association with paxillin and p50CSK in human metastatic prostate carcinoma. Int J Cancer. 1996;68(2):164–171. doi: 10.1002/(sici)1097-0215(19961009)68:2<169::aid-ijc4>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- 68.Zheng DQ, Woodard AS, Fornaro M, Tallini G, Languino LR. Prostatic carcinoma cell migration via alpha(v)beta3 integrin is modulated by a focal adhesion kinase pathway. Cancer Res. 1999;59(7):1655–1664. [PubMed] [Google Scholar]
- 69.Zeng ZZ, Jia Y, Hahn NJ, Markwart SM, Rockwood KF, Livant DL. Role of focal adhesion kinase and phosphatidylinositol 3′-kinase in integrin fibronectin receptor-mediated, matrix metallo-proteinase-1-dependent invasion by metastatic prostate cancer cells. Cancer Res. 2006;66(16):8091–8099. doi: 10.1158/0008-5472.CAN-05-4400. [DOI] [PubMed] [Google Scholar]
- 70.Slack-Davis JK, Hershey ED, Theodorescu D, Frierson HF, Parsons JT. Differential requirement for focal adhesion kinase signaling in cancer progression in the transgenic adenocarcinoma of mouse prostate model. Mol Cancer Ther. 2009;8(8):2470–2477. doi: 10.1158/1535-7163.MCT-09-0262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Hecker TP, Grammer JR, Gillespie GY, Stewart J, Jr, Gladson CL. Focal adhesion kinase enhances signaling through the Shc/extracellular signal-regulated kinase pathway in anaplastic astrocytoma tumor biopsy samples. Cancer Res. 2002;62(9):2699–2707. [PubMed] [Google Scholar]
- 72.Hecker TP, Ding Q, Rege TA, Hanks SK, Gladson CL. Overexpression of FAK promotes Ras activity through the formation of a FAK/p120RasGAP complex in malignant astrocytoma cells. Oncogene. 2004;23(22):3962–3971. doi: 10.1038/sj.onc.1207541. [DOI] [PubMed] [Google Scholar]
- 73.Natarajan M, Stewart JE, Golemis EA, Pugacheva EN, Alexandropoulos K, Cox BD, Wang W, Grammer JR, Gladson CL. HEF1 is a necessary and specific downstream effector of FAK that promotes the migration of glioblastoma cells. Oncogene. 2006;25(12):1721–1732. doi: 10.1038/sj.onc.1209199. [DOI] [PubMed] [Google Scholar]
- 74.Shi Q, Hjelmeland AB, Keir ST, Song L, Wickman S, Jackson D, Ohmori O, Bigner DD, Friedman HS, Rich JN. A novel low-molecular weight inhibitor of focal adhesion kinase, TAE226, inhibits glioma growth. Mol Carcinog. 2007;46(6):488–496. doi: 10.1002/mc.20297. [DOI] [PubMed] [Google Scholar]
- 75.Scott G, Liang H. pp125fak in human melanocytes and melanoma: expression and phosphorylation. Exp Cell Res. 1995;219(1):197–203. doi: 10.1006/excr.1995.1219. [DOI] [PubMed] [Google Scholar]
- 76.Akasaka T, van Leeuwen RL, Yoshinaga IG, Mihm MC, Jr, Byers HR. Focal adhesion kinase (p125FAK) expression correlates with motility of human melanoma cell lines. J Invest Dermatol. 1995;105(1):104–108. doi: 10.1111/1523-1747.ep12313396. [DOI] [PubMed] [Google Scholar]
- 77.Maung K, Easty DJ, Hill SP, Bennett DC. Requirement for focal adhesion kinase in tumor cell adhesion. Oncogene. 1999;18(48):6824–6828. doi: 10.1038/sj.onc.1203094. [DOI] [PubMed] [Google Scholar]
- 78.Kahana O, Micksche M, Witz IP, Yron I. The focal adhesion kinase (P125FAK) is constitutively active in human malignant melanoma. Oncogene. 2002;21(25):3969–3977. doi: 10.1038/sj.onc.1205472. [DOI] [PubMed] [Google Scholar]
- 79.Smith CS, Golubovskaya VM, Peck E, Xu LH, Monia BP, Yang X, Cance WG. Effect of focal adhesion kinase (FAK) downregulation with FAK antisense oligonucleotides and 5-fluorouracil on the viability of melanoma cell lines. Melanoma Res. 2005;15(5):357–362. doi: 10.1097/00008390-200510000-00003. [DOI] [PubMed] [Google Scholar]
- 80.Hess AR, Hendrix MJ. Focal adhesion kinase signaling and the aggressive melanoma phenotype. Cell Cycle. 2006;5(5):478–480. doi: 10.4161/cc.5.5.2518. [DOI] [PubMed] [Google Scholar]
- 81.Hamamura K, Tsuji M, Ohkawa Y, Nakashima H, Miyazaki S, Urano T, Yamamoto N, Ueda M, Furukawa K, Furukawa K. Focal adhesion kinase as well as p130Cas and paxillin is crucially involved in the enhanced malignant properties under expression of ganglioside GD3 in melanoma cells. Biochim Biophys Acta. 2008;1780(3):513–519. doi: 10.1016/j.bbagen.2007.11.002. [DOI] [PubMed] [Google Scholar]
- 82.Lee JC, Tsai CY, Kao JY, Kao MC, Tsai SC, Chang CS, Huang LJ, Kuo SC, Lin JK, Way TD. Geraniin-mediated apoptosis by cleavage of focal adhesion kinase through up-regulation of Fas ligand expression in human melanoma cells. Mol Nutr Food Res. 2008;52(6):655–663. doi: 10.1002/mnfr.200700381. [DOI] [PubMed] [Google Scholar]
- 83.Sonoda Y, Hada N, Kaneda T, Suzuki T, Ohshio T, Takeda T, Kasahara T. A synthetic glycosphingolipid-induced antiproliferative effect in melanoma cells is associated with suppression of FAK, Akt, and Erk activation. Biol Pharm Bull. 2008;31(6):1279–1283. doi: 10.1248/bpb.31.1279. [DOI] [PubMed] [Google Scholar]
- 84.Owens LV, Xu L, Dent GA, Yang X, Sturge GC, Craven RJ, Cance WG. Focal adhesion kinase as a marker of invasive potential in differentiated human thyroid cancer. Annals Surg Oncol. 1996;3(1):100–105. doi: 10.1007/BF02409059. [DOI] [PubMed] [Google Scholar]
- 85.Kim SJ, Park JW, Yoon JS, Mok JO, Kim YJ, Park HK, Kim CH, Byun DW, Lee YJ, Jin SY, Suh KI, Yoo MH. Increased expression of focal adhesion kinase in thyroid cancer: immunohistochemical study. J Korean Med Sci. 2004;19(5):710–715. doi: 10.3346/jkms.2004.19.5.710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Recher C, Ysebaert L, Beyne-Rauzy O, Mansat-De Mas V, Ruidavets JB, Cariven P, Demur C, Payrastre B, Laurent G, Racaud-Sultan C. Expression of focal adhesion kinase in acute myeloid leukemia is associated with enhanced blast migration, increased cellularity, and poor prognosis. Cancer Res. 2004;64(9):3191–3197. doi: 10.1158/0008-5472.can-03-3005. [DOI] [PubMed] [Google Scholar]